Beruflich Dokumente
Kultur Dokumente
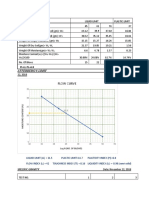
RESULTS Experiment No. 2
Hochgeladen von
Grace N MalikCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
RESULTS Experiment No. 2
Hochgeladen von
Grace N MalikCopyright:
Verfügbare Formate
RESULTS
Tube Tube Tube Tube Tube Tube Tube Tube
No.1 No.2 No.3 No.4 No.5 No.6 No.7 No.8
Initial 0.0 0.6 8.9 2.5 6.59 0.3 22.6 0.9
Final 8.33 9.22 22.45 13.75 28.61 24.45 30.75 7.8
Total 8.33 9.06 13.55 11.25 22.02 24.15 8.15 6.9
Test tube No. 1 & 2 : 6 M HCl + 5 ml distilled water
Test tube No. 3 & 4 : 6 M HCl + 5 ml pure CH3COOC2H5
Test tube No. 5 & 6 : 6 M HCl + 3 ml glacial CH3COOH + 2 ml absolute C2H5OH
Test tube No. 7 & 8: 6 M HCl + 2 ml glacial CH3COOH + 3 ml absolute C2H5OH
Weight of Samples:
SAMPLE VOLUME WEIGHT
6 N HCl 5 ml 5.14 g
CH3COOC2H5 5 ml (0.898 g/ml)(5 ml) = 4.49 g
CH3COOH 2 ml (1.05 g/ml)(2 ml) = 2.1 g
C2H5OH 2 ml (0.789 g/ml)(2 ml) = 1.578 g
CH3COOH 3 ml (1.05 g/ml)(3 ml) = 3.15 g
C2H5OH 3 ml (0.789 g/ml)(3 ml) = 2.367 g
Test tube 1 & 2: N = 0.238 N
V of H2O = 5ml 2 H2O H3O + + OH-
Mass of H2O = 5 g
Vsoln = 25 ml NaOH + 5 ml H2O + 5 ml I 55.56 0 0
HCl = 35 ml solution C -2x x x
E 55.26 2x x x
1 mol
5 g( )
NH2O = 18 g = 55.56 M H2O
0.005 L [ H 3 O ] [OH ] [ x ][ x ]
K1 = [ H 2 O]2 = [55.26 2 x ]2
Test tube 1: [0.238] 2
K1 = [55.26 2(0.238)]2 = 1.87 x 10 -5
NVsoln = NVNaOH
N (35 ml) = 1(8.33 ml)
NVsoln = NVNaOH
Test tube 2: N (35 ml) = 1(9.06 ml)
N = 0.259 N
[0.259]2
K2 = [55.26 2(1.259)]2 = 2.21 x 10 -5
Test tube 3 & 4:
CH3COOC2H5 + H2O C2H5OH + CH3COOH
1 mol
4.49 g ( )
MCH3COOC2H5 = 88 g = 10.215 M CH3COOC2H5
0.005 L
Mass of HCl = 6 mol / L soln (36.45 g/mol)(0.005 L) = 1.0935 g HCl
Mass of H2O = 5.14 g 1.0935 g = 4.0465 g H2O
1 mol
4.0465 g ( )
MH2O = 18 g = 44.96 M
0.005 L
CH3COOC2H5 + H2O C2H5OH + CH3COOH
I 10.215 44.96 0 0
C -x -x x x
E 10.215-x 44.96-x x x
Test tube 3: Test tube 4:
NVsoln = NVNaOH NVsoln = NVNaOH
N (35 ml) = 1(13.55 ml) N (35 ml) = 1(11.25 ml)
N = 0.387 N N = 0.3214 N
[0.3214]2
[ C 2 H 5 OH ][CH 3COOH ] K1 = [ 10.2150.3214 ] [44.960.3214 ]
K1 = [CH 3 COOC 2 H 5][ H 2O]
= 2.43x10 -4
[0.238]2
K1 = [ 10.2150.387 ] [44.960.387] =
3.42 x 10 -4
Das könnte Ihnen auch gefallen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (120)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- 2019 Cisile Exhibitor ListDokument5 Seiten2019 Cisile Exhibitor ListKanagarajan UmapathiNoch keine Bewertungen
- Stesdtgxzgdzyzroboydzryts in The Wild 1tgzdstrbzts6tsdytDokument2 SeitenStesdtgxzgdzyzroboydzryts in The Wild 1tgzdstrbzts6tsdytGrace N MalikNoch keine Bewertungen
- Real Estate MortgageDokument5 SeitenReal Estate MortgageBeng KalawNoch keine Bewertungen
- Cloud Services (Discussion)Dokument1 SeiteCloud Services (Discussion)Grace N MalikNoch keine Bewertungen
- Robot SIN THE Wild: Ruth R. Secretario Roed G. Ruiz Grace Lyn Y. NiadasDokument9 SeitenRobot SIN THE Wild: Ruth R. Secretario Roed G. Ruiz Grace Lyn Y. NiadasGrace N MalikNoch keine Bewertungen
- Contacts To ImportDokument1 SeiteContacts To ImportGrace N MalikNoch keine Bewertungen
- 123456interviewing Example ScriptDokument1 Seite123456interviewing Example ScriptGrace N MalikNoch keine Bewertungen
- r1565w3785686586876rf8675-12 Create An Account Validation RuleDokument2 Seitenr1565w3785686586876rf8675-12 Create An Account Validation RuleGrace N MalikNoch keine Bewertungen
- Asgtstysdgxdzyd8-4 Create A ProcessydhcxgxdyDokument2 SeitenAsgtstysdgxdzyd8-4 Create A ProcessydhcxgxdyGrace N MalikNoch keine Bewertungen
- 8-2 Create An Email TemplateDokument2 Seiten8-2 Create An Email TemplateGrace N MalikNoch keine Bewertungen
- Sdkkrutylsjlbjkxchdfh8-1 Create A Folder and LetterheadsgyxdhxzcgDokument2 SeitenSdkkrutylsjlbjkxchdfh8-1 Create A Folder and LetterheadsgyxdhxzcgGrace N MalikNoch keine Bewertungen
- Hfjgkghkhkyiyjh8-2 Create An Email TemplatejklhhkDokument2 SeitenHfjgkghkhkyiyjh8-2 Create An Email TemplatejklhhkGrace N MalikNoch keine Bewertungen
- Position Paper of LatorreDokument3 SeitenPosition Paper of LatorreGrace N MalikNoch keine Bewertungen
- Zfzstxbxydxhxcsztgzsdtg8-3 Create A New Workflow Rule With Immediate and Time Dependent ActionDokument2 SeitenZfzstxbxydxhxcsztgzsdtg8-3 Create A New Workflow Rule With Immediate and Time Dependent ActionGrace N MalikNoch keine Bewertungen
- Atoguysigykxzhvbhiozygfisyhkg5-13 Create An Opportunity Validation RuleDokument1 SeiteAtoguysigykxzhvbhiozygfisyhkg5-13 Create An Opportunity Validation RuleGrace N MalikNoch keine Bewertungen
- AssignmentDokument3 SeitenAssignmentGrace N MalikNoch keine Bewertungen
- 123456789102113142214robots in The WildsdvvbjfdgSAJGdfjgjgvbcjDokument2 Seiten123456789102113142214robots in The WildsdvvbjfdgSAJGdfjgjgvbcjGrace N MalikNoch keine Bewertungen
- 5-1 Administer Standard FieldsDokument2 Seiten5-1 Administer Standard FieldsGrace N MalikNoch keine Bewertungen
- Beginners Cal PDFDokument1 SeiteBeginners Cal PDFAsteka IoanaNoch keine Bewertungen
- Oath Taking ProgramDokument2 SeitenOath Taking ProgramKuo SarongNoch keine Bewertungen
- 5-1 Administer Standard FieldsDokument2 Seiten5-1 Administer Standard FieldsGrace N MalikNoch keine Bewertungen
- Complaint For ReplevinDokument8 SeitenComplaint For Replevinjonna campuganNoch keine Bewertungen
- Cle Position Paper PDFDokument5 SeitenCle Position Paper PDFGrace N MalikNoch keine Bewertungen
- Position Paper of LatorreDokument3 SeitenPosition Paper of LatorreGrace N MalikNoch keine Bewertungen
- 5-1 Administer Standard FieldsDokument2 Seiten5-1 Administer Standard FieldsGrace N MalikNoch keine Bewertungen
- 82 1 PDFDokument4 Seiten82 1 PDFAshish KumarNoch keine Bewertungen
- Characteristics and Attitudes of MillinnialDokument2 SeitenCharacteristics and Attitudes of MillinnialGrace N MalikNoch keine Bewertungen
- Figure 1: Flow Diagram of Disposal of Organic and Physico-Chemical SludgeDokument1 SeiteFigure 1: Flow Diagram of Disposal of Organic and Physico-Chemical SludgeGrace N MalikNoch keine Bewertungen
- OSH Standards Amended 1989 LatestDokument338 SeitenOSH Standards Amended 1989 Latestverkie100% (1)
- 190793Dokument11 Seiten190793Grace N MalikNoch keine Bewertungen
- PDFDokument34 SeitenPDFGrace N MalikNoch keine Bewertungen
- Star Education Academy: Short Questions TestDokument2 SeitenStar Education Academy: Short Questions TestMohammad AshfaqNoch keine Bewertungen
- Studies On Tribological Behaviour of Banana and Coir Hybrid Fiber Epoxy Reinforced CompositesDokument19 SeitenStudies On Tribological Behaviour of Banana and Coir Hybrid Fiber Epoxy Reinforced CompositesKirubhakaran KathiresanNoch keine Bewertungen
- Chemistry Class 11 Workbook (Tomar Chemistry Tutorial)Dokument29 SeitenChemistry Class 11 Workbook (Tomar Chemistry Tutorial)anshudeepNoch keine Bewertungen
- Thermo Scientific Pierce Protein Ladders GuideDokument5 SeitenThermo Scientific Pierce Protein Ladders GuideBerniceTanNoch keine Bewertungen
- UV-Visible Systems - Operational Qualification - Col23 PDFDokument10 SeitenUV-Visible Systems - Operational Qualification - Col23 PDFIsabelle PlourdeNoch keine Bewertungen
- Characterisation of A Thiosulphate-Sulphite Gold Electrodeposition Process PDFDokument5 SeitenCharacterisation of A Thiosulphate-Sulphite Gold Electrodeposition Process PDFLennonNoch keine Bewertungen
- Coa Grape SeedDokument1 SeiteCoa Grape SeedVinny Fitria ArdyaniNoch keine Bewertungen
- Instruction Manuals - Parr Instrument CompanyDokument7 SeitenInstruction Manuals - Parr Instrument CompanyMika SuominenNoch keine Bewertungen
- Energy Engineering: B.Sc. Chemical Engineering Session 2018 Delivered byDokument44 SeitenEnergy Engineering: B.Sc. Chemical Engineering Session 2018 Delivered bySohaibNoch keine Bewertungen
- Arsip VOC S HasanahDokument11 SeitenArsip VOC S HasanahYadi KartonoNoch keine Bewertungen
- Microbial Staining - Principles and Methods Microbial Staining - Principles and MethodsDokument33 SeitenMicrobial Staining - Principles and Methods Microbial Staining - Principles and MethodsSELVI ANoch keine Bewertungen
- Energy Units Conversion Table - 1Dokument2 SeitenEnergy Units Conversion Table - 1dwiudNoch keine Bewertungen
- Thermodynamics QPDokument22 SeitenThermodynamics QPChioma UchegbuNoch keine Bewertungen
- HANDLING OF IDLE AND STANDBY STEAM GENERATING SYSTEMS 27 Jan 2014Dokument9 SeitenHANDLING OF IDLE AND STANDBY STEAM GENERATING SYSTEMS 27 Jan 2014kleber17100Noch keine Bewertungen
- Olimpiada Judet 2022 Varianta 1 Bucuresti OlenDokument4 SeitenOlimpiada Judet 2022 Varianta 1 Bucuresti OlenRoxy RoxanaNoch keine Bewertungen
- Che 511 Lecture Note 2023Dokument5 SeitenChe 511 Lecture Note 2023Bright ChimezieNoch keine Bewertungen
- Lab CompilationDokument11 SeitenLab CompilationJanita SiddiquiNoch keine Bewertungen
- Fluoride-Doped Amorphous Calcium Phosphate Nanoparticles As A Promising Biomimetic Material For Dental RemineralizationDokument9 SeitenFluoride-Doped Amorphous Calcium Phosphate Nanoparticles As A Promising Biomimetic Material For Dental Remineralization王子瑜Noch keine Bewertungen
- Pharmaceutical Chemistry Model-Answer-Paper-Winter-2019Dokument29 SeitenPharmaceutical Chemistry Model-Answer-Paper-Winter-2019Deepak VermaNoch keine Bewertungen
- TDS Simacover EP Buildcoat (Intermediate)Dokument2 SeitenTDS Simacover EP Buildcoat (Intermediate)rrahardiandiasNoch keine Bewertungen
- Worksheet #6 - Structure, Properties, and Reactions of TriacylglycerolsDokument3 SeitenWorksheet #6 - Structure, Properties, and Reactions of TriacylglycerolsDanielle SibayNoch keine Bewertungen
- Catalogo Consumabili Elga 2017Dokument150 SeitenCatalogo Consumabili Elga 2017Eraldo MigliavaccaNoch keine Bewertungen
- 04-06-2020 - SR - LT - All - All INDIA - E-Test Series - Jee Main - MFT-02 - Key & Sol's (NjwnddnsnshsabhbDokument14 Seiten04-06-2020 - SR - LT - All - All INDIA - E-Test Series - Jee Main - MFT-02 - Key & Sol's (NjwnddnsnshsabhbSai GokulNoch keine Bewertungen
- Investigating Osmosis LabDokument5 SeitenInvestigating Osmosis LabAnanya Sharma - Lincoln Alexander SS (2132)Noch keine Bewertungen
- Adrenochrome - C9H9NO3 - PubChemDokument20 SeitenAdrenochrome - C9H9NO3 - PubChemHansley Templeton CookNoch keine Bewertungen
- Flow Chart For Analysis of Suspensibility of Deltamethrin WP FormulationDokument4 SeitenFlow Chart For Analysis of Suspensibility of Deltamethrin WP FormulationDwi KristiantoNoch keine Bewertungen
- EfkaPB2720 TDSDokument2 SeitenEfkaPB2720 TDSSebastian GonzalezNoch keine Bewertungen
- Hydroxyl Functional Resin TDSDokument4 SeitenHydroxyl Functional Resin TDSFerdika Dwi CandraNoch keine Bewertungen
- Chapter 2 - Atoms - Molecules - and IonsDokument53 SeitenChapter 2 - Atoms - Molecules - and IonsWarakorn AkarasareenonNoch keine Bewertungen