Beruflich Dokumente
Kultur Dokumente
Quantum Tunneling
Hochgeladen von
cyrimathewOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Quantum Tunneling
Hochgeladen von
cyrimathewCopyright:
Verfügbare Formate
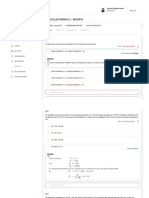
QUANTUM MECHANICAL TUNNELING
Let us consider the case of a particle (say, an electron) confined to move in a one-dimensional box with
walls of infinite and finite heights as shown below. The potential energy function V is assumed to be zero from
x =0 to x = a. But the potential energy V is
taken as at x =0 and at x = a, as V 0
(potential energy is finite). Classically the
electron should be found within the
boundaries x = 0 to x = a, and the
Potential barrier
electron wavefunction
V= V0
probability of finding the electron beyond
of finite height
x = a is to be zero. But it is observed that
when the potential barrier is finite and of
Thickness of barrier
suitable thickness, there exists a finite V
probability of finding the electron beyond
x = a. The probability decreases
exponentially with increase in barrier
a
0
thickness. This phenomenon is known as
x
electron tunnel through barrier
tunneling. Tunneling is a quantum
mechanical phenomenon. Radioactive decay is an example of tunneling. It is the process of emission of particles and
energy from the unstable nucleus of an atom to form a stable product. This is done via the tunneling of a particle out
of the nucleus (an electron tunneling into the nucleus is electron capture). Another example is the working of
scanning tunneling microscope (STM). In STM, the region from x = 0 to x = a, could be considered as tip and the
region beyond the barrier as sample. The barrier thickness could be considered as the distance d between tip
and sample surface.
Das könnte Ihnen auch gefallen
- Term SymbolDokument23 SeitenTerm SymbolCyriac Mathew73% (11)
- Physics2a 101118021050 Phpapp02Dokument315 SeitenPhysics2a 101118021050 Phpapp02umesh52Noch keine Bewertungen
- Problems in Quantum Mechanics: Third EditionVon EverandProblems in Quantum Mechanics: Third EditionBewertung: 3 von 5 Sternen3/5 (2)
- Quantum TunnelingDokument11 SeitenQuantum Tunnelingaashi kapoor100% (2)
- IMS - Integrated Management System Implementation Steps-Sterling - Rev00-240914 PDFDokument28 SeitenIMS - Integrated Management System Implementation Steps-Sterling - Rev00-240914 PDFNorman AinomugishaNoch keine Bewertungen
- TOK Assessed Student WorkDokument10 SeitenTOK Assessed Student WorkPeter Jun Park100% (1)
- AHU CatalogueDokument16 SeitenAHU CatalogueWai Ee YapNoch keine Bewertungen
- Portland Cement: Standard Specification ForDokument9 SeitenPortland Cement: Standard Specification ForHishmat Ezz AlarabNoch keine Bewertungen
- Grade 6 q2 Mathematics LasDokument151 SeitenGrade 6 q2 Mathematics LasERIC VALLE80% (5)
- Lecture Notes - Introduction To Big DataDokument8 SeitenLecture Notes - Introduction To Big Datasakshi kureley0% (1)
- SSD03Dokument25 SeitenSSD03AKHIL REDDY JAMBULANoch keine Bewertungen
- Lecture 20 - Quantum Tunneling of Electrons: Notes by MIT Student (And MZB)Dokument10 SeitenLecture 20 - Quantum Tunneling of Electrons: Notes by MIT Student (And MZB)Nabilah Calista Balqis AprilliaNoch keine Bewertungen
- 2021 PH107 Tutorial09Dokument2 Seiten2021 PH107 Tutorial09Bhakti MatsyapalNoch keine Bewertungen
- Tut Sheet 4 SCH RNT Freeb SHODokument8 SeitenTut Sheet 4 SCH RNT Freeb SHOPrithviraj NetkeNoch keine Bewertungen
- PH-107 (2018) Tutorial Sheet 10: V E. 0), For Which The ProbabilityDokument3 SeitenPH-107 (2018) Tutorial Sheet 10: V E. 0), For Which The ProbabilityVangala PorusNoch keine Bewertungen
- Changes Introduced in Quantum Confined Charge Carriers On Introduction of Limited Periodic Lattice PotentialsDokument22 SeitenChanges Introduced in Quantum Confined Charge Carriers On Introduction of Limited Periodic Lattice PotentialsarunNoch keine Bewertungen
- Quantum Tunneling NotesDokument5 SeitenQuantum Tunneling Notessaurabh gageNoch keine Bewertungen
- Visvesvaraya National Institute of Technology Nagpur: Vlsi Design MTECH 2021-23 Nano LabDokument9 SeitenVisvesvaraya National Institute of Technology Nagpur: Vlsi Design MTECH 2021-23 Nano LabRutvik PatelNoch keine Bewertungen
- 10 1D SystemDokument19 Seiten10 1D SystemRahajengNoch keine Bewertungen
- 11.9: Quantum-Mechanical Tunneling: Violating Classical MechanicsDokument4 Seiten11.9: Quantum-Mechanical Tunneling: Violating Classical MechanicsDdumbaNoch keine Bewertungen
- PHYSICSDokument13 SeitenPHYSICSRianie Taguiam CiprianoNoch keine Bewertungen
- X-Rays As Inverse of Photoelectric EffectDokument5 SeitenX-Rays As Inverse of Photoelectric Effectphysics LoverNoch keine Bewertungen
- Beta Decay As A Virtual Particle Interaction Analogous To Hawking RadiationDokument5 SeitenBeta Decay As A Virtual Particle Interaction Analogous To Hawking Radiationjaswinder singhNoch keine Bewertungen
- Sample Theory With Ques.-Quantum Mechanics (NET PH Unit-6) PDFDokument21 SeitenSample Theory With Ques.-Quantum Mechanics (NET PH Unit-6) PDFKuchibhotla MahatiNoch keine Bewertungen
- The Quantum Tunneling of Particles Through PDokument26 SeitenThe Quantum Tunneling of Particles Through PSayyad aliNoch keine Bewertungen
- Solid State Physics: Statistical Mechanics Band Theory of SolidsDokument23 SeitenSolid State Physics: Statistical Mechanics Band Theory of SolidsShivanshu PandeyNoch keine Bewertungen
- Equilibrium of Diffusion and Field Currents: 2.4.2 Debye LengthDokument5 SeitenEquilibrium of Diffusion and Field Currents: 2.4.2 Debye LengthjawaidaligNoch keine Bewertungen
- Photoelectric Effect: Classical PredictionDokument7 SeitenPhotoelectric Effect: Classical PredictionOliver58Noch keine Bewertungen
- Hall Effect PDFDokument11 SeitenHall Effect PDFArum WulandariNoch keine Bewertungen
- Quiz On Quantum MechanicsDokument3 SeitenQuiz On Quantum MechanicsAnge1196Noch keine Bewertungen
- Quantum Mechanics Practice TestDokument7 SeitenQuantum Mechanics Practice TestMatthew HolmesNoch keine Bewertungen
- A Research Assingment On The Behaviour of A ParticDokument14 SeitenA Research Assingment On The Behaviour of A ParticlmNoch keine Bewertungen
- Part 1Dokument5 SeitenPart 1pelegmeir4Noch keine Bewertungen
- Quantum 03Dokument29 SeitenQuantum 03manishkumawat1420Noch keine Bewertungen
- Class 6 CH101 06092012 PCDokument6 SeitenClass 6 CH101 06092012 PCSudheer ChauhanNoch keine Bewertungen
- Lab 1 Plancks Constant DCEDokument5 SeitenLab 1 Plancks Constant DCERaja KhanNoch keine Bewertungen
- Hall EffectDokument10 SeitenHall EffectNidaul Muiz Aufa100% (1)
- Electron States in A Crystal: Solid State PhysicsDokument23 SeitenElectron States in A Crystal: Solid State PhysicsXylarNoch keine Bewertungen
- Tut9 Band TheoryDokument2 SeitenTut9 Band TheoryaanjaneyakNoch keine Bewertungen
- Engineering PhysicsDokument10 SeitenEngineering PhysicsThilshathNoch keine Bewertungen
- Electron in A BoxDokument5 SeitenElectron in A BoxsonirocksNoch keine Bewertungen
- Tunnel Effect: A Particle Without The Energy To Pass Over A Potential Barrier May Still Tunnel Through ItDokument1 SeiteTunnel Effect: A Particle Without The Energy To Pass Over A Potential Barrier May Still Tunnel Through ItasiyahNoch keine Bewertungen
- Mt-201Electronic Conduction2023Dokument150 SeitenMt-201Electronic Conduction2023RashmiNoch keine Bewertungen
- Question 1Dokument8 SeitenQuestion 1abdul wahabNoch keine Bewertungen
- 1-Ion Channels-08!06!2022 (08-Jun-2022) Material II 08-06-2022 Goldman EquationDokument21 Seiten1-Ion Channels-08!06!2022 (08-Jun-2022) Material II 08-06-2022 Goldman Equationfiseha tadesseNoch keine Bewertungen
- Waves FunctionDokument45 SeitenWaves FunctionDrakalopNoch keine Bewertungen
- Phy Unit 3 QuantumMechanics PDFDokument18 SeitenPhy Unit 3 QuantumMechanics PDFJayanth GuntupalliNoch keine Bewertungen
- Particle in A 1-Dimensional Box - ChemWiki PDFDokument4 SeitenParticle in A 1-Dimensional Box - ChemWiki PDFSri PuduNoch keine Bewertungen
- Turnneling EffectDokument33 SeitenTurnneling EffectmiarcNoch keine Bewertungen
- Summary: The Classical Electron Conductors: Suggested ReadingDokument13 SeitenSummary: The Classical Electron Conductors: Suggested ReadingsidhajiNoch keine Bewertungen
- 26.electron & PhotonDokument27 Seiten26.electron & Photonrakeshece0701Noch keine Bewertungen
- Quantum TunnelingDokument9 SeitenQuantum TunnelingVedant NigadeNoch keine Bewertungen
- Mechanics ProblemDokument2 SeitenMechanics ProblemShikhar MehrotraNoch keine Bewertungen
- Quantum Tunnelling - WikipediaDokument68 SeitenQuantum Tunnelling - WikipediaAnonymous gUjimJKNoch keine Bewertungen
- Quantum Mechanics L3Dokument22 SeitenQuantum Mechanics L3shafinsq15Noch keine Bewertungen
- Set of Problems in Quantum MechanicsDokument4 SeitenSet of Problems in Quantum MechanicsAishwarya MauryaNoch keine Bewertungen
- HW1Dokument3 SeitenHW1Asghar FarhadiNoch keine Bewertungen
- Electronics (Bansal)Dokument11 SeitenElectronics (Bansal)RoNNoch keine Bewertungen
- X-RayDokument23 SeitenX-RayKunal kumarNoch keine Bewertungen
- Properties of X-Rays: Cullity Chapter 1Dokument47 SeitenProperties of X-Rays: Cullity Chapter 1neveed100% (2)
- Problems On ElectrostaticsDokument3 SeitenProblems On ElectrostaticsBen QuachtranNoch keine Bewertungen
- Jour4 339 56 RDokument19 SeitenJour4 339 56 RAli Raza ShahabNoch keine Bewertungen
- Tutorial 7 The Final Tutorial For PHI-101 Autumn 2023-24Dokument2 SeitenTutorial 7 The Final Tutorial For PHI-101 Autumn 2023-24drragravatNoch keine Bewertungen
- Manfra - CH 6 - Quantum Mechanics IIDokument32 SeitenManfra - CH 6 - Quantum Mechanics IIJohn DoeNoch keine Bewertungen
- 4 Tut 7 - 8 - 9 Q.M.Dokument3 Seiten4 Tut 7 - 8 - 9 Q.M.adarshpandey1515016Noch keine Bewertungen
- Nuclear Qudrupole Resonance Spectroscopy: Eq Xyzr DDokument2 SeitenNuclear Qudrupole Resonance Spectroscopy: Eq Xyzr DcyrimathewNoch keine Bewertungen
- Mossbauer SpectrosDokument7 SeitenMossbauer SpectroscyrimathewNoch keine Bewertungen
- Liquid CrystalsDokument3 SeitenLiquid CrystalscyrimathewNoch keine Bewertungen
- EprDokument8 SeitenEprcyrimathewNoch keine Bewertungen
- Infrared SpectrosDokument17 SeitenInfrared SpectroscyrimathewNoch keine Bewertungen
- LLLL SSSS: Uantum Echanical PINDokument5 SeitenLLLL SSSS: Uantum Echanical PINcyrimathewNoch keine Bewertungen
- Point GroupsDokument3 SeitenPoint GroupscyrimathewNoch keine Bewertungen
- Group Theory in A NutshellDokument7 SeitenGroup Theory in A NutshellcyrimathewNoch keine Bewertungen
- Distribution of Continuous R.V.: Normal Distribution (CH 1.4) TopicsDokument7 SeitenDistribution of Continuous R.V.: Normal Distribution (CH 1.4) TopicsPhạm Ngọc HòaNoch keine Bewertungen
- DocsDokument4 SeitenDocsSwastika SharmaNoch keine Bewertungen
- Analog Electronics-2 PDFDokument20 SeitenAnalog Electronics-2 PDFAbhinav JangraNoch keine Bewertungen
- Basics PDFDokument21 SeitenBasics PDFSunil KumarNoch keine Bewertungen
- Boq Cme: 1 Pole Foundation Soil WorkDokument1 SeiteBoq Cme: 1 Pole Foundation Soil WorkyuwonoNoch keine Bewertungen
- Solutions Jet FuelDokument4 SeitenSolutions Jet FuelkevinNoch keine Bewertungen
- (Database Management Systems) : Biag, Marvin, B. BSIT - 202 September 6 2019Dokument7 Seiten(Database Management Systems) : Biag, Marvin, B. BSIT - 202 September 6 2019Marcos JeremyNoch keine Bewertungen
- Directorate of Technical Education, Maharashtra State, MumbaiDokument57 SeitenDirectorate of Technical Education, Maharashtra State, MumbaiShubham DahatondeNoch keine Bewertungen
- Week 2 - Sulphur DyesDokument5 SeitenWeek 2 - Sulphur DyesRR TNoch keine Bewertungen
- Read The Dialogue Below and Answer The Following QuestionDokument5 SeitenRead The Dialogue Below and Answer The Following QuestionDavid GainesNoch keine Bewertungen
- English 2 Q3 Week 7 DLLDokument7 SeitenEnglish 2 Q3 Week 7 DLLEste R A BulaonNoch keine Bewertungen
- SAP Environment, Health, and Safety (EHS)Dokument13 SeitenSAP Environment, Health, and Safety (EHS)SAFETY VOFPLNoch keine Bewertungen
- Civil Engineering Literature Review SampleDokument6 SeitenCivil Engineering Literature Review Sampleea442225100% (1)
- Ems Speed Sensor Com MotorDokument24 SeitenEms Speed Sensor Com MotorKarina RickenNoch keine Bewertungen
- Introduction To Templates in C++Dokument16 SeitenIntroduction To Templates in C++hammarbytpNoch keine Bewertungen
- sp.1.3.3 Atoms,+Elements+&+Molecules+ActivityDokument4 Seitensp.1.3.3 Atoms,+Elements+&+Molecules+ActivityBryaniNoch keine Bewertungen
- Supply Chain Management 101Dokument36 SeitenSupply Chain Management 101Trần Viết ThanhNoch keine Bewertungen
- List of Institutions With Ladderized Program Under Eo 358 JULY 2006 - DECEMBER 31, 2007Dokument216 SeitenList of Institutions With Ladderized Program Under Eo 358 JULY 2006 - DECEMBER 31, 2007Jen CalaquiNoch keine Bewertungen
- GeminiDokument397 SeitenGeminiJohnnyJC86Noch keine Bewertungen
- Geology, Logging, Drilling ReportDokument53 SeitenGeology, Logging, Drilling Reportwisam alkhooryNoch keine Bewertungen
- Beginning Cosmetic ChemistryDokument1 SeiteBeginning Cosmetic ChemistrySergio Rugerio0% (1)
- 96 Dec2018 NZGeoNews PDFDokument139 Seiten96 Dec2018 NZGeoNews PDFAditya PrasadNoch keine Bewertungen
- Comsol - Guidelines For Modeling Rotating Machines in 3DDokument30 SeitenComsol - Guidelines For Modeling Rotating Machines in 3DtiberiupazaraNoch keine Bewertungen
- CompTIA A+ Lesson 3 Understanding, PATA, SATA, SCSIDokument8 SeitenCompTIA A+ Lesson 3 Understanding, PATA, SATA, SCSIAli Ghalehban - علی قلعه بانNoch keine Bewertungen