Beruflich Dokumente
Kultur Dokumente
2b - NMR For Oil Gas Prospecting PDF
Hochgeladen von
Gheorghe Alexandru Gabriel0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
74 Ansichten10 SeitenOriginaltitel
2b_NMR for oil gas prospecting.pdf
Copyright
© © All Rights Reserved
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
74 Ansichten10 Seiten2b - NMR For Oil Gas Prospecting PDF
Hochgeladen von
Gheorghe Alexandru GabrielCopyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 10
NMR for oil gas prospecting and exploration
Alexander Yurchuk Owner, Institute geophysics and problems of the
Earth
Applications of nuclear magnetic resonance for oil gas prospecting and
exploration gives unique results. Company Halliburton and Schlumberger
used NMR in logging surveys in the wells (only to a distance of 20-30
meters).
We use NMR properties from ground surface (without drilling) and see the
hydrocarbons directly to a depth of 5 km and possibly deeper. This is our
big advantage over the Schlumberger and Halliburton.
Our technology provides a direct detection of hydrocarbons and shows the
effectiveness of exploratory surveys to 98% and significantly exceeds the
aeromagnetic and aerogravity and seismic surveys. (These surveys provide
the anomalies).
Our technology is based on effect of Nuclear Magnetic Resonance (NMR,
in medicine is called MRI),
http://en.wikipedia.org/wiki/Nuclear_magnetic_resonance
Video of NMR, look: http://www.youtube.com/watch?v=7aRKAXD4dAg
Kirlian effect, http://en.wikipedia.org/wiki/Kirlian_photography
Kirlian effect is widely used around the world to visualize the images
invisible to the eye, is what we use when a transfer the image from the
infrared range to the visible eye image.
And the property of radio waves at terahertz range, THz (1012 - 1015 Hz)
http://en.wikipedia.org/wiki/Terahertz_radiation
Terahertz range of radio waves used in airports to detect explosives in
passengers baggage and passengers inside at distance of tens of meters.
MRI sees the human body through (no matter how body was- thick or
thin), and NMR in geophysics allows technology to see to great depth.
Nuclear magnetic resonance
From Wikipedia, the free encyclopedia
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei
in a magnetic field absorb and re-emit electromagnetic radiation. This energy is at
a specific resonance frequency which depends on the strength of the magnetic
field and the magnetic properties of the isotope of the atoms;
In practical applications, the frequency is similar to VHF and UHF television
broadcasts (601000 MHz). NMR allows the observation of specific quantum
mechanical magnetic properties of the atomic nucleus. Many scientific techniques
exploit NMR phenomena to study molecular physics, crystals, and non-crystalline
materials through NMR spectroscopy.
NMR is also routinely used in advanced medical imaging techniques, such as in
magnetic resonance imaging (MRI).
All isotopes that contain an odd number of protons and/or of neutrons (see
Isotope) have an intrinsic magnetic moment and angular momentum, in other
words a nonzero spin, while all nuclides with even numbers of both have a total
spin of zero. The most commonly studied nuclei are 1H and 13C, although nuclei
from isotopes of many other elements (e.g. 2H, 6Li, 10B, 11B, 14N, 15N, 17O,
19F, 23Na, 29Si, 31P, 35Cl, 113Cd, 129Xe, 195Pt) have been studied by high-
field NMR spectroscopy as well.
A key feature of NMR is that the resonance frequency of a particular substance is
directly proportional to the strength of the applied magnetic field. It is this feature that
is exploited in imaging techniques; if a sample is placed in a non-uniform magnetic field
then the resonance frequencies of the sample's nuclei depend on where in the field
they are located. Since the resolution of the imaging technique depends on the
magnitude of magnetic field gradient, many efforts are made to develop increased field
strength, often using superconductors. The effectiveness of NMR can also be improved
using hyperpolarization, and/or using two-dimensional, three-dimensional and higher-
dimensional multi-frequency techniques.
The principle of NMR usually involves two sequential steps:
The alignment (polarization) of the magnetic nuclear spins in an applied, constant
magnetic field H0.
The perturbation of this alignment of the nuclear spins by employing an electro-
magnetic, usually radio frequency (RF) pulse. The required perturbing
frequency is dependent upon the static magnetic field (H0) and the nuclei of
observation.
The two fields are usually chosen to be perpendicular to each other as this
maximizes the NMR signal strength. The resulting response by the total
magnetization (M) of the nuclear spins is the phenomenon that is exploited in
NMR spectroscopy and magnetic resonance imaging. Both use intense applied
magnetic fields (H0) in order to achieve dispersion and very high stability to
deliver spectral resolution, the details of which are described by chemical shifts,
the Zeeman effect, and Knight shifts (in metals).
NMR phenomena are also utilized in low-field NMR, NMR spectroscopy and
MRI in the Earth's magnetic field (referred to as Earth's field NMR), and in
several types of magnetometers.
Data acquisition in the petroleum industry
Main article: NMR in porous media
Another use for nuclear magnetic resonance is data acquisition in the petroleum
industry for petroleum and natural gas exploration and recovery.
A borehole is drilled into rock and sedimentary strata into which nuclear magnetic
resonance logging equipment is lowered.
Nuclear magnetic resonance analysis of these boreholes is used to measure rock
porosity, estimate permeability from pore size distribution and identify pore fluids
(water, oil and gas). These instruments are typically low field NMR spectrometers.
Low field NMR spans a range of different nuclear magnetic resonance
(NMR) modalities, going from NMR conducted in permanent magnets,
supporting magnetic fields of a few T, all the way down to zero field
NMR, where the Earth's field is carefully shielded such that magnetic
fields of nT are achieved where nuclear spin precession is close to zero.
In a broad sense, "Low-field NMR" is the branch of nuclear magnetic
resonance that is NOT conducted in superconducting high-field
magnets. Low field NMR also includes Earth's field NMR where simply
the Earth's field is exploited to cause nuclear spin-precession which is
detected. With magnetic fields on the order of T and below
magnetometers such as SQUIDs or atomic magnetometers (among
others) are used as detectors. "Normal" high field NMR relies on the
detection of spin-precession with inductive detection with a simple
coil. However, this detection modality becomes less sensitive as the
magnetic field and the associated frequencies decrease. Hence the
push toward alternative detection methods at very low fields.
When a sample is placed in a constant magnetic field and stimulated (perturbed) by a
pulsed or alternating magnetic field, NMR active nuclei resonate at characteristic
frequencies. Examples of such nuclei are the isotopes carbon-13, and hydrogen-1 also
referred to as protons. The resonant frequency of each isotope is directly proportional to
the strength of the applied magnetic field, and the magnetogyric or gyromagnetic ratio of
that isotope. The signal strength is proportional both to the stimulating magnetic field and
the number of nuclei of that isotope in the sample. Thus in the 21 tesla magnetic field that
may be found in high resolution laboratory NMR spectrometers, protons resonate at
900 MHz. However in the Earth's magnetic field the same nuclei resonate at audio
frequencies of around 2 kHz and generate very weak signals.
The location of a nucleus within a complex molecule affects the 'chemical environment' (i.e.
the rotating magnetic fields generated by the other nuclei) experienced by the nucleus.
Thus different hydrocarbon molecules containing NMR active nuclei in different positions
within the molecules produce slightly different patterns of resonant frequencies.
EFNMR signals can be affected by both magnetically noisy laboratory environments and
natural variations in the Earth's field, which originally compromised its usefulness. However
this disadvantage has been overcome by the introduction of electronic equipment which
compensates changes in ambient magnetic fields.
Whereas chemical shifts are important in NMR, they are insignificant in the Earth's field.
The absence of chemical shifts causes features such as spin-spin multiplets (that are
separated by high fields) to be superimposed in EFNMR. Instead, EFNMR spectra are
dominated by spin-spin coupling (J-coupling) effects. Software optimised for analysing
these spectra can provide useful information about the structure of the molecules in the
sample.
Das könnte Ihnen auch gefallen
- Nuclear Magnetic Resonance (NMR) Spectroscopy - Instrumentation - Microbe NotesDokument2 SeitenNuclear Magnetic Resonance (NMR) Spectroscopy - Instrumentation - Microbe Notesjimgitonga100% (2)
- NMR Spectroscopy)Dokument3 SeitenNMR Spectroscopy)smart ptmNoch keine Bewertungen
- NMR ReichiDokument186 SeitenNMR ReichiJanni ManojkumarNoch keine Bewertungen
- Bioenergetic AssignmentDokument16 SeitenBioenergetic Assignmentshanza FaheemNoch keine Bewertungen
- Nuclear Magnetic Resonance IntroDokument17 SeitenNuclear Magnetic Resonance Introlisan2053Noch keine Bewertungen
- Nuclear Magnetic ResonanceDokument23 SeitenNuclear Magnetic ResonanceDavid OsasNoch keine Bewertungen
- Chm580 NMRDokument8 SeitenChm580 NMRNorHidayu Azmi50% (2)
- NMR Principles and ApplicationsDokument2 SeitenNMR Principles and Applicationsahmad054Noch keine Bewertungen
- Nuclear Magnetic Resonance - WikiDokument16 SeitenNuclear Magnetic Resonance - Wiki12BAcNoch keine Bewertungen
- Nuclear Magnetic Resonance and Radiation TechniquesDokument15 SeitenNuclear Magnetic Resonance and Radiation TechniquesMathavaraja JeyaramanNoch keine Bewertungen
- Written Report NMR SpectrometryDokument6 SeitenWritten Report NMR SpectrometryRye M. BirungNoch keine Bewertungen
- Spectroscopic Atomic Nuclei Radio Waves Nuclear Magnetic ResonanceDokument3 SeitenSpectroscopic Atomic Nuclei Radio Waves Nuclear Magnetic Resonancejaved khanNoch keine Bewertungen
- NMR - A Non Destructive Food Evaluation Technique: Ramesh. VDokument44 SeitenNMR - A Non Destructive Food Evaluation Technique: Ramesh. VAnkit GoyalNoch keine Bewertungen
- Nuclear Magnetic Resonance SpectrosDokument25 SeitenNuclear Magnetic Resonance SpectrosFalak Naz100% (1)
- Nuclear Magnetic Resonance SpectrosDokument21 SeitenNuclear Magnetic Resonance SpectrosDawoodNoch keine Bewertungen
- Infrared Spectroscopy: Conformational IsomersDokument13 SeitenInfrared Spectroscopy: Conformational Isomersmai elewaNoch keine Bewertungen
- About The Possibility of IdentDokument10 SeitenAbout The Possibility of Ident雷鸣Noch keine Bewertungen
- NMR Workshop ReportDokument18 SeitenNMR Workshop ReportmrtharamNoch keine Bewertungen
- NMR SpectrosDokument45 SeitenNMR SpectrosfatemaNoch keine Bewertungen
- LU4 Nuclear Magnetic Resonance IDokument22 SeitenLU4 Nuclear Magnetic Resonance IYit JuanNoch keine Bewertungen
- NMR Spectroscopy: PrincipDokument38 SeitenNMR Spectroscopy: Principandi evi febriantiNoch keine Bewertungen
- Magnetic Resonance Spectroscopy: Christopher J. RhodesDokument52 SeitenMagnetic Resonance Spectroscopy: Christopher J. RhodesSonia Ayu AndiniNoch keine Bewertungen
- NMR (Nuclear Magnetic Resonance) SpectrosDokument5 SeitenNMR (Nuclear Magnetic Resonance) Spectrossophia del rosarioNoch keine Bewertungen
- NMR - A ThesisDokument4 SeitenNMR - A ThesisJ KNoch keine Bewertungen
- NMR GoodDokument52 SeitenNMR GoodSiju N. Antony100% (1)
- Nuclear Magnetic Resonance SpectrosDokument10 SeitenNuclear Magnetic Resonance SpectrosLaura CelisNoch keine Bewertungen
- Nuclear Magnetic Resonanace Spectroscopy (NMR)Dokument29 SeitenNuclear Magnetic Resonanace Spectroscopy (NMR)boyapati venupriyaNoch keine Bewertungen
- H NMRDokument34 SeitenH NMRbsmalah11alroxnamNoch keine Bewertungen
- Nuclear Magnetic Resonance (NMR) : Spinning NucleusDokument3 SeitenNuclear Magnetic Resonance (NMR) : Spinning NucleusyashbhardwajNoch keine Bewertungen
- NMR - Nuclear Magnetic ResonanceDokument3 SeitenNMR - Nuclear Magnetic ResonanceSam ShohetNoch keine Bewertungen
- NMR NsuDokument73 SeitenNMR NsuhujjatullahhbNoch keine Bewertungen
- Chemical Analysis NMRDokument5 SeitenChemical Analysis NMROmar AbdulahNoch keine Bewertungen
- Nuclear Magnetic Resonance Spectroscopy 3be574aa E12a 4ba6 80f6 573389d5c75dDokument11 SeitenNuclear Magnetic Resonance Spectroscopy 3be574aa E12a 4ba6 80f6 573389d5c75dBeamlak MarelignNoch keine Bewertungen
- Nuclear Magnetic Resonance Spectroscopy: Absorption inDokument21 SeitenNuclear Magnetic Resonance Spectroscopy: Absorption inFiona OyatsiNoch keine Bewertungen
- NMR For BSCDokument13 SeitenNMR For BSCmalluchaithra88_3587Noch keine Bewertungen
- NMR JDMDokument5 SeitenNMR JDMFahad ShameemNoch keine Bewertungen
- NMRDokument17 SeitenNMRSourabhNoch keine Bewertungen
- 7 Nuclear Magnetic Resonance SpectrosDokument69 Seiten7 Nuclear Magnetic Resonance SpectrosKazim ZarghamNoch keine Bewertungen
- Fundamentals of NMR - James PDFDokument31 SeitenFundamentals of NMR - James PDFSoundarya ChandramouleeswaranNoch keine Bewertungen
- NMR Spectroscopy: The TheoryDokument4 SeitenNMR Spectroscopy: The Theoryoliv1aNoch keine Bewertungen
- A New Very High Sensitivity Potassium Magnetometer For Near Surface Geophysical MappingDokument15 SeitenA New Very High Sensitivity Potassium Magnetometer For Near Surface Geophysical MappinglivrosNoch keine Bewertungen
- Research Paper On NMR SpectrosDokument8 SeitenResearch Paper On NMR Spectrosfzgz6hyt100% (1)
- Chem 3052 CHAPTER 7 (Nuclear Magnetic Resonance Spectroscopy (NMR) )Dokument6 SeitenChem 3052 CHAPTER 7 (Nuclear Magnetic Resonance Spectroscopy (NMR) )ashenafiNoch keine Bewertungen
- EXPERIMENT 4: NMR Analysis of A Constitutional IsomerDokument18 SeitenEXPERIMENT 4: NMR Analysis of A Constitutional IsomerDhiyyah Mardhiyyah100% (1)
- Proton Nuclear Magnetic Resonance Spectroscopy (1H NMRDokument15 SeitenProton Nuclear Magnetic Resonance Spectroscopy (1H NMRJes AnthonyNoch keine Bewertungen
- AI Unit-IIIDokument75 SeitenAI Unit-IIIchakrimvnNoch keine Bewertungen
- NMR (VP)Dokument59 SeitenNMR (VP)Vishnu PriyaNoch keine Bewertungen
- 13C NMR SpectrosDokument32 Seiten13C NMR Spectrosamit chavan100% (1)
- McMurry9e PPT CH13Dokument72 SeitenMcMurry9e PPT CH13Ibrahim MNoch keine Bewertungen
- NMR FinalDokument50 SeitenNMR Finalpharmacologist786Noch keine Bewertungen
- 2D NMR Spectroscopy - 1-D and 2-D NMR, Noesy and Cosy, Hetcor, INADEQUATE TechniquesDokument21 Seiten2D NMR Spectroscopy - 1-D and 2-D NMR, Noesy and Cosy, Hetcor, INADEQUATE TechniquesАрсений БекишевNoch keine Bewertungen
- 2DNMRSpectroscopy AdvancedspectralanalysisDokument21 Seiten2DNMRSpectroscopy Advancedspectralanalysisابراهيم الاسطىNoch keine Bewertungen
- FinalDokument50 SeitenFinalarfa2009Noch keine Bewertungen
- 1H NMRDokument64 Seiten1H NMRhussemadl41Noch keine Bewertungen
- Nuclear Magnetic Resonance Spectroscopy: 1.1. BackgroundDokument4 SeitenNuclear Magnetic Resonance Spectroscopy: 1.1. BackgroundFalak NazNoch keine Bewertungen
- NMR Spectroscopy: Koradiya Ketan N. Inorganic Chemistry, Reg. Roll No-5, Bhavnagar University, Bhavnagar-05Dokument41 SeitenNMR Spectroscopy: Koradiya Ketan N. Inorganic Chemistry, Reg. Roll No-5, Bhavnagar University, Bhavnagar-05Koradiya Ketan NNoch keine Bewertungen
- SpectrosDokument2 SeitenSpectrosVishal SahuNoch keine Bewertungen
- NMR Case Studies: Data Analysis of Complicated MoleculesVon EverandNMR Case Studies: Data Analysis of Complicated MoleculesBewertung: 5 von 5 Sternen5/5 (1)
- Nanomagnetism and SpintronicsVon EverandNanomagnetism and SpintronicsTeruya ShinjoNoch keine Bewertungen
- DSX s300btx User ManualDokument2 SeitenDSX s300btx User ManualAngelLazarte100% (1)
- Voltage Monitoring Series SM 501Dokument5 SeitenVoltage Monitoring Series SM 501srinivasgateNoch keine Bewertungen
- Submitted By:: Physics Investigatory ProjectDokument19 SeitenSubmitted By:: Physics Investigatory Projectblack kobraNoch keine Bewertungen
- 02 - Modulo-5 CounterDokument4 Seiten02 - Modulo-5 CounterUsman QadeerNoch keine Bewertungen
- Solid-State LaserDokument7 SeitenSolid-State LaserAdel AbdallahNoch keine Bewertungen
- Introduction To Semiconductor ModuleDokument42 SeitenIntroduction To Semiconductor ModuleR Y100% (1)
- Free Chlorine Analyser (Swan)Dokument2 SeitenFree Chlorine Analyser (Swan)Văn Tuấn NguyễnNoch keine Bewertungen
- Circuit Theory: DR Paul S SpencerDokument13 SeitenCircuit Theory: DR Paul S SpencerMathew ClewlowNoch keine Bewertungen
- 2CSG257153R4051 Anr96prf 230 Network AnalyserDokument2 Seiten2CSG257153R4051 Anr96prf 230 Network AnalyserLuis EduardoNoch keine Bewertungen
- D74942GC40 15947 UsDokument4 SeitenD74942GC40 15947 UsWilliam LeeNoch keine Bewertungen
- mcp3911 3.3v Two Channel Analog Front End ds20002286dDokument74 Seitenmcp3911 3.3v Two Channel Analog Front End ds20002286dJosueEspinozaNoch keine Bewertungen
- Lecture Notes For Physics 229: Quantum Information and ComputationDokument321 SeitenLecture Notes For Physics 229: Quantum Information and ComputationIvan CheungNoch keine Bewertungen
- Norma Transformadores IeeeDokument96 SeitenNorma Transformadores IeeeedamycNoch keine Bewertungen
- FDAS Technical Data SheetsDokument6 SeitenFDAS Technical Data SheetsPhel FloresNoch keine Bewertungen
- 7SG14 - Duobias M Complete Technical Manual PDFDokument142 Seiten7SG14 - Duobias M Complete Technical Manual PDFhizbi70% (1)
- PLLDP BSCDokument6 SeitenPLLDP BSCmounhacNoch keine Bewertungen
- CD4020BC - CD4040BC - CD4060BC 14-Stage Ripple Carry Binary Counters - 12-Stage Ripple Carry Binary Counters - 14-Stage Ripple Carry Binary CountersDokument9 SeitenCD4020BC - CD4040BC - CD4060BC 14-Stage Ripple Carry Binary Counters - 12-Stage Ripple Carry Binary Counters - 14-Stage Ripple Carry Binary CountersNitish KumarNoch keine Bewertungen
- Abb Ag: RTU560 / RTU211Dokument2 SeitenAbb Ag: RTU560 / RTU211Cosmic Garash 2Noch keine Bewertungen
- Fiitjee: JEE (Main), 2014Dokument23 SeitenFiitjee: JEE (Main), 2014Gnana Deepak JuvvaNoch keine Bewertungen
- Chapter 7Dokument69 SeitenChapter 7Amit DostNoch keine Bewertungen
- Datasheet PDFDokument10 SeitenDatasheet PDFRi Cha RdNoch keine Bewertungen
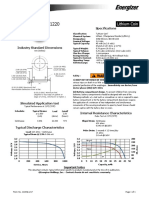
- Energizer Cr1220: Product DatasheetDokument1 SeiteEnergizer Cr1220: Product DatasheetZARCO_MX77Noch keine Bewertungen
- Lenovo G505s (Compal LA-A091PDokument60 SeitenLenovo G505s (Compal LA-A091PIsuru SharadaNoch keine Bewertungen
- Uniconn Operation Manual Addendum Subject: Operation of Phoenix Select Gauge With Uniconn Firmware: 1.200R1 Wellview Version 2.400R1Dokument11 SeitenUniconn Operation Manual Addendum Subject: Operation of Phoenix Select Gauge With Uniconn Firmware: 1.200R1 Wellview Version 2.400R1ahmed elsheikhNoch keine Bewertungen
- LCD InterfacingDokument5 SeitenLCD InterfacingRokibul hasanNoch keine Bewertungen
- Third Year 5 Semester Syllabus: (Applicable From The Academic Session 2018-2019)Dokument23 SeitenThird Year 5 Semester Syllabus: (Applicable From The Academic Session 2018-2019)Tignangshu ChatterjeeNoch keine Bewertungen
- Datasheet IDG500Dokument8 SeitenDatasheet IDG500Bharadwaj NandakumarNoch keine Bewertungen
- Cisco Netacad Chapter 5Dokument59 SeitenCisco Netacad Chapter 5Carlos IzaNoch keine Bewertungen
- Xilinx System Generator-Based Rapid Prototyping of Solid-State Transformer For On-Grid Renewable Energy IntegrationDokument9 SeitenXilinx System Generator-Based Rapid Prototyping of Solid-State Transformer For On-Grid Renewable Energy Integrationusher jamesNoch keine Bewertungen