Beruflich Dokumente
Kultur Dokumente
Guide To Solving Spectroscopy Problems
Hochgeladen von
JenOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Guide To Solving Spectroscopy Problems
Hochgeladen von
JenCopyright:
Verfügbare Formate
Guide to Solving Spectroscopy Problems
by Jessica Reel, Fall 2016
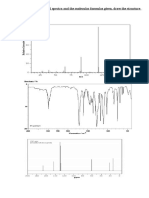
Shown below are the MS, IR, 1H NMR, and 13C NMR spectra of an unknown compound.
What is the structure of this compound?
These types of problems are common in undergraduate organic chemistry. The easiest way to go about
solving these is to make a list of all the information that you can glean from the provided spectra, and
then solve the structure bit by bit, similar to a jigsaw puzzle.
1. MS: gives molecular weight, presence of halogens
The mass spec molecular ion peak, designated as M or M+, gives the molecular weight.
An M+2 peak roughly the same height as the M peak indicates Br; an M+2 peak that is 1/3 the
height of the M peak indicates Cl.
If the molecular (or empirical) formula is not given, use the molecular weight to make a list of
possible formulas. C has a mass of 12, H = 1, O = 16. Use other spectra to refine the list.
2. Molecular (or empirical) formula: degree of unsaturation
If the compound has only C, H, and (optionally) O atoms, its fully saturated if the formula fits
the pattern
Cn H2n+2 O(any)
If its not fully saturated, you can calculate the degree of unsaturation (DoU) as follows:
+
= + = 1 + (
)
2
where C = number of carbons, H = number of hydrogens, X = number of halogens, and N =
number of nitrogens. (Note that oxygen has no effect on degree of unsaturation.)
An alternate method for determining DoU: Draw any structure that contains the same number
of C, N, O, X, etc. atoms as the molecular formula, disregarding Hs. Do not include any rings or
-bonds in your drawing, and use as many hydrogens as needed to complete the octet for each
atom. Count the number of Hs in your structure and subtract the number of Hs in the actual
molecular formula. The difference, divided by two, equals the degree of unsaturation.
If the degree of unsaturation is at least 4, its reasonable to assume a benzene
sub-structure (1 ring + 3 bonds)
3. IR: what functional groups are present or absent?
Dont try to assign every peak in the spectrum. Look for the presence or absence of a few key
peaks; use the DoU to rule out possible functional groups (such as C=O and C=C) beforehand.
The table below lists the most informative IR peaks from left-to-right; the most important peaks to
check for first are highlighted in blue.
Guide to Solving Spectroscopy Problems
Stretch
OH
4.
Approx. cm-1
35003100
33002500
NH
35003300
CH
=CH
CH
O=CH
CC, CN
aromatic
C=O
C=C
3300
31003010
29502850
28302700
22602100
20001600
18001650
16801600
13
Appearance
Very broad, smooth hump.
Very broad but jagged. May overlap
with CH stretches.
Two somewhat broad peaks.
One somewhat broad peak.
Strong, sharp peak.
Medium intensity.
Medium to strong intensity.
Small sharp peak.
Relatively weak, but easy to spot.
A group of fairly weak peaks.
Very strong, sharp peak.
Intensity may vary.
J. Reel, Fall 2016
Indicates
Alcohol
Carboxylic acid
(if C=O also present)
1 amine/amide
2 amine/amide
Terminal alkyne
Alkene/aromatic
Alkyl groups
Aldehyde
Triple bond
Aromatic
Carbonyl
Alkene
C NMR: how many, and what types, of carbons?
Number of peaks = number of chemically inequivalent carbons. These are carbons that cannot
be exchanged or interconverted via symmetry or bond rotation.
Peak height (integration) is not useful information in 13C NMR.
The chemical shift (ppm) gives information about the functional group containing the carbon.
Carbons near electronegative atoms (O, N, F, etc.) are more deshielded (higher ppm).
Approx. ppm
< 0 ppm (rarely seen)
040 ppm
3080 ppm
65100 ppm
77 ppm, 1:1:1 triplet
100150 ppm
160210 ppm
Type of carbon
carbon attached to a metal
sp3 carbon NOT attached to an electronegative heteroatom.
sp3 carbon attached to an electronegative atom (O, N, X, etc.)
alkyne sp carbons ( CC )
signal for CDCl3 solvent; not part of the structure youre solving
sp2 carbons (alkenes and aromatics)
carbonyl (C=O) carbons
13
DEPT-135 spectra will show CH and CH3 peaks above the baseline, CH2 below the baseline, and
omit quaternary carbons (including C=O). DEPT-90 will show only CH peaks (above the baseline).
These can be useful for determining sub-structures (puzzle pieces).
C NMR spectra are often acquired in proton decoupled mode. However, if you are given a
proton coupled spectrum, you can use it to determine how many Hs are attached to each C, via
the splitting patterns: a carbon with N attached hydrogens will be split into N+1 peaks.
5. 1H NMR: how many, and what types, of hydrogens?
Number of peaks =number of chemically inequivalent hydrogens.
H NMR spectra tend to take the most time to analyze, so you may want to do that last.
Guide to Solving Spectroscopy Problems
J. Reel, Fall 2016
Peak height (integration) gives a ratio of how many Hs produced each signal. Divide all
integrations by the smallest integration to give the ratio of protons for each peak.
As with 13C NMR, the chemical shift gives information about the functional group:
Approx. ppm
02 ppm
23 ppm
35 ppm
56 ppm
68 ppm
910 ppm
1012 ppm
Type of proton
sp3 CH attached to another sp3 C
sp3 CH attached to C=O, C=C, or C=N,
OR terminal alkyne CCH (singlet)
sp3 CH attached to O, N, or halogen
alkene sp2 C=CH
aromatic sp2 C=CH
aldehyde O=CH
carboxylic acid COOH
The splitting pattern tells how many non-equivalent protons are on the same carbon or adjacent
carbons. A signal will be split into N+1 peaks by N non-equivalent neighboring protons. (Each
non-equivalent neighboring H adds 1 to the multiplicity.) A signal which is not split (a singlet)
means the proton has no adjacent non-equivalent H atoms, indicating that the carbon which
bears the proton is likely connected to a heteroatom (O, N, etc.) or a 4 carbon (including C=O).
Some common splitting patterns are provided in the table below:
Common 1H NMR patterns
3H singlet between 04 ppm
2H quartet with a 3H triplet
1H sextet with a 6H doublet
9H singlet between 03 ppm
two 1H doublets around 6 ppm
three 1H doublet of doublets
Indicates this
( indicates a heteroatom or 4 C)
methyl group
CH3
ethyl group
CH2CH3
isopropyl group
CH(CH3)2
tert-butyl group
C(CH3)3
alkene with 2 different
CH=CH
substituents (see below)
C=CH2
vinyl group
CH=CH2
(may be hard to distinguish)
two 2H doublets around 7 ppm
one 1H s, two 1H d, one 1H dd
two 1H d, two 1H dd
para-disubstituted benzene
meta-disubstituted benzene
ortho-disubstituted benzene
(these may be difficult
to discern; may appear
as a multiplet)
When comparing two H atoms on a ring or double bond, the two protons are equivalent only if
they are cis and trans to the same groups; see the examples below.
These dichloroethenes each produce one singlet
in the 1H NMR spectrum.
These bromochloroethenes each produce two doublets
in the 1H NMR spectrum.
Chlorocyclopropane gives rise to 3 separate 1H NMR signals.
Guide to Solving Spectroscopy Problems
J. Reel, Fall 2016
A special note about OH and NH proton signals: They may not be visible on the spectrum, may
appear over a wide ppm range, may be broad, tend not to split, and may not integrate to the
expected ratio. They will also exchange with D2O, which will cause them to no longer show up
on the spectrum.
6. Put the pieces together!
Draw all the known sub-structures or functional groups; dont worry about connecting them yet.
Double-check that all the pieces add up to the molecular formula (or molecular weight).
Account for degree(s) of unsaturation. If the DoU > 0 and none of the spectra indicate bonds,
you probably have a cyclic compound.
Try to connect pieces, based on 1H NMR splitting patterns (neighboring Hs)
Chemical shift can tell you if a C or H is next to an O, F, Cl, Br, N, C=O, etc.
The mass spec fragmentation pattern (if provided) can give clues. A series of high-intensity
peaks all separated by 14 (corresponding to CH2) is characteristic of long-chain hydrocarbons.
Assemble the pieces into an educated guess for the complete structure.
Once you make a complete structure, double-check! Does this structure make sense? Would
this structure have produced exactly these spectra?
Das könnte Ihnen auch gefallen
- Report NMRDokument13 SeitenReport NMRsarahNoch keine Bewertungen
- Spec Prob Set 315 CurrentDokument20 SeitenSpec Prob Set 315 CurrentUmang Agarwal57% (7)
- PMR Spectroscopy: Solved Problems Volume : IIVon EverandPMR Spectroscopy: Solved Problems Volume : IIBewertung: 5 von 5 Sternen5/5 (3)
- NMR Solving StrategyDokument2 SeitenNMR Solving Strategysorrow Lemon100% (1)
- NMR HandoutDokument23 SeitenNMR HandoutVirendra Singh RajputNoch keine Bewertungen
- Nuclear Magnetic Resonance SpectrosDokument11 SeitenNuclear Magnetic Resonance Spectrosilias1973Noch keine Bewertungen
- 2d NMRDokument32 Seiten2d NMRDelicz TanNoch keine Bewertungen
- Spectroscopic Solutions of StructureDokument21 SeitenSpectroscopic Solutions of StructureKassimNoch keine Bewertungen
- Chemistry 344: Spectroscopy and Spectrometry Problem Set 1Dokument12 SeitenChemistry 344: Spectroscopy and Spectrometry Problem Set 1Fasiha RazaNoch keine Bewertungen
- IR - HNMR ProblemsDokument33 SeitenIR - HNMR Problemsbsakaly112100% (1)
- UV-visible Spectrum of A Transition Metal Complex (d3 - d8) From Internet - Assign The Peaks and Using The Appropriate Tanabe - Sugano Diagram Calculate Do For The Complex.Dokument5 SeitenUV-visible Spectrum of A Transition Metal Complex (d3 - d8) From Internet - Assign The Peaks and Using The Appropriate Tanabe - Sugano Diagram Calculate Do For The Complex.peptidesynthesizerNoch keine Bewertungen
- Silverstein Chapter 1 Mass SpectrometryDokument71 SeitenSilverstein Chapter 1 Mass SpectrometryNikita GroverNoch keine Bewertungen
- NMR 1Dokument3 SeitenNMR 1amitNoch keine Bewertungen
- 13C NMR SpectrosDokument16 Seiten13C NMR Spectrosapi-3723327100% (4)
- Ir SpectrosDokument32 SeitenIr SpectrosAnonymous vr1l9YlnsNoch keine Bewertungen
- OSE Answer BookDokument35 SeitenOSE Answer BookDdd Bbb100% (1)
- Spectroscopy Problems Part 1Dokument49 SeitenSpectroscopy Problems Part 1Partha Samanta100% (1)
- NMR SpectrosDokument29 SeitenNMR Spectroshareesh13h100% (1)
- Electroanalysis: Theory and Applications in Aqueous and Non-Aqueous Media and in Automated Chemical ControlVon EverandElectroanalysis: Theory and Applications in Aqueous and Non-Aqueous Media and in Automated Chemical ControlNoch keine Bewertungen
- Reductions in Organic ChemistryDokument13 SeitenReductions in Organic ChemistryNikola PudjaNoch keine Bewertungen
- Solving UnknownsDokument36 SeitenSolving UnknownsluyawinNoch keine Bewertungen
- (UV Vis) SpectrosDokument4 Seiten(UV Vis) SpectrosGarion Charles0% (1)
- McMurry OC8e EV CH13 PDFDokument28 SeitenMcMurry OC8e EV CH13 PDFCrizel Ricaro100% (1)
- NMR Organic ChemistryDokument17 SeitenNMR Organic Chemistrysallymoon34Noch keine Bewertungen
- NMR SpectrosDokument185 SeitenNMR SpectrosBathir JafarNoch keine Bewertungen
- Essays on Analytical Chemistry: In Memory of Professor Anders RingbomVon EverandEssays on Analytical Chemistry: In Memory of Professor Anders RingbomErkki WänninenNoch keine Bewertungen
- Combined Problems and Solution On Organic Spectros PDFDokument2 SeitenCombined Problems and Solution On Organic Spectros PDFJill29% (7)
- Molecular Spectroscopy: BackgroundDokument45 SeitenMolecular Spectroscopy: Backgroundsavvy_as_98100% (1)
- Ws10 AnswersDokument4 SeitenWs10 AnswersKassimNoch keine Bewertungen
- Hybrid Retrosynthesis: Organic Synthesis using Reaxys and SciFinderVon EverandHybrid Retrosynthesis: Organic Synthesis using Reaxys and SciFinderNoch keine Bewertungen
- Resonance and Inductive Effects PresentationDokument36 SeitenResonance and Inductive Effects Presentationeagl33yeNoch keine Bewertungen
- Isolobal AnalogyDokument4 SeitenIsolobal Analogyindu priyaNoch keine Bewertungen
- Nucleophilic Substitution ReactionDokument17 SeitenNucleophilic Substitution ReactionRojo JohnNoch keine Bewertungen
- Structure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsVon EverandStructure and Properties of Inorganic Solids: International Series of Monographs in Solid State PhysicsNoch keine Bewertungen
- Organic Structure Elucidation WorkbookDokument498 SeitenOrganic Structure Elucidation WorkbookKajan Muneeswaran75% (4)
- Answer: (A) and (B)Dokument18 SeitenAnswer: (A) and (B)Germaine Manangan100% (1)
- AromaticityDokument12 SeitenAromaticityV G Viju KumarNoch keine Bewertungen
- Clusters and Catenation in P-Block: Allotropes of CarbonDokument15 SeitenClusters and Catenation in P-Block: Allotropes of Carbonrajender kumarNoch keine Bewertungen
- Synthesis Characterization and Biological Evaluation of Somethiazolidinone Derivatives As Antimicrobial AgentsDokument8 SeitenSynthesis Characterization and Biological Evaluation of Somethiazolidinone Derivatives As Antimicrobial Agentssunaina agarwalNoch keine Bewertungen
- Model Answer: The Following Questions Answer Choose The Correct Answer: (20Dokument4 SeitenModel Answer: The Following Questions Answer Choose The Correct Answer: (20Khalid AbeedNoch keine Bewertungen
- PhotochemistryDokument24 SeitenPhotochemistryVijay PradhanNoch keine Bewertungen
- Enzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisVon EverandEnzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisBewertung: 4 von 5 Sternen4/5 (2)
- Aromaticity CompleteDokument104 SeitenAromaticity Completewahidalwahdi100% (1)
- Module8 PDFDokument40 SeitenModule8 PDFFaizan AhmadNoch keine Bewertungen
- Hückel's MO Treatment of BenzeneDokument12 SeitenHückel's MO Treatment of BenzeneRichard Allen0% (1)
- Electronic Absorption Spectra and Geometry of Organic Molecules: An Application of Molecular Orbital TheoryVon EverandElectronic Absorption Spectra and Geometry of Organic Molecules: An Application of Molecular Orbital TheoryBewertung: 5 von 5 Sternen5/5 (1)
- Lecture Notes 2 Nano MaterialsDokument21 SeitenLecture Notes 2 Nano MaterialsHuzaifa ShabbirNoch keine Bewertungen
- Experiments in Physical Chemistry: Second Revised and Enlarged EditionVon EverandExperiments in Physical Chemistry: Second Revised and Enlarged EditionNoch keine Bewertungen
- Polytechnic TRB Syllabus of ChemistryDokument4 SeitenPolytechnic TRB Syllabus of ChemistrysanjeevNoch keine Bewertungen
- Chalcone Synthesis, Structure DiversityDokument13 SeitenChalcone Synthesis, Structure DiversityDini Elsi ANoch keine Bewertungen
- Huckel Theory For Conjugated Systems: CH 105: Organic ChemistryDokument72 SeitenHuckel Theory For Conjugated Systems: CH 105: Organic ChemistryRaunaq Bhirangi100% (1)
- Retrosynthetic Analysis PDFDokument6 SeitenRetrosynthetic Analysis PDFNoleNoch keine Bewertungen
- Experiment I1 Preparation of Some Cobaltammine ComplexesDokument7 SeitenExperiment I1 Preparation of Some Cobaltammine ComplexesIftitah HauriyahNoch keine Bewertungen
- 1311 2181 SyllabusDokument5 Seiten1311 2181 SyllabusJenNoch keine Bewertungen
- Content ServerDokument23 SeitenContent ServerJenNoch keine Bewertungen
- Racial Climate PDFDokument16 SeitenRacial Climate PDFJenNoch keine Bewertungen
- 1 PDFDokument36 Seiten1 PDFJenNoch keine Bewertungen
- 3 Facets of SelfesteemDokument6 Seiten3 Facets of SelfesteemRichieDaisyNoch keine Bewertungen
- N XPLRTR TD FTH RLTNHP BT NTLVD PRT V N HBT ND NFRT T LN NRDokument27 SeitenN XPLRTR TD FTH RLTNHP BT NTLVD PRT V N HBT ND NFRT T LN NRJenNoch keine Bewertungen
- 10 1 1 476 8200 PDFDokument17 Seiten10 1 1 476 8200 PDFJorge R FreitasNoch keine Bewertungen
- Sample Paper-03 CHEMISTRY (Theory) Class - XI: Material Downloaded From andDokument4 SeitenSample Paper-03 CHEMISTRY (Theory) Class - XI: Material Downloaded From andSarthakNoch keine Bewertungen
- 14 BishopDokument44 Seiten14 Bishopnfalkdrf alkfalkNoch keine Bewertungen
- Coal PropertiesDokument11 SeitenCoal Propertiesgujrati guyNoch keine Bewertungen
- Problem, Failure and Safety Analysis of Ammonia Plant-A ReviewDokument16 SeitenProblem, Failure and Safety Analysis of Ammonia Plant-A ReviewATUL SONAWANE67% (3)
- Properties of MatterDokument22 SeitenProperties of MatterNicole Joyce Catabay FloresNoch keine Bewertungen
- P Block Elements-Group 16Dokument41 SeitenP Block Elements-Group 16Sanskriti Keshkar100% (5)
- Power Plant ChemistryDokument0 SeitenPower Plant ChemistrySHIVAJI CHOUDHURY75% (4)
- Small Molecules and The Chemistry of LifeDokument46 SeitenSmall Molecules and The Chemistry of LifeDiabyNoch keine Bewertungen
- Experiment # 8 Six-Step Synthesis Aniline To 1-Bromo-3cholor-5iodobenzeneDokument10 SeitenExperiment # 8 Six-Step Synthesis Aniline To 1-Bromo-3cholor-5iodobenzeneColin CheNoch keine Bewertungen
- Experiments H-TecDokument40 SeitenExperiments H-TecAmélia MoreiraNoch keine Bewertungen
- Jin Spirit Human (Reincarnation)Dokument202 SeitenJin Spirit Human (Reincarnation)Emre100% (20)
- Canon ES700Dokument8 SeitenCanon ES700d.ramadhanNoch keine Bewertungen
- Shashi NFL Report 2011Dokument93 SeitenShashi NFL Report 2011Shashi VermaNoch keine Bewertungen
- Icpat 8Dokument204 SeitenIcpat 8MisgatesNoch keine Bewertungen
- CHT Reviewer OChem 5Dokument112 SeitenCHT Reviewer OChem 5Chastine CruzNoch keine Bewertungen
- Chemical Cleaning and Degassing Refinery EquipmentDokument4 SeitenChemical Cleaning and Degassing Refinery EquipmentWilfred OzerecNoch keine Bewertungen
- Copper Cycle LabDokument4 SeitenCopper Cycle LabShubham ChattopadhyayNoch keine Bewertungen
- Discussion Exp 1Dokument6 SeitenDiscussion Exp 1Dhirah Yuhans67% (3)
- On-Site Electrolytic Chlorination Skid-Mounted OSEC B-Pak SystemDokument4 SeitenOn-Site Electrolytic Chlorination Skid-Mounted OSEC B-Pak SystemgohviccNoch keine Bewertungen
- Failure of Heat Exchanger in Sulphur Recovery Unit PDFDokument8 SeitenFailure of Heat Exchanger in Sulphur Recovery Unit PDFticaram rNoch keine Bewertungen
- Index of Hydrogen DeficiencyDokument10 SeitenIndex of Hydrogen Deficiencymaheen aurangzaibNoch keine Bewertungen
- ICSE-Chemistry Sample Paper-1-Class 10 Question PaperDokument5 SeitenICSE-Chemistry Sample Paper-1-Class 10 Question PaperFirdosh Khan100% (4)
- Chemistry The Central Science 1Dokument4 SeitenChemistry The Central Science 1Ariane Caranto100% (2)
- O - LEVEL CHEMISTRY WORKSHEETS - Reactions QuestionsDokument21 SeitenO - LEVEL CHEMISTRY WORKSHEETS - Reactions QuestionsFahim Ahmed75% (4)
- Design of An Organic Waste Power Plant Coupling Anaerobic Digestion and Solid Oxide Fuel Cell TechnologiesDokument9 SeitenDesign of An Organic Waste Power Plant Coupling Anaerobic Digestion and Solid Oxide Fuel Cell TechnologiesMaria Fernanda Paillie PintoNoch keine Bewertungen
- Gas Dynamic Resonance Ignition For Repetitive StartsDokument8 SeitenGas Dynamic Resonance Ignition For Repetitive StartsBrunno VasquesNoch keine Bewertungen
- Hydrogen Production - ElectrolysisDokument4 SeitenHydrogen Production - ElectrolysisakshayNoch keine Bewertungen
- Compressed Gas CylindersDokument9 SeitenCompressed Gas Cylindersmomkok221Noch keine Bewertungen