Beruflich Dokumente
Kultur Dokumente
Effect of Type and Concentration of Different Water Soluble Polymer Solutions On Rheological Properties
Hochgeladen von
Madhukar ScribdOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Effect of Type and Concentration of Different Water Soluble Polymer Solutions On Rheological Properties
Hochgeladen von
Madhukar ScribdCopyright:
Verfügbare Formate
Nahrain University, College of Engineering Journal (NUCEJ) Vol.12 No.1, 2009 pp.
26-37
Dr. Muhanned A.R. Areej Jasim Mohammed
Mohammed
Assist. Prof. in Chemical Former Post graduat student
Engineering Chemical Engineering Dept.
Nahrain Unversity Nahrain Unversity
Effect of Type and Concentration of Different Water Soluble
Polymer Solutions on Rheological Properties
Dr. Muhanned A.R. Mohammed and Areej Jasim Mohammed
Abstract
1. Introduction
This research deals with experimental
study of the effect of concentration of Rheology is the study of the deformation
polymer solutions on rheological properties. and flow of matter. Deformation is the relative
All polymers studied in this work are water displacement of points of a body. It can be
soluble, which are: XC-polymer, divided into two types: flow and elasticity.
Carboxymethyl cellulose (two types), Flow is irreversible deformation; when the
Hydroxyethyl cellulose and Polyvinyl alcohol. stress is removed the material does not revert
The rheological properties of these polymer to its original form. This means that the work
solutions was investigated using a Couette converted to heat. Elasticity is reversible
coaxial cylinder rotational viscometer ( Fann deformation; the deformed body recovers its
model 35A ), by measuring shear stresses original shape, and the applied work is largely
versus shear rates (i.e. the flow curve). 55 recoverable. Vescoelastic materials show both
experiments were performed with different flow and elasticity [1].
polymer solutions concentrations at
temperature 30 C ( 1 C). It was found that Water Soluble Polymers
as polymer concentration increased, the flow Polymers are large organic molecules
behavior index (n) decreased and the compose of seed extracts (guar, starch),
consistency index (k) increased. This modified cellulose (CMC, HEC), biosynthetic
behavior reflects the fact that as polymer gums (Xanthan), and synthetic polymers
concentration increases the solution become (PVA) [2].
far from Newtonian fluid. Correlations were
found which describe the effect of polymer Sodium Carboxymethyl Cellulose (CMC)
concentration on n and k (for each polymer CMC is prepared by the reaction of
used in this study) and presented in a linear cellulose with chloroacetic acid in the presence
and exponential form respectively. of sodium hydroxide. It is containing strong
carboxyl groups which place it in the anionic
Key words: polymer, non-Newtonian fluids, polyelectrolyte category.CMC is mainly
Rheology. consumed in detergent, paint, textile, pulp and
paper, ceramics and oil drilling industries [3].
NUCEJ Vol.12, No.1 Water Soluble Polymer Solutions 26
Hydroxyethyl Cellulose (HEC) orientation. In dilute solutions, the rheology of
HEC is prepared by reaction of alkali the solution is dependent solely on the
cellulose with ethylene oxide in the presence dynamics of an individual chain and the
of isopropyl alcohol. number of chains (i.e. the concentration) in the
HEC is used in industry as dispersing system. At higher concentrations in the
agent, thickening, film-forming etc. In entangled region, interactions between
petroleum exploitation, it is used as stabilizer polymer molecules due to entanglements
and thickening agent, lubricating agent for impact the rheology in a significant way [2].
well drilling, completing and consolidating to
The Power Law Model
give slurry a good fluidity and stability.
The power law model is widely used as a
Besides, it finds wide application in ink, textile
model for non-Newtonian fluids.
dyeing, paper making, pharmaceuticals, food,
n
agriculture etc [4]. k 1
XC Polymer (Xanthan gum) where ; : is shear stress, : is shear rate, n :
XC polymer, also known as Xanthan gum, is flow behavior index, and k : is consistency
is an anionic polysaccharide derived from the index.
fermentation of the plant bacteria The power law model holds many solutions
Xanthamonas campestris. It is soluble in hot or and can describe Newtonian, shear-thinning,
cold water and gives visually hazy, neutral pH and shear-thickening behavior, depending on
solution [4]. the power factor, n, also called the flow
Xanthan gum is widely used as an effective behavior index. For a Newtonian fluid, n=1
stabilizer or a suitable thickener for various and the equation reduces to Newtonian model.
kinds of water-based systems. Its numerous If n is less than 1, the fluid is shear thinning; if
area of application cover a broad range it is greater than 1, the fluid is shear
including food, pharmaceutical ,cosmetic, thickening. A test of whether the power law
agricultural, textile, ceramic, and petroleum applies and a means to determining n is to plot
industries [5]. the log shear stress vs the log shear rate. If the
Polyvinyl Alcohol (PVA) plot is linear the power low applies. The value
Unlike most vinyl polymers, PVA is not of n, which is the slope of the line, can be used
prepared by polymerization of the as a measure of the degree of shear thinning or
corresponding monomer. PVA instated is shear thickening.
prepared by partial or complete hydrolysis of 2. Experimental Work
polyvinyl acetate to remove acetate group [6]. Polymers Used
Some uses of PVA include [7]: All polymers studied in this investigation
- Adhesive and thickener material in latex are water soluble and used in industries as a
paints, paper coatings, shampoos and glue. rheology control additive (rheology modifiers).
- Hoses, gaskets, pipe and gloves where its oil Five polymers were used in this study, these
and solvent barrier properties are important. are: XC-polymer, Carboxymethyl cellulose
- As an additive for strength to concrete and (two types), Hydroxyethyl cellulose and
cements. Polyvinyl alcohol, as follow:
Polymer Solutions Sets of Experiments
Polymer solutions can be considered as 55 experiments were performed to study the
liquid mixtures made of long macromolecular rheological properties of the aqueous solutions
chains, and small, light molecules of solvent of polymers used in this study. Experiments
[2]. were performed at 30 C ( 1 C). List of
Dilute solutions are those in which each experiments is shown in Table (1).
polymer chain is believed (or assumed) to be
completely isolated from the other polymer Viscometer
chains, and forms a coil at equilibrium. When The Fann viscometer model 35 is direct
the concentration is increased polymer reading instrument which has six speeds: 600,
molecules begin to interact by becoming 300, 200, 100, 6 and 3 rpm. It is a Couette
entangled. The concentration (known as the coaxial cylinder rotational viscometer [9].
critical concentration) required for a solution This instrument is a form of concentric
to become entangled will decrease as the cylinder viscometer that enables the variation
molecule becomes longer and occupies a larger of shearing stress with shear rate to be
equilibrium volume. observed.
In response to a deformation, the polymer
molecule itself can change both its shape and
NUCEJ Vol.12 No.1 Muhanned and Areej 27
electronicbalance, and added to 500 ml
Preparation of Polymer Solution
ofdistilled water in the Hamilton beach
Preparation of CMC, HEC and XC polymer
cup.The polymer lightly sprinkled into the
solutions
water and stirring continued for one hour to
The method for preparing a sample of
ensure completely polymer dissolution. The
polymeric solution at a certain concentration
prepared solution was kept at rest at room
was as follow:
temperature for 24 hr prior to conducting the
A previously dried polymer powder was
rheology measurements.
weighted to the nearest 0.001g using
Table (1) List of Experiments
Exp.No. Polymer Conc.(g/l) (g/l) Exp.No. Polymer Conc.(g/l) (g/l)
1 XC 4 29 CMC 48
2 XC 8 30 CMC 56
3 XC 12 31 CMC 64
4 XC 16 32 CMC 72
5 XC 20 33 CMC-2 4
6 XC 24 34 CMC-2 8
7 XC 32 35 CMC-2 12
8 XC 40 36 CMC-2 16
9 HEC 4 37 CMC-2 20
10 HEC 8 38 CMC-2 24
11 HEC 12 39 CMC-2 32
12 HEC 16 40 CMC-2 40
13 HEC 20 41 CMC-2 48
14 HEC 24 42 CMC-2 56
15 HEC 32 43 CMC-2 64
16 HEC 40 44 PVA 4
17 HEC 48 45 PVA 8
18 HEC 56 45 PVA 12
19 HEC 64 47 PVA 16
20 HEC 72 48 PVA 20
21 CMC 4 49 PVA 24
22 CMC 8 50 PVA 32
23 CMC 12 51 PVA 40
24 CMC 16 52 PVA 48
25 CMC 20 53 PVA 56
26 CMC 24 54 PVA 64
27 CMC 32 55 PVA 72
NUCEJ Vol.12, No.1 Water Soluble Polymer Solutions 28
Preparation of PVA solution versus shear rate are plotted on logarithmic
There are two methods for preparing scale.
aqueous solution of polyvinyl alcohol, the Selected flow curves, for lower and upper
conventional heating method and the polymer concentrations on logarithmic scale
microwave heating method. are shown in figures (6) to (10).
The microwave oven method is the The power law index (n) is the slope of the
preferred method for preparing the PVA line, while the power law consistency is the
solution [6,10], so it was considered in this intercept of the line with shear rate equal to
investigation as follow: one.
Dry PVA powder was weighted to the The effects of polymer concentration on
nearest 0.001g using electronic balance and the flow behavior index (n) for all polymer
added to 500 ml of distilled water in a Pyrex solution used are shown in figure (11).
beaker with stirring. The beaker placed in a
From this figure one can conclude that as
microwave oven and turned on high for three
the polymer concentration increased, n
minutes. The prepared solution was kept at rest
decreased. This behavior reflect the fact that as
at room temperature for 24 hr prior to
the polymer concentration increases the
conducting the rheological measurements
solution become more non-Newtonian, since
the polymers act as a thickening agent due to
Rheological Measurements
the interaction between the polymer molecules.
The procedure for measuring the
rheological properties of polymer solutions, Equations that describe the effect of
using the Fann viscometer model 35 was as polymer concentration on the flow behavior
follow: The sample cup was filled with index can be presented in a linear form with
polymer solution to the scribed line, and the correlation coefficients as shown in equations
rotor was immersed to the proper immersion below:
depth. nXC = - 0.02030 CXC + 0.8791 R2 =0.9938 2
The instrument was operated at 300 rpm
for three minutes to equalize the temperature nHEC= - 0.00240 CHEC + 0.6737 R2 = 0.8081 3
of the bob, rotor and polymer solution.
nCMC1= - 0.0006 CCMC1 + 0.7482 R2 = 0.1150 4
The instrument speed switched on to 600,
300, 200, 100, 6 and 3 rpm and the dial
reading was recorded. nCMC2= - 0.0029 CCMC2 + 0.7157 R2 = 0.8673 5
3. Results and Discussion nPVA= - 0.0009CPVA + 0.6964 R2 =0.6165 6
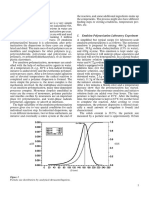
Sample of selected experimental results
are presented in figures (1) to (5), which are a where ; R : is the correlation coefficient.
plot of shear stress versus shear rate. In each
figure two curves were drawn, one at the lower From the above equations and from figure
polymer concentration used, and the other at (11) it can be noticed that n for XC polymer
the maximum polymer concentration which is more effected by concentration than other
can be measured in the Fann viscometer polymer used in this study. While PVA and
instrument. CMC (type 1) is less effected than other. and it
From these figures, one can notice that can be concluded that, in the range of polymer
the shear stress increases with increasing shear concentrations used in this study, n affected
rate in a non-linear shape. The curves are by concentration in the order:
bending down which reflects pseudo-plastic n XC > n CMC2 > n HEC > n CMC1 > n
behavior. As polymer concentration increases, PVA
the values of the shear stresses increased, since The effects of polymer concentration on the
the polymer molecules begin to interact by consistency index (k) for all polymer solutions
becoming entangled. used are shown in figure (12).
Determination of Rheological Properties
To determine the values of the power law
parameters, namely, flow behavior index (n)
and consistency index (k), the shear stress
NUCEJ Vol.12 No.1 Muhanned and Areej 29
12 180
160
10
140
Shear stress ( Pa ) 8 120
Shear stress ( Pa )
100
6
80
4 60
40
2
20
0 0
0 200 400 600 800 1000 1200 0 200 400 600 800 1000 1200
Shear rate ( 1 / s ) Shear rate ( 1 / s )
Figure (1) Flow curves for XC polymer solution at
concentrations 4 and 24 g/l.
3
140
2.5 120
100
2
Shear stress ( Pa )
Shear stress ( Pa )
80
1.5
60
1
40
0.5
20
0 0
0 200 400 600 800 1000 1200 0 200 400 600 800 1000 1200
Shear rate ( 1 / s ) Shear rate ( 1 / s )
Figure (2) Flow curves for HEC solution at concentrations 4 and 56 g/l.
3.5
180
3 160
140
2.5
120
Shear stress ( Pa )
Shear stress ( Pa )
2
100
1.5 80
60
1
40
0.5
20
0
0
0 200 400 600 800 1000 1200
0 200 400 600 800 1000 1200
Shear rate ( 1 / s )
Shear rate ( 1 / s )
Figure (3) Flow curves for CMC-1 solution at concentrations 4 and 72 g/l.
NUCEJ Vol.12, No.1 Water Soluble Polymer Solutions 30
3 160
140
2.5
120
2
Shear stress ( Pa )
Shear stress ( Pa )
100
1.5 80
60
1
40
0.5
20
0 0
0 200 400 600 800 1000 1200 0 200 400 600 800 1000 1200
Shear rate ( 1 / s ) Shear rate ( 1 / s )
Figure (4) Flow curves for CMC-2 solution at concentrations 4 and 48 g/l.
3 160
140
2.5
120
2
Shear stress ( Pa )
Shear stress ( Pa )
100
1.5 80
60
1
40
0.5
20
0 0
0 200 400 600 800 1000 1200 0 200 400 600 800 1000 1200
Shear rate ( 1 / s ) Shear rate ( 1 / s )
Figure (5) Flow curves for PVA solution at concentrations 4 and 72 g/l.
100 1000
10 100
Shear stress ( Pa )
Shear stress ( Pa )
1 10
y = 0.0551x 0.793 y = 13.364x 0.3774
R2 = 0.9917 R2 = 0.9814
0.1 1
1 10 100 1000 10000 1 10 100 1000 10000
Shear rate ( 1 / s ) Shear rate ( 1 / s )
Figure (6) Flow curves for XC polymer solution at concentrations 4 and 24 g/l.
NUCEJ Vol.12 No.1 Muhanned and Areej 31
10 1000
100
Shear stress ( Pa )
Shear stress ( Pa )
1 10
y = 0.022x 0.7127 y = 2.9478x 0.5494
R2 = 0.9791 R2 = 0.9575
0.1 0.1
1 10 100 1000 10000 1 10 100 1000 10000
Shear rate ( 1 / s ) Shear rate ( 1 / s )
Figure (7) Flow curves for HEC solution at concentrations 4 and 56 g/l.
10 1000
1 100
Shear stress ( Pa )
Shear stress ( Pa )
0.1 10
y = 0.0104x 0.8242 y = 1.253x 0.7104
R2 = 0.9896 R2 = 0.9966
0.01 1
1 10 100 1000 10000 1 10 100 1000 10000
Shear rate ( 1 / s ) Shear rate ( 1 / s )
Figure (8) Flow curves for CMC-1 solution at concentrations 4 and 72 g/l.
10 1000
100
Shear stress ( Pa )
Shear stress ( Pa )
1 10
y = 0.0169x 0.71
y = 3.8227x 0.5651
R2 = 0.9918
R2 = 0.9733
0.1 0.1
1 10 100 1000 10000 1 10 100 1000 10000
Shear rate ( 1 / s ) Shear rate ( 1 / s )
Figure (9) Flow curves for CMC-2 solution at concentrations 4 and 48 g/l.
NUCEJ Vol.12, No.1 Water Soluble Polymer Solutions 32
10 1000
Shear stress ( Pa ) 100
Shear stress ( Pa )
1 10
y = 0.0169x 0.71
R2 = 0.9918 y = 1.6835x 0.6311
R2 = 0.9886
0.1 0.1
1 10 100 1000 10000 1 10 100 1000 10000
Shear rate ( 1 / s ) Shear rate ( 1 / s )
Figure (10) Flow curves for PVA solution at concentrations 4 and 72 g/l.
thinning fluid, in which the viscosity
From this figure one can conclude that as the
decreases as the shear rate increases, or
polymer concentration increased, k increased.
the shear stress increases as shear rate
This behavior reflect the fact that as the
increases.
polymer concentration increases the solution
become more thicker.
2. As polymer concentration increased, the
Equations that describe the effect of flow behavior index (n) decreased. This
polymer concentration on the power law behavior reflects the fact that as polymer
consistency can be presented in an exponent concentration increases the solution
form with correlation coefficients as shown in become far from Newtonian behavior,
equations below: since the molecules of polymer interact to
kXC = 0.0243 e 0.2743 C R2 = 0.9837 7 become entangled. The XC polymer is
more effected by concentration than other
kHEC = 0.0310 e 0.0845 C R2 = 0.9581 8 polymers used in this study.
kCMC1 = 0.0119 e 0.0674 C R2 = 0.9776 9 3. Correlations were found, which describe
the effect of polymer concentration on the
kCMC2 = 0.0101 e 0.1316 C R2 = 0.9805 10 flow behavior index ( n ) for each
polymer used in this study.
kPVA = 0.0149 e 0.0672 C R2 = 0.9883 11
4. As the polymer concentration is increased,
where ; C: is the concentration of the polymer
the consistency index (k) is increased.
used.
This behavior reflects the fact that as the
Also from the above equations and from polymer concentration increases the
figure (12) it can be noticed that k for XC solution become thicker, since the
polymer is more effected by concentration than polymers act as thickening agents. The
other polymer used in this study. And it can be XC polymer is more effected by
concluded that, in the range of polymer concentration than other polymers used in
concentrations used in this study, k affected this study.
by concentration in the order:
5. Correlations were found, which describe the
k XC > k CMC2 > k HEC > k PVA > k CMC1 effect of polymer concentration on the
consistency index ( k ) for each polymer
4. Conclusions used in this study.
1. All polymer solutions used in this work
(XC-polymer, Carboxymethyl cellulose
(two types), Hydroxyethyl cellulose and
Polyvinyl alcohol) behave as shear-
NUCEJ Vol.12 No.1 Muhanned and Areej 33
1
1
0.9
0.9
0.8
0.8
0.7
Flow behavior index ( n )
0.7
Flow behavior index ( n )
0.6
0.6
0.5
0.5
0.4
0.4
0.3
0.3
0.2
0.2
0.1 y = -0.0203x + 0.8791
0.1 y = -0.0024x + 0.6737
R2 = 0.9938
R2 = 0.8081
0
0
0 5 10 15 20 25 30
0 20 40 60 80
Concentration ( g / l )
Concentration ( g / l )
1
1
0.9
0.9
0.8
0.8
0.7
Flow behavior index ( n )
0.7
Flow behavior index ( n )
0.6
0.6
0.5
0.5
0.4
0.4
0.3
0.3
0.2
0.2
0.1 y = -0.0006x + 0.7482
0.1 y = -0.0029x + 0.7157
R2 = 0.115
R2 = 0.8673
0
0
0 20 40 60 80
0 20 40 60 80
Concentration ( g / l )
Concentration ( g / l )
0.9
0.8
0.7
Flow behavior index ( n )
0.6
0.5
0.4
0.3
0.2
0.1 y = -0.0009x + 0.6964
R2 = 0.6165
0
0 20 40 60 80
Concentration ( g / l )
Figure (11)Effect of polymer concentratio onflow behavior inde
NUCEJ Vol.12, No.1 Water Soluble Polymer Solutions 34
20 4
18
3.5
16
3
14
Consistency index ( k )
Consistency index ( k )
2.5
12
10 2
8 1.5
6
1
4
0.5
2 y = 0.031e0.0845x
y = 0.0243e0.2743x
R2 = 0.9581
R2 = 0.9837
0
0
0 20 40 60 80
0 5 10 15 20 25 30
Concentration ( g / l )
Concentration ( g / l )
1.6 6
1.4
5
1.2
Consistency index ( k )
Consistency index ( k )
4
1
0.8 3
0.6
2
0.4
1
0.2
y = 0.0119e0.0674x y = 0.0101e0.1316x
R2 = 0.9776 R2 = 0.9805
0 0
0 20 40 60 80 0 20 40 60 80
Concentration ( g / l ) Concentration ( g / l )
2
1.8
1.6
1.4
Consistency index ( k )
1.2
0.8
0.6
0.4
0.2 y = 0.0149e0.0672x
R2 = 0.9883
0
0 20 40 60 80
Concentration ( g / l )
Figure (12)Effect of polymer concentrationon consistency index.
NUCEJ Vol.12 No.1 Muhanned and Areej 35
5. NOMENCLATUR
Symbol Meaning Unit
k Consistency index -
n Flow behavior index -
Greek Meaning Unit
letters
Shear rate s-1
Viscosity Pa. s
Newtonian limiting
Pa.s
viscosity
Shear stress Pa
Shear stress at mean
m Pa
viscosity
o Yield stress Pa
Abbreviations
Carboxymethyl
CMC
Cellulose
PVA Polyvinyl Alcohol
HEC Hydroxyethyl Cellulose
Xanthamonas
XC
Campestris
6. References 4. Polyvinyl Alcohol.
1. Kirk-Othemer, "Encyclopedia of http://en.wikipedia.org/wiki/Polyvinyl_alc
chemical technology", 4th edition, Vol.21, ohol
John Wiley and sons (1998). 5. Chhabra, R.P. and Richardson, " Non-
2. Gowariker, V.R.,Viswanathan, N.V. Newtonian flow in the process
and Sreedhar, J., "Polymer science", John industries", Elsevier Publishing Ltd.
Wiley and sons (1986). (1999).
AQUALON Sodium Carboxymethyl 6. Fann Viscometer, Model 35A
cellulose", Hercules Incorporated (1999). Manual.
http://www.herc.com/aqualon/product_dat http://www.expotechusa.com/manuals/fan
a/brochures/250_10.pdf n/35496.pdf
3. Vanderbilt Company, Inc., "Rheology 7. Flinn Scientific Inc., "Preparation of
control additive". polyvinyl alcohol" (2003).
http://www.rtvanderbilt.com/wwwprd http://www.flinnsci.com
/No920CD.pdf 8. Mohammed, M. AR. and
4. Vanderbilt Company, Inc., "Rheology Mohammed, A.J, "Effect of
control additive". concentration of water-soluble
http://www.rtvanderbilt.com/wwwprd/No polymer solutions on rheological
920CD.pdf properties.", M.Sc. thesis, Nahrain
2. Xanthan Gum. University (2008).
http://en.wikipedia.org/wiki/Xanthan_gum
3. Katz, D.A. "Polyvinyl alcohol slime",
(2005).
http://www.chymist.com/PVA%20Slime.p
df
NUCEJ Vol.12, No.1 Water Soluble Polymer Solutions 36
XC
(shear stress) (Fann VG-35A)
(shear rate)
n
k
k
NUCEJ Vol.12 No.1 Muhanned and Areej 37
This document was created with Win2PDF available at http://www.daneprairie.com.
The unregistered version of Win2PDF is for evaluation or non-commercial use only.
Das könnte Ihnen auch gefallen
- The Golden Book of Chemistry ExperimentsDokument114 SeitenThe Golden Book of Chemistry ExperimentsT4urus-VegaNoch keine Bewertungen
- GlassDokument22 SeitenGlassAnonymous RgKKzKGIX3Noch keine Bewertungen
- Techniques of SolubilizationDokument33 SeitenTechniques of SolubilizationSreekanth NamaNoch keine Bewertungen
- Iec60092-350 (Ed2 0) enDokument7 SeitenIec60092-350 (Ed2 0) enAzad RahmanNoch keine Bewertungen
- Chemical Enhanced Oil RecoveryDokument83 SeitenChemical Enhanced Oil RecoveryBryan Xavier Medina RodriguezNoch keine Bewertungen
- Brand Name Generic Name/Composition Primary Function Manufacturer/SupplierDokument27 SeitenBrand Name Generic Name/Composition Primary Function Manufacturer/Supplieriqbalpec8947Noch keine Bewertungen
- Midas Concrete DamageDokument9 SeitenMidas Concrete DamageShishir Kumar Nayak100% (1)
- Methods of Repairing Concrete StructuresDokument22 SeitenMethods of Repairing Concrete StructuresManzeer Fasaludeen100% (3)
- Determination of In-Situ Unit Weight of Soil: Experiment No 2 & 3 Soil Mechanics Laboratory CE PC 594Dokument30 SeitenDetermination of In-Situ Unit Weight of Soil: Experiment No 2 & 3 Soil Mechanics Laboratory CE PC 594SumanHaldar100% (1)
- Blended CementsDokument170 SeitenBlended CementsShruti KonkaNoch keine Bewertungen
- Characteristics of Steel Slags and Their Use in Cement and ConcreteDokument11 SeitenCharacteristics of Steel Slags and Their Use in Cement and ConcreteArulvijayNoch keine Bewertungen
- Complete Report On Formation of Poly Lac PDFDokument119 SeitenComplete Report On Formation of Poly Lac PDFMadhukar Scribd0% (1)
- Surface Chemistry of Surfactants and PolymersVon EverandSurface Chemistry of Surfactants and PolymersBewertung: 5 von 5 Sternen5/5 (1)
- Rheology of MeltsDokument6 SeitenRheology of Meltszeqs9Noch keine Bewertungen
- Degradation of Polyvinyl Alcohol Using Acoustic CavitationDokument19 SeitenDegradation of Polyvinyl Alcohol Using Acoustic CavitationMohammad AtifNoch keine Bewertungen
- Ankur Gupta, 2020Dokument14 SeitenAnkur Gupta, 2020Dimi lira santa rosaNoch keine Bewertungen
- Miniemulsion Polymerization-A Comparative Study Preparative VariablesDokument8 SeitenMiniemulsion Polymerization-A Comparative Study Preparative VariablesFelipe Vázquez DávilaNoch keine Bewertungen
- Rheological Properties of Carboxymethyl Cellulose: Mamdouh T. Ghannam, M. Nabil EsmailDokument13 SeitenRheological Properties of Carboxymethyl Cellulose: Mamdouh T. Ghannam, M. Nabil Esmailtrinh phamNoch keine Bewertungen
- 1 s2.0 S1226086X14002020 MainDokument5 Seiten1 s2.0 S1226086X14002020 MainHNoch keine Bewertungen
- Journal of Industrial and Engineering Chemistry: Hee Yeon Jang, Ke Zhang, Bo Hyun Chon, Hyoung Jin ChoiDokument3 SeitenJournal of Industrial and Engineering Chemistry: Hee Yeon Jang, Ke Zhang, Bo Hyun Chon, Hyoung Jin ChoiHNoch keine Bewertungen
- 1295 1 OnlineDokument20 Seiten1295 1 OnlineBarisNoch keine Bewertungen
- Synthesis of A New Three Dimensional Network Co Polymer and Studying The Ability of Drug Delivery SystemDokument7 SeitenSynthesis of A New Three Dimensional Network Co Polymer and Studying The Ability of Drug Delivery SystemNadherdaman AlshamaryNoch keine Bewertungen
- Oil-In-Water Nanoemulsions For Pesticide FormulationsDokument6 SeitenOil-In-Water Nanoemulsions For Pesticide FormulationsiswanadaNoch keine Bewertungen
- Synthesis and Characterization of Low Molecular Weight Cut Off Ultrafiltration Membranes From Cellulose Propionate PolymerDokument10 SeitenSynthesis and Characterization of Low Molecular Weight Cut Off Ultrafiltration Membranes From Cellulose Propionate PolymerGokul VenugopalNoch keine Bewertungen
- Inaccessible Pore VolumeDokument15 SeitenInaccessible Pore VolumeAprilya RamadantiNoch keine Bewertungen
- 3 s2.0 B0080431526004939 MainDokument6 Seiten3 s2.0 B0080431526004939 MainMohammed JamaliNoch keine Bewertungen
- Theory of ColloidsDokument16 SeitenTheory of ColloidsMarissa HarahapNoch keine Bewertungen
- Solubility of Drugs (Write Any 2)Dokument3 SeitenSolubility of Drugs (Write Any 2)ahmadzul8957Noch keine Bewertungen
- Influence of PH On The Stability of Oil-In-Water Emulsions Stabilized by A Splittable SurfactantDokument7 SeitenInfluence of PH On The Stability of Oil-In-Water Emulsions Stabilized by A Splittable Surfactantsfar yassineNoch keine Bewertungen
- Performance Study of Ceramic Micro FiltrationDokument7 SeitenPerformance Study of Ceramic Micro FiltrationAntonio SempereNoch keine Bewertungen
- Inverse-Emulsion Copolymerization of Acrylamide and Quaternary Ammonium CationicDokument10 SeitenInverse-Emulsion Copolymerization of Acrylamide and Quaternary Ammonium CationicSkolastika ErnaNoch keine Bewertungen
- Pseudoplastic& Thixotropic Behaviour: Presented byDokument27 SeitenPseudoplastic& Thixotropic Behaviour: Presented bysrugeeNoch keine Bewertungen
- SchaeferDokument7 SeitenSchaeferBundanufa CiciNoch keine Bewertungen
- Rheological Properties of Carboxymethyl Cellulose (CMC) SolutionsDokument8 SeitenRheological Properties of Carboxymethyl Cellulose (CMC) SolutionsSiti MujdalipahNoch keine Bewertungen
- Study of Physiochemical Properties of Sodium Dodecyl Sulphate Surfactant It's Micellization, Oil in Water Emulsification and Industrial ApplicationsDokument6 SeitenStudy of Physiochemical Properties of Sodium Dodecyl Sulphate Surfactant It's Micellization, Oil in Water Emulsification and Industrial ApplicationsInternational Journal of Innovative Science and Research TechnologyNoch keine Bewertungen
- Costa 2012Dokument7 SeitenCosta 2012Cristofer Newton ChigualaNoch keine Bewertungen
- 1 s2.0 S0927776521000485 MainDokument10 Seiten1 s2.0 S0927776521000485 MainPreyansh SrivastavaNoch keine Bewertungen
- 1 s2.0 S0272884223005655 MainDokument20 Seiten1 s2.0 S0272884223005655 MainafrizalsatyapNoch keine Bewertungen
- Sorption Studies of Methylene Blue On Silica GelDokument5 SeitenSorption Studies of Methylene Blue On Silica GelmtanaydinNoch keine Bewertungen
- (Bioplastic) Liquid, Injectable, Hydrophobic and Biodegradable Polymers As Drug Delivery VehiclesDokument11 Seiten(Bioplastic) Liquid, Injectable, Hydrophobic and Biodegradable Polymers As Drug Delivery VehiclesThanhTung NguyenNoch keine Bewertungen
- 11 Increasing Oil Recovery ThroughDokument12 Seiten11 Increasing Oil Recovery ThroughRika NazmilaNoch keine Bewertungen
- 2005 Wiley-Vch Verlag GMBH & Co. Kgaa, Weinheim Chem. Eur. J. 2005, 11, 1366 - 1373Dokument8 Seiten2005 Wiley-Vch Verlag GMBH & Co. Kgaa, Weinheim Chem. Eur. J. 2005, 11, 1366 - 1373Ade AnjaniNoch keine Bewertungen
- Polylactic Acid Synthesis With PolymerizationDokument9 SeitenPolylactic Acid Synthesis With PolymerizationnierzaNoch keine Bewertungen
- Novel Treatment Process of Harmful Organic Materials in Waste Water Using Temperature-Sensitive Gel Synthesized From PVADokument5 SeitenNovel Treatment Process of Harmful Organic Materials in Waste Water Using Temperature-Sensitive Gel Synthesized From PVAArash AbbasiNoch keine Bewertungen
- Rheological Properties of Carboxymethyl Cellulose (CMC) SolutionsDokument8 SeitenRheological Properties of Carboxymethyl Cellulose (CMC) SolutionsGeetanjali ChauhanNoch keine Bewertungen
- Effect of Solvent, InhibitorDokument12 SeitenEffect of Solvent, InhibitorNisreen MohamedNoch keine Bewertungen
- Controlling The Emulsion Stability of Cosmetics Through Shear Mixing ProcessDokument7 SeitenControlling The Emulsion Stability of Cosmetics Through Shear Mixing ProcessPark JiminNoch keine Bewertungen
- Ijca 47a (7) 1014-1019Dokument6 SeitenIjca 47a (7) 1014-1019Eliton Medeiros Candido de MacêdoNoch keine Bewertungen
- Chemical Injection PolymerDokument44 SeitenChemical Injection PolymerSyasya Ja’afarNoch keine Bewertungen
- C72IA001EN-C Application Report ChitosanDokument4 SeitenC72IA001EN-C Application Report ChitosanMartín PerezNoch keine Bewertungen
- Article 4Dokument7 SeitenArticle 4zulhairiNoch keine Bewertungen
- 1 s2.0 S014486171500096X MainDokument5 Seiten1 s2.0 S014486171500096X MainAhmad Sofyan SulaemanNoch keine Bewertungen
- Foaming in Micellar Solutions: E Ffects of Surfactant, Salt, and Oil ConcentrationsDokument11 SeitenFoaming in Micellar Solutions: E Ffects of Surfactant, Salt, and Oil Concentrationsjulio adrian tzoni patlaniNoch keine Bewertungen
- Oral Osmotic Pump: By: Rana Muhammad Awais Khan 06331613002Dokument45 SeitenOral Osmotic Pump: By: Rana Muhammad Awais Khan 06331613002abdullahNoch keine Bewertungen
- Pervaporation Membrane Reactors: 2010 Elsevier B.V. All Rights ReservedDokument29 SeitenPervaporation Membrane Reactors: 2010 Elsevier B.V. All Rights ReservedrukwavuNoch keine Bewertungen
- Physicochemical and Rheological CharacteDokument11 SeitenPhysicochemical and Rheological CharactegessicapalaoroNoch keine Bewertungen
- Rheology and Viscoelastic Properties of FluidsDokument29 SeitenRheology and Viscoelastic Properties of FluidsVetPhoenix StkNoch keine Bewertungen
- Rheological Properties of Carboxymethyl Cellulose (CMC) SolutionsDokument9 SeitenRheological Properties of Carboxymethyl Cellulose (CMC) SolutionsSantiago LopezNoch keine Bewertungen
- Jurnal FixDokument7 SeitenJurnal FixEva IndrianiNoch keine Bewertungen
- Poly (Dimethylsiloxane) Coatings For Controlled Drug Release. II. Mechanism of The Crosslinking Reaction in EmulsionDokument9 SeitenPoly (Dimethylsiloxane) Coatings For Controlled Drug Release. II. Mechanism of The Crosslinking Reaction in EmulsionMateoNoch keine Bewertungen
- Modification of The Stability of Oil-In-Water Nano-Emulsions by Polymers With Different StructuresDokument10 SeitenModification of The Stability of Oil-In-Water Nano-Emulsions by Polymers With Different StructuresAnand aashishNoch keine Bewertungen
- Firp Booklet # E201-ADokument30 SeitenFirp Booklet # E201-Aandrea.cipagautaNoch keine Bewertungen
- Effect of Molecular Weight On Drag Reducing AgentDokument9 SeitenEffect of Molecular Weight On Drag Reducing AgentWaltoy DinizNoch keine Bewertungen
- Cellulose Ethers: 2006 Wiley-Vch Verlag GMBH & Co. Kgaa, WeinheimDokument18 SeitenCellulose Ethers: 2006 Wiley-Vch Verlag GMBH & Co. Kgaa, WeinheimjaimeNoch keine Bewertungen
- Thermogelation of Methylcellulose: Rheological ConsiderationsDokument11 SeitenThermogelation of Methylcellulose: Rheological ConsiderationsGeetanjali ChauhanNoch keine Bewertungen
- 09 Shear Rheology-1Dokument5 Seiten09 Shear Rheology-1Siti MujdalipahNoch keine Bewertungen
- Asnacios 2000Dokument9 SeitenAsnacios 2000brouuorbNoch keine Bewertungen
- Gan Ley 2017Dokument9 SeitenGan Ley 2017franklinNoch keine Bewertungen
- 3-Pharmaceutical PolymersDokument49 Seiten3-Pharmaceutical Polymersahmed hotyNoch keine Bewertungen
- Chapter 9Dokument1 SeiteChapter 9Madhukar ScribdNoch keine Bewertungen
- Chapter 7Dokument1 SeiteChapter 7Madhukar ScribdNoch keine Bewertungen
- First Write UpDokument2 SeitenFirst Write UpMadhukar ScribdNoch keine Bewertungen
- Chapter 2Dokument1 SeiteChapter 2Madhukar ScribdNoch keine Bewertungen
- Chapter 4Dokument1 SeiteChapter 4Madhukar ScribdNoch keine Bewertungen
- Simulation Lab 19-08-14Dokument1 SeiteSimulation Lab 19-08-14Madhukar ScribdNoch keine Bewertungen
- Chapter 3Dokument1 SeiteChapter 3Madhukar ScribdNoch keine Bewertungen
- Distillation Column Minimizing Energy Requirements With APCDokument12 SeitenDistillation Column Minimizing Energy Requirements With APCMadhukar ScribdNoch keine Bewertungen
- Ch13 MCQDokument14 SeitenCh13 MCQMadhukar ScribdNoch keine Bewertungen
- 2506Dokument74 Seiten2506Madhukar ScribdNoch keine Bewertungen
- An Update On Solar Central Receiver Systems, Projects, and TechnologiesDokument11 SeitenAn Update On Solar Central Receiver Systems, Projects, and TechnologiesMadhukar ScribdNoch keine Bewertungen
- Government of Telangana C.T.Department: OriginalDokument3 SeitenGovernment of Telangana C.T.Department: OriginalMadhukar ScribdNoch keine Bewertungen
- An Introductory Course of Modeling and Computation For Chemical EngineersDokument15 SeitenAn Introductory Course of Modeling and Computation For Chemical EngineersMadhukar ScribdNoch keine Bewertungen
- MATLAB Manual by Dr. Narahari MarneniDokument72 SeitenMATLAB Manual by Dr. Narahari MarneniMadhukar ScribdNoch keine Bewertungen
- Government of Telangana C.T.Department: OriginalDokument3 SeitenGovernment of Telangana C.T.Department: OriginalMadhukar ScribdNoch keine Bewertungen
- Eoi - Website Publication - Modhera - Baola-210316 PDFDokument11 SeitenEoi - Website Publication - Modhera - Baola-210316 PDFMadhukar ScribdNoch keine Bewertungen
- All The Best : ##Reservoir Engineering##Dokument1 SeiteAll The Best : ##Reservoir Engineering##Madhukar ScribdNoch keine Bewertungen
- Press Release Bid SubmissionDokument8 SeitenPress Release Bid SubmissionMadhukar ScribdNoch keine Bewertungen
- Pngstat PDFDokument232 SeitenPngstat PDFMadhukar ScribdNoch keine Bewertungen
- Packaging Code: GRM31BR73A222KW01 - (1206, X7R:EIA, 2200pF, DC1000V) Reference SheetDokument26 SeitenPackaging Code: GRM31BR73A222KW01 - (1206, X7R:EIA, 2200pF, DC1000V) Reference Sheetakshay rajNoch keine Bewertungen
- 712-1010-001 - Avkcms - en 2205Dokument6 Seiten712-1010-001 - Avkcms - en 2205Vieru GabrielNoch keine Bewertungen
- Rubber JointDokument1 SeiteRubber JointAymen AyedNoch keine Bewertungen
- Polymer Chemistry For B.SC - Sem-6th PDFDokument49 SeitenPolymer Chemistry For B.SC - Sem-6th PDFSohel Ansari0% (1)
- ISRO Scientist Engineer Civil Paper 7 January 2024Dokument31 SeitenISRO Scientist Engineer Civil Paper 7 January 2024SATHISH KUMAR SNoch keine Bewertungen
- Glazed Alum Curtain Wall - Vendors ListDokument3 SeitenGlazed Alum Curtain Wall - Vendors ListKiran Kumar AkulaNoch keine Bewertungen
- Finite Element Simulation of Temperature and Strain Distribution in Al2024 Aluminum Alloy by Friction Stir WeldingDokument5 SeitenFinite Element Simulation of Temperature and Strain Distribution in Al2024 Aluminum Alloy by Friction Stir Weldingabhilash sharanNoch keine Bewertungen
- 22gg0023 - MZ Builders - 90 CDDokument2 Seiten22gg0023 - MZ Builders - 90 CDJan JanNoch keine Bewertungen
- Unit-I Chemical Bonding and Molecular Structure: (18 Contact Hours)Dokument3 SeitenUnit-I Chemical Bonding and Molecular Structure: (18 Contact Hours)Imran Afzal BhatNoch keine Bewertungen
- Mason1954 Mason, W., & Wick, R. (1954) - Ferroelectrics and The Dielectric Amplifier. Proceedings of The IRE, 42 (11), 1606Dokument15 SeitenMason1954 Mason, W., & Wick, R. (1954) - Ferroelectrics and The Dielectric Amplifier. Proceedings of The IRE, 42 (11), 1606Magdy Hussein Mourad MohammadNoch keine Bewertungen
- 1Dokument4 Seiten1Anonymous 6MI1wMNoch keine Bewertungen
- Recent Advances in Active Metal Brazing of Ceramics and Process-S12540-019-00536-4Dokument12 SeitenRecent Advances in Active Metal Brazing of Ceramics and Process-S12540-019-00536-4sebjangNoch keine Bewertungen
- ArcelorMittal - Quarto Plates PDFDokument7 SeitenArcelorMittal - Quarto Plates PDFDilip PatilNoch keine Bewertungen
- Geotechnical Engineering IiDokument72 SeitenGeotechnical Engineering IiGhail Rivas Gha ILNoch keine Bewertungen
- MTPDF4 - Module 4 Main PDF LessonDokument35 SeitenMTPDF4 - Module 4 Main PDF LessonEunnicePanaliganNoch keine Bewertungen
- Cylindrical Mandrel TesterDokument1 SeiteCylindrical Mandrel TesterAbiem SebastyanNoch keine Bewertungen
- AbcdDokument3 SeitenAbcdNikesh ShahNoch keine Bewertungen
- PCM Free Cooling With DEC PDFDokument13 SeitenPCM Free Cooling With DEC PDFqaiserNoch keine Bewertungen
- 2603988Dokument14 Seiten2603988Don RahulNoch keine Bewertungen
- Solid Waste ManagementDokument19 SeitenSolid Waste Managementayush maheshwariNoch keine Bewertungen
- 1998 Zhang Biodegradability ofDokument8 Seiten1998 Zhang Biodegradability ofAlejandro Castro YaruroNoch keine Bewertungen