Beruflich Dokumente
Kultur Dokumente
UV-Vis Exercise 1 - Food Dye AnalysisTeacher Resource Pack - ENGLISH PDF
Hochgeladen von
Kizzy Anne Boatswain CarbonOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
UV-Vis Exercise 1 - Food Dye AnalysisTeacher Resource Pack - ENGLISH PDF
Hochgeladen von
Kizzy Anne Boatswain CarbonCopyright:
Verfügbare Formate
1
Exercise 1
Food dye analysis
Introduction
The electromagnetic spectrum ranges from radio waves
with wavelengths the size of buildings down to gamma rays,
the size of atomic nuclei. White light forms a small part of this
spectrum and is composed of a range of different wavelengths
which can be dispersed using a prism into its component
colours. The colour an object, or a solution, appears will depend
on which light is transmitted or reflected in the visible spectrum
and which light is absorbed. Using a UV-visible spectrometer
and a range of food dyes you will test how the absorbance
wavelength value relates to the colour of the solution.
UV-Visible Spectrometer
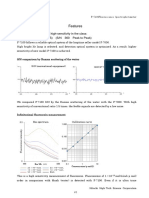
UV-visible spectrometers can be used to measure the absorbance of ultra violet or visible light by a sample.
The spectrum produced is a plot of absorbance versus wavelength (nm) in the UV and visible section of the
electromagnetic spectrum. Instruments can be used to measure at a single wavelength or perform a scan over
a range in the spectrum. The UV region ranges from 190 to 400 nm and the visible region from 400 to 800 nm.
The technique can be used both quantitatively and qualitatively.
Copyright 2009 Royal Society of Chemistry www.rsc.org
If a substance 450 nm
400 nm
800 nm - Visible Region
absorbs here...
Ultraviolet - Visible Spectroscopy (UV) Exercise 1 - Food dye analysis 2
Violet
Blue Red
Method 495 nm Visible Region Colour Wheel 620 nm
1. Prepare a dilute sample for each colour to be tested using 4. Use the colour wheel to predict absorbance values
a cuvette and distilled water (approximately 1 drop food for each solution and recordGreen
your predictions in the
Orange
colouring to 100 ml distilled water). table provided.
2. For each colour sample fill a plastic cuvette and stopper
Yellow
5. Set up the spectrometer to scan the visible region from ...it appe
with a lid. 350-800 nm and run each sample. Print out the spectrum as this c
and note the wavelength for570each
nm
of the absorbance 590
peaks.
nm
3. Prepare a blank sample cuvette containing distilled water
Compare these with your predictions.
only and stopper with a lid.
Red 620-750 nm
Orange 590-620 nm
UV Region Yellow 570-590 nm
Green 496-570 nm
Blue 450-495 nm
Violet 380-450 nm
190 nm 400 nm
If a substance 450 nm
400 nm
800 nm - Visible Region
absorbs here...
Violet
Blue Red
495 nm Visible Region Colour Wheel 620 nm
Green Orange
Yellow ...it appears
as this colour
570 nm 590 nm
Materials Red 620-750 nm
Orange 590-620 nm
Chemicals Instrument
Yellow 570-590 nm
Food colouring samples - Green 496-570 nmSpectrometer (integral printer and paper)
UV-visible
Red, yellow, green, blue, pink, black Laptop (optional)
De-ionised/distilled water Blue 450-495
Printer nm
(optional)
Apparatus
Violet 380-450 nm cables x 2 (optional)
Connection
Set up for laptop and printer use:
Disposable plastic cuvettes and stoppers
Wash bottles x 4 Connect UV-vis to laptop via left hand front
100 ml beakers x 10 USB port (Com 5)
1 box pasteur pipettes and teats Connect printer to any USB port
(Plastic for younger children) From spectrometer menu Select printer /
Tissues auto print on / Computer USB / OK
Open PVC program, set auto print to on or off depending
on requirements.
Copyright 2009 Royal Society of Chemistry www.rsc.org
Ultraviolet - Visible Spectroscopy (UV) Exercise 1 - Food dye analysis 3
Results
Colour Predicted Absorbance Actual Absorbance Notes
Value (nm) Value (nm)
Red 496 - 570 519 & 528 Absorbs Green
Yellow 380 - 450 428 Absorbs Violet
Green 620 - 750 427 & 635 Absorbs Red
Blue 590 - 620 409 & 628 Absorbs Orange
496 - 470 Absorbs possibly
Pink 510
570 - 590 Green/Yellow
Note: This absorbs both
in the Red and Green which
Black ? 519 & 635 are directly opposite, solution
appears black
Higher Lower
Frequency
Visible Spectrum Frequency
VIOLET BLUE GREEN YELLOW ORANGE RED
UV IR
400 500 600 700 800
wavelength (nm)
Copyright 2009 Royal Society of Chemistry www.rsc.org
Ultraviolet - Visible Spectroscopy (UV) Exercise 1 - Food dye analysis 4
Model Spectra
Red: 519 and 528 nm
Yellow: 428 nm
Copyright 2009 Royal Society of Chemistry www.rsc.org
Ultraviolet - Visible Spectroscopy (UV) Exercise 1 - Food dye analysis 5
Green: 635 nm
Blue: 628 nm
Copyright 2009 Royal Society of Chemistry www.rsc.org
Ultraviolet - Visible Spectroscopy (UV) Exercise 1 - Food dye analysis 6
Pink: 510 nm
Black: 519 and 635 nm
Copyright 2009 Royal Society of Chemistry www.rsc.org
Ultraviolet - Visible Spectroscopy (UV) Exercise 1 - Food dye analysis 7
Student Work Sheet
Colour Predicted Absorbance Actual Absorbance Notes
Value (nm) Value (nm)
Red
Yellow
Green
Blue
Pink
Black
Higher Lower
Frequency
Visible Spectrum Frequency
VIOLET BLUE GREEN YELLOW ORANGE RED
UV IR
400 500 600 700 800
wavelength (nm)
Copyright 2009 Royal Society of Chemistry www.rsc.org
Das könnte Ihnen auch gefallen
- Ecological Pyramids WorksheetDokument4 SeitenEcological Pyramids WorksheetTrilok SawantNoch keine Bewertungen
- CSEC Chemistry June 2009 P1Dokument14 SeitenCSEC Chemistry June 2009 P1Kizzy Anne Boatswain Carbon83% (18)
- Basic LightingDokument5 SeitenBasic Lightingzax418Noch keine Bewertungen
- Instruments and Techniques for Questioned Document ExaminationDokument1 SeiteInstruments and Techniques for Questioned Document Examinationgracia62% (13)
- CHM260 Lab Report SubmissionDokument38 SeitenCHM260 Lab Report Submissionasta yuno100% (1)
- Alternate Light Source Imaging Forensic Photography TechniquesDokument99 SeitenAlternate Light Source Imaging Forensic Photography Techniquesjadrio1556Noch keine Bewertungen
- Brochure Newton 7 0 in Vivo ImagingDokument6 SeitenBrochure Newton 7 0 in Vivo ImagingPrasad KulkarniNoch keine Bewertungen
- UV PosterDokument1 SeiteUV PosterYossuara Pitti100% (1)
- 1993 - Schaffler - Rapid Analysis of SugarDokument8 Seiten1993 - Schaffler - Rapid Analysis of SugarOpal Priya WeningNoch keine Bewertungen
- Lambda Max KMnO4Dokument9 SeitenLambda Max KMnO4Rayeha90% (10)
- Basics of Color in Dentistry - A Review PDFDokument8 SeitenBasics of Color in Dentistry - A Review PDFDacone TubeNoch keine Bewertungen
- CHM260 Lab Report SubmissionDokument37 SeitenCHM260 Lab Report Submissionasta yuno100% (1)
- 1.E - Quantum Theory (Exercises) - Chemistry LibreTexts PDFDokument8 Seiten1.E - Quantum Theory (Exercises) - Chemistry LibreTexts PDFabdooufNoch keine Bewertungen
- Wavelengths of ColorsDokument1 SeiteWavelengths of ColorsSwas PrhrywnNoch keine Bewertungen
- Infrared LED Lights Product Lineup: Abundant Lineup, Total of 56 Models Available For Various ApplicationsDokument12 SeitenInfrared LED Lights Product Lineup: Abundant Lineup, Total of 56 Models Available For Various ApplicationsKaiser MonterreyNoch keine Bewertungen
- Uv Vis Spectroscopy QuestionsDokument6 SeitenUv Vis Spectroscopy QuestionsFahra Aqilla AzzurahNoch keine Bewertungen
- 6.UV Visible LatestDokument61 Seiten6.UV Visible Latestdimitra shenoyNoch keine Bewertungen
- Understanding photodiode response time and spectral output of light sourcesDokument13 SeitenUnderstanding photodiode response time and spectral output of light sourcesBhagyashri Jog LimayeNoch keine Bewertungen
- MATTHEW NAZARRO - Chem 2208 Lab Experiment No. 8-Visible Spectrophotometry of Nickel (II) ChlorideDokument7 SeitenMATTHEW NAZARRO - Chem 2208 Lab Experiment No. 8-Visible Spectrophotometry of Nickel (II) ChlorideMATTHEW NAZARRONoch keine Bewertungen
- Performance Task 5 - SPECTROPHOTOMETRYDokument2 SeitenPerformance Task 5 - SPECTROPHOTOMETRYKeith BagangNoch keine Bewertungen
- 7 Spectrophotometry Loops - ManualDokument5 Seiten7 Spectrophotometry Loops - ManualJerry WangNoch keine Bewertungen
- Features: 1. The Highest Level of The High-Sensitivity in The Class: S/N 1200 (RMS) (S/N 360 Peak To Peak)Dokument2 SeitenFeatures: 1. The Highest Level of The High-Sensitivity in The Class: S/N 1200 (RMS) (S/N 360 Peak To Peak)PrianurraufikachmadNoch keine Bewertungen
- Lighting the Way with Diagnostics Analyser InnovationsDokument8 SeitenLighting the Way with Diagnostics Analyser InnovationsOo Kenx OoNoch keine Bewertungen
- 3 CC1Dokument6 Seiten3 CC1marianNoch keine Bewertungen
- Fotometer: Foto: Cahaya Meter: PengukuranDokument38 SeitenFotometer: Foto: Cahaya Meter: PengukuranRahmatika IntianiNoch keine Bewertungen
- John Kunes - DL - Spectral LinesDokument3 SeitenJohn Kunes - DL - Spectral Linesapi-549570937Noch keine Bewertungen
- 3 SpectrophotometryDokument3 Seiten3 SpectrophotometryRonald Angelo CironNoch keine Bewertungen
- FOTOMETER: Light Measurement ToolDokument38 SeitenFOTOMETER: Light Measurement ToolDiduunNoch keine Bewertungen
- Molecular SpectroDokument11 SeitenMolecular SpectroNikita SharmaNoch keine Bewertungen
- 2019 NatureElec M. Holler Et Al. - Three-Dimensional Imaging of Integrated CircuitsDokument7 Seiten2019 NatureElec M. Holler Et Al. - Three-Dimensional Imaging of Integrated CircuitsMaximeNoch keine Bewertungen
- Pharmaceutical Standards Guide For UV-Vis SpectrophotometersDokument8 SeitenPharmaceutical Standards Guide For UV-Vis SpectrophotometersGeorgescu MugurelNoch keine Bewertungen
- RA1611004010Dokument27 SeitenRA1611004010Aarthi ShivaNoch keine Bewertungen
- Fundamentals of Optics Ebook FinalDokument38 SeitenFundamentals of Optics Ebook FinalSuman RachaNoch keine Bewertungen
- Spectrophotometry: Calderon, Edna J. Britanico, Gyle Lynette P. Peralta, Angelo M. Sente, Thiesli Marc Y.Dokument11 SeitenSpectrophotometry: Calderon, Edna J. Britanico, Gyle Lynette P. Peralta, Angelo M. Sente, Thiesli Marc Y.Lyn BritanicoNoch keine Bewertungen
- Siskomoptik: Ref: Berbagai SumberDokument23 SeitenSiskomoptik: Ref: Berbagai SumberFariNoch keine Bewertungen
- Blue Modern Company Profile PresentationDokument24 SeitenBlue Modern Company Profile PresentationPrasya Mauliya Anbar KhairaniNoch keine Bewertungen
- FP 8000E Presentation ABLE SmallDokument99 SeitenFP 8000E Presentation ABLE SmallAliNoch keine Bewertungen
- Publication 10 11891 250 UV-Vis2Dokument12 SeitenPublication 10 11891 250 UV-Vis2abhinavNoch keine Bewertungen
- Clinical Chemistry Laboratory - SpectrophotometryDokument2 SeitenClinical Chemistry Laboratory - SpectrophotometrynikkiNoch keine Bewertungen
- Avantes ApplicationsDokument24 SeitenAvantes ApplicationsphmartNoch keine Bewertungen
- Schott Puravis Datasheet enDokument6 SeitenSchott Puravis Datasheet enNicholas TanNoch keine Bewertungen
- vis-uvvis-spectroscopy-solutions-industrial-labs-FL52993 Genesys 30Dokument2 Seitenvis-uvvis-spectroscopy-solutions-industrial-labs-FL52993 Genesys 30ReneNoch keine Bewertungen
- Spectrophotometry Lab GuideDokument3 SeitenSpectrophotometry Lab GuideDimple May Gianne DumaguitNoch keine Bewertungen
- Everything you need to comply with USP Chapter <857> updateDokument6 SeitenEverything you need to comply with USP Chapter <857> updateNeelima CherukupalliNoch keine Bewertungen
- Fluorescence Spectrophotometer LFS A10 and LFSDokument4 SeitenFluorescence Spectrophotometer LFS A10 and LFSlabtrondigitalNoch keine Bewertungen
- Paper For Upload PDFDokument6 SeitenPaper For Upload PDFAnonymous uJpBMLqHNoch keine Bewertungen
- UV VIS SpectrosDokument37 SeitenUV VIS SpectrosDiana JeronimoNoch keine Bewertungen
- Qc2 m1 Spectrometric Method of Analysis of Drug TransesDokument7 SeitenQc2 m1 Spectrometric Method of Analysis of Drug TransesKrisel AguilarNoch keine Bewertungen
- Uv-V ApplicationDokument42 SeitenUv-V ApplicationTare ye TesfuNoch keine Bewertungen
- Calibration Spectrophotometer PDFDokument29 SeitenCalibration Spectrophotometer PDFpiagiopersempreNoch keine Bewertungen
- Iub Pha404 Autumn 2022 AasDokument40 SeitenIub Pha404 Autumn 2022 AasTanvir FahimNoch keine Bewertungen
- 6 Dan 7. Bahan Kuliah GLP, SpektroDokument30 Seiten6 Dan 7. Bahan Kuliah GLP, SpektroFildzah HusnaNoch keine Bewertungen
- Tech Note 14Dokument2 SeitenTech Note 14meteohrNoch keine Bewertungen
- Validacion Uv ShimadzuDokument2 SeitenValidacion Uv Shimadzuchegue head hunterNoch keine Bewertungen
- DEOXYRIBOSE, RIBOSEPHOSPHATE, ATP AND DNA BY DIRECT (150-190 NM) AND FAR-UV (190-260 NM) REGIONS USING SYNCHROTRON RADIATION AS A LIGHT SOURCEDokument4 SeitenDEOXYRIBOSE, RIBOSEPHOSPHATE, ATP AND DNA BY DIRECT (150-190 NM) AND FAR-UV (190-260 NM) REGIONS USING SYNCHROTRON RADIATION AS A LIGHT SOURCE石子Noch keine Bewertungen
- OM 19 Brochure STD 2009Dokument2 SeitenOM 19 Brochure STD 2009Borza DorinNoch keine Bewertungen
- INDEX FinalDokument13 SeitenINDEX FinalGazi Mehedi HasanNoch keine Bewertungen
- F. UUVis - Colours in Organic CompoundsDokument8 SeitenF. UUVis - Colours in Organic Compoundsharsheen kaurNoch keine Bewertungen
- WTW TurbidityDokument6 SeitenWTW TurbidityIndra AditamaNoch keine Bewertungen
- Myco 2Dokument19 SeitenMyco 2pecqueur.thomasNoch keine Bewertungen
- High-Performance Systems: Lambda UV/Vis/NIR and UV/Vis Spectrophotometers - 950, 850, and 650Dokument8 SeitenHigh-Performance Systems: Lambda UV/Vis/NIR and UV/Vis Spectrophotometers - 950, 850, and 650primadanaNoch keine Bewertungen
- Spectroscopy Lab Reveals Star CompositionDokument5 SeitenSpectroscopy Lab Reveals Star CompositionKaylee LambertNoch keine Bewertungen
- Solar Energy Lab V.10 07102020 - CompressedDokument2 SeitenSolar Energy Lab V.10 07102020 - CompressedMuhammad HamzaNoch keine Bewertungen
- 2 - SpectrophotometryDokument17 Seiten2 - SpectrophotometryQasmNoch keine Bewertungen
- Shimadzu SpectrophotometerDokument30 SeitenShimadzu Spectrophotometerfadli091Noch keine Bewertungen
- Application of Spectral Studies in Pharmaceutical Product development: (Basic Approach with Illustrated Examples) First Revised EditionVon EverandApplication of Spectral Studies in Pharmaceutical Product development: (Basic Approach with Illustrated Examples) First Revised EditionNoch keine Bewertungen
- BIO 11 Lab 02 2004 Enz. Copy - DoDokument10 SeitenBIO 11 Lab 02 2004 Enz. Copy - DoKizzy Anne Boatswain CarbonNoch keine Bewertungen
- Bonding Structure and Periodicity Assessed HWDokument17 SeitenBonding Structure and Periodicity Assessed HWKizzy Anne Boatswain CarbonNoch keine Bewertungen
- Molecular Shapes - SiCl4, BF3, NF3 shapes and bond anglesDokument1 SeiteMolecular Shapes - SiCl4, BF3, NF3 shapes and bond anglesDaneilla BanksNoch keine Bewertungen
- Organic Chemistry Delivery GuideDokument43 SeitenOrganic Chemistry Delivery GuideKizzy Anne Boatswain Carbon100% (1)
- Form 1 Scheme of Work 2015-2016Dokument7 SeitenForm 1 Scheme of Work 2015-2016Kizzy Anne Boatswain CarbonNoch keine Bewertungen
- Notes On The MoleDokument22 SeitenNotes On The MoleKizzy Anne Boatswain CarbonNoch keine Bewertungen
- Bonding Structure and Periodicity Assessed HW MsDokument10 SeitenBonding Structure and Periodicity Assessed HW MsKizzy Anne Boatswain CarbonNoch keine Bewertungen
- The Superheroes of The Atomic Model Activity Teacher InstructionsDokument7 SeitenThe Superheroes of The Atomic Model Activity Teacher InstructionsKizzy Anne Boatswain CarbonNoch keine Bewertungen
- Balancing Equations Teacher InstructionsDokument7 SeitenBalancing Equations Teacher InstructionsKizzy Anne Boatswain CarbonNoch keine Bewertungen
- Balancing Equations Teacher InstructionsDokument7 SeitenBalancing Equations Teacher InstructionsKizzy Anne Boatswain CarbonNoch keine Bewertungen
- Exam Report 2015 To The Nation. UPDATED. 1Dokument20 SeitenExam Report 2015 To The Nation. UPDATED. 1alixNoch keine Bewertungen
- BIOL2161 Lec2 RE 2017Dokument23 SeitenBIOL2161 Lec2 RE 2017Kizzy Anne Boatswain CarbonNoch keine Bewertungen
- Mechanism FlashcardsDokument8 SeitenMechanism FlashcardsKizzy Anne Boatswain CarbonNoch keine Bewertungen
- Mass Spec Exercise 1 - Murder Mystery - Student Resource Pack - ENGLISHDokument7 SeitenMass Spec Exercise 1 - Murder Mystery - Student Resource Pack - ENGLISHKizzy Anne Boatswain CarbonNoch keine Bewertungen
- What Is This MoleculeDokument2 SeitenWhat Is This MoleculeKizzy Anne Boatswain CarbonNoch keine Bewertungen
- St Benedict's Chemistry Club Boosts Exam Pass RateDokument2 SeitenSt Benedict's Chemistry Club Boosts Exam Pass RateKizzy Anne Boatswain CarbonNoch keine Bewertungen
- Topic 4.10 Organic Synthesis and AnalysisDokument12 SeitenTopic 4.10 Organic Synthesis and AnalysisPawat SilawattakunNoch keine Bewertungen
- Balancing Chemical EquationsDokument13 SeitenBalancing Chemical EquationsKizzy Anne Boatswain CarbonNoch keine Bewertungen
- BIOL 2163 Assignment 2Dokument5 SeitenBIOL 2163 Assignment 2Kizzy Anne Boatswain CarbonNoch keine Bewertungen
- Topic 4.10 Organic Synthesis and AnalysisDokument12 SeitenTopic 4.10 Organic Synthesis and AnalysisPawat SilawattakunNoch keine Bewertungen
- 4.11 NotesDokument24 Seiten4.11 NotesKeri Gobin SamarooNoch keine Bewertungen
- Balancing Equations Using Periodic Table Puzzle PiecesDokument5 SeitenBalancing Equations Using Periodic Table Puzzle PiecesKizzy Anne Boatswain CarbonNoch keine Bewertungen
- 4.9 NotesDokument17 Seiten4.9 NotesKizzy Anne Boatswain CarbonNoch keine Bewertungen
- 4.2 TeacherDokument8 Seiten4.2 TeacherHardianty HamzahNoch keine Bewertungen
- 5.4 NotesDokument24 Seiten5.4 Notesmuhajireen0% (1)
- 1.4 NotesDokument9 Seiten1.4 NotesUmer SalmanNoch keine Bewertungen
- 4.11 NotesDokument24 Seiten4.11 NotesKeri Gobin SamarooNoch keine Bewertungen
- Topic 1 NotesDokument25 SeitenTopic 1 NotesKizzy Anne Boatswain CarbonNoch keine Bewertungen
- Science Module 5 PDFDokument30 SeitenScience Module 5 PDFerwinNoch keine Bewertungen
- Chapter 7 Quantum Theory and Atomic Structure: Follow-Up ProblemsDokument32 SeitenChapter 7 Quantum Theory and Atomic Structure: Follow-Up Problems원철이Noch keine Bewertungen
- Chapter 33 The Nature and Propagation of LightDokument66 SeitenChapter 33 The Nature and Propagation of LightMark Reyes0% (1)
- Ftir SpectrosDokument36 SeitenFtir SpectrosHafiz Mudaser AhmadNoch keine Bewertungen
- Thermography PPT 110405102354 Phpapp02Dokument24 SeitenThermography PPT 110405102354 Phpapp02surbhi pareekNoch keine Bewertungen
- TYDB-D172719DEH4-33FT2 8 Port Dual BeamDokument2 SeitenTYDB-D172719DEH4-33FT2 8 Port Dual Beambudi wirahmiNoch keine Bewertungen
- Deuterium PDFDokument8 SeitenDeuterium PDFRICHIHOTS2Noch keine Bewertungen
- Quantum AnsDokument12 SeitenQuantum AnslemathaNoch keine Bewertungen
- Electromagnetic Waves Folio SPMDokument40 SeitenElectromagnetic Waves Folio SPMKhairul Akmal88% (8)
- Additive color mixing explainedDokument23 SeitenAdditive color mixing explainedtamil selviNoch keine Bewertungen
- Comparing In Vivo and In Vitro Testing of Sunscreen FormulasDokument8 SeitenComparing In Vivo and In Vitro Testing of Sunscreen FormulasAnisaLastriNoch keine Bewertungen
- Electromagnetic RadiationDokument5 SeitenElectromagnetic RadiationrendakianNoch keine Bewertungen
- AWB DevicesDokument4 SeitenAWB Devicesinsanehb1123Noch keine Bewertungen
- What is the Electromagnetic SpectrumDokument13 SeitenWhat is the Electromagnetic SpectrumJones JonnyNoch keine Bewertungen
- Light WavesDokument12 SeitenLight WavesMpforgeNoch keine Bewertungen
- (SH) Intelligence: Fa '/) ReferenceDokument38 Seiten(SH) Intelligence: Fa '/) ReferenceAnais MartinNoch keine Bewertungen
- Juno Lighting Architectural Lighting Products Catalog 1996Dokument88 SeitenJuno Lighting Architectural Lighting Products Catalog 1996Alan MastersNoch keine Bewertungen
- Resistor color codingDokument1 SeiteResistor color codingMEL ANGELESNoch keine Bewertungen
- Radiography Testing Level I and IIDokument16 SeitenRadiography Testing Level I and IIJoshnewfoundNoch keine Bewertungen
- Physics For The Technician-ZhdanovDokument643 SeitenPhysics For The Technician-Zhdanovdurant2616Noch keine Bewertungen
- Electromagnetic WaveDokument32 SeitenElectromagnetic WaveClrs BchnNoch keine Bewertungen
- Pmled 6 5K 10a 66Dokument6 SeitenPmled 6 5K 10a 66Eduardo SalgadoNoch keine Bewertungen
- How Does A Rainbow FormDokument6 SeitenHow Does A Rainbow FormJaylord lamdagNoch keine Bewertungen
- 5G Channel Characterization at Bhagad City MmwaveDokument6 Seiten5G Channel Characterization at Bhagad City MmwaveNirmalaNoch keine Bewertungen
- Galaxy Lighting 2021-2022 灯饰目录Dokument240 SeitenGalaxy Lighting 2021-2022 灯饰目录qq1691492197Noch keine Bewertungen
- Quotation For LED Light ProductsDokument17 SeitenQuotation For LED Light Productssalaar12Noch keine Bewertungen