Beruflich Dokumente
Kultur Dokumente
Supplemental Information: Han Et Al., Supplementary Information Page 1
Hochgeladen von
Baoz PingOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Supplemental Information: Han Et Al., Supplementary Information Page 1
Hochgeladen von
Baoz PingCopyright:
Verfügbare Formate
Cell Stem Cell, volume 12
Supplemental Information
Forebrain Engraftment
by Human Glial Progenitor Cells Enhances
Synaptic Plasticity and Learning in Adult Mice
Xiaoning Han, Michael Chen, Fushun Wang, Martha Windrem, Su Wang, Steven Shanz, Qiwu
Xu, Nancy Ann Oberheim, Lane Bekar, Sarah Betstadt, Alcino J. Silva, Takahiro Takano, Steven
A. Goldman, and Maiken Nedergaard
Inventory of Supplementary Information Page 1
Supplementary Figure S1 Page 2
Related to Figure 1
Supplementary Figure S2 Page 4
Related to Figure 2
Supplementary Figure S3 Page 5
Related to Figure 4
Supplementary Figure S4 Page 6
Related to Figure 5
Supplementary Figure S5 Page 7
Related to Figure 6
Supplementary Experimental Procedures Page 9
References for Supplementary Methods Page 18
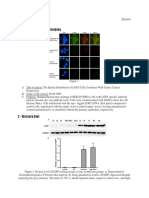
Han et al., Supplementary Information page 1
Supplementary Figures
Figure S1, related to Figure 1
Human glial chimerization accomplished by neonatal engraftment is not associated with a
detectable host microglial response
Han et al., Supplementary Information page 2
(A) Immunolabeling of cultured human glial progenitor cells in a rag2-/- immunodeficient host, 11
days post-MACS selection of A2B5+/PSA-NCAM- cells. By this time-point, in high-density culture,
most of the cells expressed hGFAP (red) and A2B5 (white), as well as EGFP (green). Histogram
illustrates the relative percentage of DAPI+ cells that expressed GFAP, A2B5, and EGFP (n = 7). A
similar expression pattern of hGFAP and A2B5 was consistently found across all the preparations used
for engraftment. Data presented as means SEM. (B) No evidence of microglial activation was noted
after neonatal engraftment of human glial cells. Immunohistochemical analysis showed no sign of
microglial activation, in human glial chimeric rag2-/- mice compared with unengrafted littermate controls
(n = 5; 7-13 months). CD68 (red) expression in the dentate gyrus in control and chimeric mice. Right
panel, high magnification. (C) Iba1 (green) expression in the DG in unengrafted and chimeric mice.
Right, high magnification. (D) CD68 (red) expression in the DG in rag1-/- chimeric mice. Right, high
magnification. (E) Iba1 (green) expression in the DG in rag1-/- chimeric mice. Right, high magnification.
(F) As a positive control, microglial activation was assessed in sections derived from spinal cord-injured
mice. Activated microglia are visualized by CD68 immunolabeling, 3 days after acute spinal cord injury.
CD68 (red), Iba1 (green), hNuclei (white), and DAPI (blue). Scale bars: (A): 100 m. (B-F): 50 m.
Han et al., Supplementary Information page 3
Figure S2, related to Figure 2
Human glial progenitors mature infrequently as oligodendrocytes in normally myelinated brain
Transferrin-immunoreactive human oligodendrocytes were essentially absent in human glial
chimeric mice (10-12 months old, n=8). (A) Non-overlap of human nuclei+ cells (hN, green) and
transferrin (Tf)+ oligodendrocytes (red), as imaged in in the dentate region of hippocampus of a
representative 10-month old chimeric mouse. All cells, DAPI (blue). Scale bar, 20 m. (B) Cell counts in
the inferior blade of the dentate gyrus (DG). Data graphed as means SEM.
Han et al., Supplementary Information page 4
Figure S3, related to Figure 4
Comparison of fEPSP slopes and paired-pulse facilitation in chimeric and unengrafted controls
(A) The slope of fEPSPs plotted as a function of fiber volley amplitude. The difference in slopes
of fEPSPs between chimeric and unengrafted littermates persisted after normalization to the fiber volley
amplitude (n=7 per group, 13.81.1vs12.60.4 months old respectively, **, p <0.001, two-way ANOVA,
F= 59.7). (B) Paired-pulse stimulation failed to identify differences between unengrafted control and
chimeric mice. Inter-pulse interval was 20 msec. (C) Paired pulse ratio plotted as a function of inter-
pulse intervals in chimeric and littermate control mice. (n=6, 12.10.5 months; p>0.280, two-way
ANOVA) (D-E) Comparison of D-Serine and serine racemase immunolabeling in chimeric and
unengrafted littermate controls. Neither D-serine (D) nor serine racemase (E) immunolabeling (red) in
mouse brain were altered by engraftment of human glial cells. DAPI, blue. Quantitative
immunohistochemistry showed no difference in intensity of labeling in chimeric mice compared with
unengrafted littermate controls. Small white arrows indicated hNuclei+ (white) cells (n = 5, 7-16 months,
p>0.05, t test; means SEM). Scale bar: 100 m.
Han et al., Supplementary Information page 5
Figure S4, related to Figure 5
Human glia express relatively high levels of TNFthan do resident glia
(A) Human glial chimeric brains express high levels of a human isoform of TNF human-specific
(hTNF), along with species non-specific mammalian TNF mRNA. From left: Lanes 1-2, Human glial
chimeric brains 1 and 2, each a rag2-/- mouse brain transplanted on the first postnatal day with 105
PSA-NCAM-/A2B5+ human fetal GPCs, and killed at 49 weeks of age. Lanes 3-4, Unengrafted controls
1 and 2, also killed 49 wks post-neonatal transplant. Lane 5, Control mouse cells: GL261 mouse glioma
cells used as a source of mouse mRNA. Lane 6, Human fetal cortical tissue: 20 wk g.a. human
neocortex, as a positive control for human-specific mRNA. Lane 7, Human CD11b+ microglia, as a
positive control for human TNF mRNA. Lane 8, Control mouse brain tissue: adult mouse forebrain
cortex as a control for mouse TNF mRNA. (B) Phosphorylation of GluR1 at the Ser845 site (red) was
not significantly altered by neonatal engraftment of human glial cells (hNuclei, white). DAPI, blue. (C)
Quantitative immunohistochemistry showed no changes in Ser845-phosphoTNF immunofluorescence
in human glial chimeric hippocampi, or in mice treated with thalidomide. (n = 6, 9-16 months, p>0.05, t
test; means SEM). Scale bar: 100 m.
Han et al., Supplementary Information page 6
Figure S5, related to Figure 6
Behavior and performance increments of human glial chimeric mice
(A-B) No difference in engraftment success (hNuclei+ cells) or glial differentiation (hGFAP vs.
hNG2) was noted between rag2 and rag1 mice. DAPI: blue; hNuclei: white; hNG2: red; hGFAP: green.
Han et al., Supplementary Information page 7
Scale bar: 200 m. (C) Comparison of responses to foot shock. As a control for potential deficits in
sensory perception between human glial chimeras and controls, the velocity index was calculated.
Velocity of movement before and immediately after foot shock was analyzed using a tracking program.
No significant differences between chimeric and control littermate were noted in either the rag1 or rag2
mice (n = 5, p > 0.05, two-way ANOVA with Bonferroni test; rag1-/- mice, 7.6 0.12 months old; rag2-/-
mice, 9.6 0.95 months old).
(D-E) No effect of human glial chimerization on thermal or mechanical sensitivity was noted in
either rag1- or rag2-null hosts (12.6 0.14 months old, n = 6, one way ANOVA, Tukey). (F) While
chimeric mice exhibited superior cognitive performance as a function of training (n=6, *, p<0.01,
Students t-test), reflected in significantly more rapid task acquisition, the probe test for Barnes Maze
demonstrated that with sufficient training, unengrafted control mice were eventually able to complete
the maze as well as chimeric mice (7.4 0.12 months old, n = 6, *, p < 0.05; t test).
(G) The social interaction levels of rag1 null mice were not affected by engraftment of human
GPCs. Both unengrafted and chimeric animals displayed a significant preference for social interaction
(6.9 0.12 months old, n = 5, *; p < 0.01, Students t-test), but indistinguishably so from one another.
Bars represent total time mice spent in contact. (H) Similarly, social novelty preference was also
unaffected by chimerization. Both unengrafted and chimeric animals displayed a strong preference for
social interactions with novel mice relative to familiar mice (6.9 0.12 months old, n=5, **, p<0.01;
Students t-test). Bars represent total time mice spent in contact.
B-H, All data plotted as means SEM.
Han et al., Supplementary Information page 8
Supplementary Experimental Procedures
Isolation of human and murine glial progenitor cells
Fetal glial cells progenitors were extracted from 17 to 22-week-old human fetuses obtained at
abortion. The forebrain ventricular and subventricular zones were dissected free on ice and then were
dissociated using papain/DNAase as described (Windrem et al., 2004). All samples were obtained with
consent under approved protocols of the University of Rochester Research Subjects Review Board.
After incubated overnight in DMEM/F12/N1 with 20 ng/ml FGF, to isolate glial progenitor cells, the
dissociated cells were sorted using a dual immunomagnetic sorting strategy. The antibodies for MACS
(Miltenyi) were anti-PSA-NCAM (Chemicon, 1:100), MAb A2B5 (clone105; ATCC, Manassas, VA),
microbead-tagged rat anti-mouse IgM (Miltenyi Biotech). All incubations were done as described
(Nunes et al., 2003) and A2B5+/PSA-NCAM- cells were eluted. Murine A2B5+/PSA-NCAM- cells were
isolated from newborn Tg(CAG-EGFP)B5Nagy/J mice pups (mixed background, Jackson Laboratory).
After sorting, the A2B5+/PSA-NCAM- human or murine cells were cultured for 1-2 days in
DMEM/F12/N1 with 20 ng/ml bFGF.
Transfection and differentiation
A2B5+/PSA-NCAM- cells were transfected to express enhanced EGFP by VSVg-pseudotyped
lentiviral-CMV-EGFP infection. The vectors were produced by transfection of 293 Ft cells as described
(Windrem et al., 20002). The cells were mixed with lentivirus overnight followed by one day of recovery.
The infected cells were cultured in DMEM/F12/N1 with 20% fetal bovine serum (FBS; GIBCO) and
passaged up to 3 times. The cells were inspected prior to engraftment, and preparations with less than
80% EGFP expression were not used. Immunolabeling showed that >95% of the cells expressed
GFAP, while approximately 30% continued to co-express A2B5, at the time of implantation (Fig. S1A).
Transplantation
Human glial chimeras were prepared as described (Windrem et al., 2008; Goldman and
Windrem, 2009). Briefly, A2B5+/PSA-NCAM- cells were dissociated from culture using 0.05% trypsin
and resuspended in the HBSS (-MgCl2,-CaCl) at a concentration of 100,000 cells/l and maintained on
ice (Windrem et al., 2004). Newborn pups of rag1 (B6.129S7-Rag1tm1Mom on a C57/Bl6 background,
Jackson Lab) immune-deficient or homozygous rag2 (Rag2tm1FwaN12, on a C3H background,
Taconic) mice were transplanted within a day of birth (postnatal day 1). The pups were first
cryoanesthetized and 1 x 105 donor cells were injected in equal amount into the forebrain at 2 locations:
1.0 mm posterior bregma and 1.0 mm bilaterally from the midline and 1.2mm to 1.5mm depth. All cells
were injected through glass micropipettes, inserted directly through the skull into the target sites (Sim et
al., 2011; Windrem et al., 2004; Windrem et al., 2008). The pups were weaned at 21 days and
Han et al., Supplementary Information page 9
maintained in caged with a small number (2-3) of mice of the same sex. All experiments approved by
Rochester University Animal Care and Use Committee.
A subset of mice was treated with thalidomide 5-10 days prior to preparation of acute
hippocampal slices or behavioral analysis (Ryu and McLarnon, 2008). Thalidomide (Sigma, T144) was
dissolved in 0.5% carboxy methylcellulose (Sigma, C9481) 250-500 mg/kg, i.p., volume ~0.25-0.3 ml
administered daily. The control chimeric group received vehicle injection (0.5% carboxymethyl
cellulose, volume ~0.25-0.3 ml) (Hammond et al., 2007; Tsenova et al., 1999; Tweedie et al., 2007). No
differences in engraftment success (hNuclei+ abundance), progenitor distribution (NG2+ cell dispersal),
or glial differentiation (hGFAP+ cells) were noted between rag2 and rag1 mice (Figs. S5B-C).
Quantitative immunohistochemistry
Chimeric mice and littermate controls (age range 2 weeks - 20 months) were perfusion-fixed
with 4% paraformaldehyde. The brains were removed and processed as 100 m vibratome sections or
as cryosections (14 m). Immunofluorescence antibodies used: mouse anti-human nuclei (1:200,
MAB1281, Chemicon), monoclonal antibody against glial fibrillary acidic protein (GFAP, stain both
mouse and human GFAP) (1:500, G3893, Sigma), rabbit anti GFAP (1:500, Z0334, DAKO),
monoclonal antibody to human GFAP (SMI21, 1:500, Sternberger), chicken anti-laminin (1:400,
ab14055, Abcam), monoclonal antibody to human mitochondria (1:200, M3985, US Biological), rabbit
anti Iba1(1:500, 019-19741, Wako), rabbit anti connexin 43 (1:500, AB1728, Chemicon), Rabbit anti-
transferrin (1:800, ab9538 , Abcam), rabbit anti human NG2 (1:500, MAB2029, Chemicon),rat anti
CD68 (1:100, MCA1957, Serotec), rabbit anti Glu1R alpha (1:300, AB1504, Chemicon), monoclonal
antibody against NMDAR1 (1:2000, MAB363, Chemicon), goat anti human TNF (1:30, BAF210, R&D
systems), rabbit anti TNF (1:500, AAM19GA, Serotec), rabbit anti D-serine (1:3000, ab6472, Abcam),
goat anti serine racemase (1:100, sc-5751, Santa Cruz), rabbit anti phospho-ser845 GluR1 (1:1000,
p1160-845, Phosphosolutions) and rabbit anti phospho-ser831 GluR1( 1:1000, 36-8200, Invitrogen). All
secondary antibodies were used at 1:250 (Jackson Immunoresearch). After the immunolabeling, the
section was counter-stained with 4,6-diamidino-2-phenylindole (DAPI, 1:10,000; D-21490, Invitrogen).
Fluorescent signals were detected with a confocal microscope by a blinded observer (FV500,
Olympus). Stacks of images with 1 m increment in depth were collected using 40x oil objective lens
(NA 1.3) with FluoView (Olympus) software and subsequently analyzed with Image J (NIH) software.
Quantitative immunohistochemistry was employed in these studies due to the limited availability of adult
chimeric mice. The protocol for quantitative immunofluorescence was adapted from previous studies
analyzing the effect noxious stimulation on the phosphorylation state of GluR1 phosphorylation and
others (McHugh et al., 2007; Nagy et al., 2004; Wiltgen and Silva, 2007). Cryosections not incubated in
the primary antibody were used to estimate the background fluorescence. Pixel intensities of a unit
Han et al., Supplementary Information page 10
square area (150 m X 150 m) were quantified in the dentate gyrus using Fluoview software. At least
3 sections from each mouse in which measurements were collected from a minimum of 3 areas were
analyzed per mouse. All sections were stained in parallel (chimeric, littermate controls, and omission of
primary antibody). The laser power intensity, PMT, offset, and all other acquisition parameters were
held constant for every batch of immunostained slides. For analysis of success of engraftment and
differentiation of GPCs, randomly selected coronal sections were labeled for human nuclei (hNuclei),
hGFAP, GFAP, and DAPI. Four to five sections including the DG were assessed per mouse, for the
number of DAPI+, hNuclei+ and hNG2+ immunoreactive cells. Since it was often not possible to assign
the long hGFAP+ processes to individual hNuclei+ cells, we calculated the fraction of hGFAP+ labeling
among all GFAP+ signal, estimated as the proportion of anti-human GFAP pixels among all GFAP+
pixels (including both human and mouse GFAP) in each imaged dentate gyrus. The diameter of the
astrocytes was determined by defining measuring the longest axis through the nucleus using the GFAP
antibody that labeled both human and mouse astrocytes (Colombo, 1996; Oberheim et al., 2009). For
dot-maps of hNuclei+ cells, three 20 m coronal sections from the brain of a 10 month-old engrafted
mouse were used. Sections were photographed on a Leica LMD 6500 and mapped using Stereo
Investigator (Microbrightfield, Williston, VT).
Electrophysiological characterization of human glial cells in chimeric mice
Chimeric mice and littermate controls (4 to 10 months) were decapitated and the whole brains
were rapidly removed and immersed in an ice-cold solution containing (in mM): 230 sucrose, 2.5 KCl,
0.5 CaCl2, 10 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, and 10 glucose and saturated with 95% O2 and 5%
CO2. Coronal slices (400 m) were cut using a vibratome and transferred to oxygenated artificial
cerebrospinal fluid (ACSF) that contained (in mM): 126 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 26 NaHCO3,
1.25 NaH2PO4, and 10 glucose (Lovatt et al., 2012; Torres et al., 2012). The slices were examined for
EGFP+ cells under fluorescent microscope, and then transferred to a submerged chamber associated
with two-photon microscope for whole-cell recordings. Nineteen chimeric mice for whole-cell
recordings, whole-cell recordings of EGFP expressing cells were low-pass filtered at 2 kHz and
digitized at 10 kHz. The pipette solution contained 140 mM K-gluconate, 5 mM Na-phosphocreatine, 2
mM MgCl2, 10 mM HEPES, 4 mM ATP, 0.3 mM GTP and 50 M rhod-2 tripotassium salt (pH adjusted
to 7.2 with KOH). The junction potential and pipette resistance was first compensated with pipette
compensation as soon as the pipette was placed in the solution. Patch clamp was performed under
two-photon microscope. Low pressure was added to the pipettes before and during inserting the pipette
in the tissue, in order to make the pipette visible under two-photon microscope, and also to
decontaminate the pipette tip. High pressure was avoided to reduce dye leakage, which would make
the EGFP cell invisible. Electrodes with seal resistances under 1G were rejected. After whole-cell
Han et al., Supplementary Information page 11
recording formed, I-V curves were plotted with a series of holding membrane potentials vs. induced
currents. Current-clamp recordings were used for the analysis of plasma membrane potential, and input
resistance was calculated by injecting +100pA currents.
Long term potentiation
Chimeric mice and littermate controls (age range 720 months) were used for recordings of field
excitatory postsynaptic potentials (fEPSPs), and analysis of activity-dependent changes in synaptic
strength. Slices were prepared similar to above. fEPSPs were evoked using a single 0.10 ms biphasic
pulse delivered through a constant isolated current source (IsoFlex Isolator, and Master-8, AMPI,
Israel) and applied to medial perforant-path fibers using with a concentric platinum/ iridium bipolar
electrode (CBARC75, FHC, Brunswick, ME), and recorded with a pipette filled with ACSF or saline and
positioned in the dentate. fEPSPs were recorded by an amplifier (700B, Axon Instruments Inc.), and a
pCLAMP 10.1 program and DigiData 1440 interface with an interval of 20 s. Synaptic efficacy was
monitored at 60% of the intensity necessary to evoke a maximum fEPSP response as determined from
input/output curve construction. Individual fEPSPs were evoked and recorded every 30 s and a stable
15 min baseline collected in all experiments. After baseline recording, we applied conditioning high
frequency stimulation (HFS, 2 train containing 100 pulses at 100 Hz, 30 s between bursts) to induce
LTP. After application of the conditioning protocol, fEPSPs were again recorded for 1 h. After recording,
slices were fixed in 4% paraformaldehyde, then transfer into 30% sucrose, and 14 m cryosections
were prepared for immunohistological examination to validate the presence of EGFP+ human or murine
glial cells. Only slices containing engrafted EGFP+ cells were included in the analysis.
Ca2+ Imaging and photolysis of caged Ca2+
Chimeric mice (age range 4 to 10 months) were used for Ca2+ imaging experiments. EGFP cells
were loaded with the cell-impermeable Ca2+ indicator, rhod-2 (50 M), tripotassium and caged Ca2+
compound o-nitrophenyl ethylene glycol bis (2-aminoethyl ether)-N,N,N'N'-tetrapotassium salt (NP-
EGTA) (200M) through whole-cell patch clamp. Fluorescence was monitored continuously to avoid
delivery of excessive amounts of the dye solution. A custom-built microscope attached to
Tsunami/Millenium laser (10 W, SpectraPhysics) and a scanning box (FV 300, Olympus) using
Fluoview software and 40X objective (0.9 NA, Olympus) was used for two-photon laser scanning
microscopy (Lovatt et al., 2012; Torres et al., 2012). Time-lapse images of astrocytic Ca2+ signaling
were recorded every second, except for line scans (2-4 ms sampling rate depending on the length of
the line selected). A nitrogen-pulsed laser (355 nm; DPSS) controlled by a shutter (Uniblitz, Vincent
Associate, 10 ms duration with an interval of 50 ms) was focused through the 40X water-immersion
objective to an optical spot of 4 m diameter to uncage NP-EGTA. To trigger Ca2+ elevation in
astrocytes, a train of 4 X 10 ms UV-laser pulses was delivered to the targeted astrocytes, and the
Han et al., Supplementary Information page 12
increase in rhod-2 fluorescence was used to monitor the effect on intracellular Ca2+. UV laser intensity
was below 20 milliwatt before entering microscopy and below 3.5 milliwatt after passing through the
40X objective. All experiments were performed in the presence of 1 M tetrodotoxin (TTX). Human
cortical tissues from 3 patients (30.6 8.8 years) were obtained with patient consent and by the
approval of the University of Rochester Research Subjects Review Board; samples were collected
directly at surgical resection for medication-refractory epilepsies in distant cortical sites, then prepared
for physiological assessment and analyzed as described for the rodent studies.
Detection of human TNF in human glial chimeras
PCR primer design PCR primers for both human specific and pan-specific were designed
according to available nucleic acid sequences of human and mouse TNF mRNA. Two available
mRNA sequences were aligned with the Vector NTI Advance 9.0 program (Invitrogen).
Oligonucleotides specific for human TNF were selected based on aligned human TNF mRNA
sequence. The resultant sense primer was 5-AAG CAA CAA GAC CAC CAC TTC G-3, and the
antisense primer was 5-TAA GGT CCA CTT GTG TCA ATT TCT AGG-3. The pan-specific primers
were selected from the sequence region shared in both human and mouse TNF mRNA. The pan-
TNF sense primer was 5-AGG GAA TGG GTG TTC ATC CAT TC-3, and the pan-TNF antisense
primer: 5-CTT GAT GGT GGT GCA TGA GAG GCC-3.
RNA isolation Total RNA samples were extracted from human or mouse brain tissues as well as
cultured cells with RNeasy Mini Kit (QIAGEN) following the manufacturers instructions.
RT and PCR reaction Reverse transcription was performed using the high-capacity cDNA
reverse transcription kit (Applied Biosystems) according to the manufacturers recommendation. Total
RNA (250 ng) was reverse transcribed in a 25 l reaction volume using random primers. PCR was
performed with a DNA thermal cycler (BIO-RED), and the amplification was followed by initial
denaturing at 94 C for 5 min, then 40 cycles were 94 C for 30 sec, 54 C for 30 sec, 72 C for 2 min;
and finally 72 C for 10 min after the last cycle. PCR products were analyzed on 1.4% agarose gels,
and bands were visualized with SYBR Safegel stain using a Carestream MI image analysis system
(Kodak).
Auditory fear conditioning
Two commercial conditioning chambers (H10-11M-TC, Coulbourn Instruments) were adapted
for auditory fear conditioning. The mice were placed in the first chamber (chamber 1) at day one and
allowed to acclimate for 3 min before receiving a sound stimulus (420 Hz, 50 dB, 30 second). Upon
termination of the tone, the mouse received a 2 second 0.3 mA foot shock delivered by stainless steel
rods in the floor. The following day, the mouse was placed inside a container in the second conditional
chamber (chamber 2). The container was cleaned with 75% alcohol and fresh mouse bedding between
Han et al., Supplementary Information page 13
each trial. A video recording was collected for the entire 6 min period. The mice were only exposed to
tone, no foot shock in chamber 2. The mice were retrained 2 hours later in first conditioning chamber
using the same training protocol (pairing the tone with the foot shock). Testing and re-training were
repeated for 4 consecutive days following the same protocol. Overtraining Like the regular training, the
mice were placed in the first chamber (chamber 1) at day one and allowed to acclimate for 3 min before
receiving a sound stimulus (420 Hz, 50 dB, 30 seconds). Upon termination of the tone, the mouse
received a 2 second 0.3 mA foot shock delivered by stainless steel rods in the floor. The mice were
then given a 1-minute rest period before receiving another tone stimulus (420 Hz, 50 dB, 30 seconds)
followed by a 2 second 0.3 mA foot shock. This was done three times for a total of 3 shocks. The
following day, the mouse was placed inside a container in the second chamber (chamber 2). The
containing was cleaned with 75% alcohol and fresh mouse bedding between each trial. Video recording
was collected for the entire 6 min period. The mice were retrained 2 hours later in first conditional
chamber using the same training protocol (pairing 3 sound stimuli and 3 foot shocks). Testing and re-
training were repeated for 4 consecutive days following the same protocol. Over-trained mice were
housed and transported in identical fashion to the regular train mice. FreezeView software (ActiMetric
Software, Inc) was used to score freezing of the last 30 seconds of acclimation and the first 30 seconds
of sound stimulus. Again motion below 20 was considered freezing.
Velocity index To compare shock reactivity between the chimeric mice and control littermates,
we calculated the velocity index before and immediately after the CS, using the Anymaze tracking
program.
Contextual Conditioning
To highlight the hippocampus learning capabilities of the mice two commercial conditioning
chambers (H10-11M-TC, Coulbourn Instruments) were adapted for contextual conditioning. The mice
were placed in the first chamber (chamber 1) at day one and allowed to acclimate for 3 min before
receiving a sound stimulus (420 Hz, 50 dB, 30 second). Upon termination of the tone, the mouse
received a 2 second 0.3 mA foot shock delivered by stainless steel rods in the floor. The following day
the mice were retrained in the same chamber and freezing percentage was noted as a measure of
hippocampus dependent spatial learning. Immediately after retraining, animals were introduced to an
alternative context (chamber 2) to determine baseline anxiety and generalized fear (Wiltgen and Silva,
2007). Training and testing continued for 4 consecutive days following baseline. To ensure consistency,
all mice were housed in individual cages for 2 months in a single room prior to testing. Transportation to
the behavior room occurred at the same time of the day in their own cage and they were immediately
thereafter returned. The acclimation period was used for freezing scoring using FreezeView software
(ActiMetric Software, Inc). The software generates an index of mouse motion by quantifying the number
of pixels that changes from frame-to-frame factoring out noise. The software generated data that
Han et al., Supplementary Information page 14
closely corresponded to blind manual scoring the movies. Motion index below 20 were scored as
freezing (Anagnostaras et al., 2000).
Overtraining Like the regular training, the mice were placed in the first chamber (chamber 1) at
day one and allowed to acclimate for 3 min before receiving a sound stimulus (420 Hz, 50 dB, 30
seconds). Upon termination of the tone, the mouse received a 2 second 0.3 mA foot shock delivered by
stainless steel rods in the floor. The mice were then given a one-minute rest period before receiving
another tone stimulus (420 Hz, 50 dB, 30 seconds) followed by a 2 second 0.3 mA foot shock. This was
done three times for a total of 3 shocks. The following day mice were retrained in the same chamber.
The containing was cleaned with 75% alcohol between each trial. As was done in the normal training,
video recording was collected for the entire 6 min period. Testing and re-training were repeated for 4
consecutive days following the same protocol. Over-trained mice were housed and transported in
identical fashion to the regular train mice. FreezeView software (ActiMetric Software, Inc) was used to
score freezing of the last 30 seconds of acclimation and the first 30 seconds of sound stimulus. Again
motion below 20 was considered freezing.
Discrimination Ratio To determine if the mice were able to distinguish the two chambers
(training and novel chamber) from one another, a discrimination ratio (training context/training context +
novel environment) was obtained (Wiltgen and Silva, 2007). A ratio of one indicates that the mice were
able to completely discriminate between the two contexts, as the mouse would have only exhibited
freezing in only one of the two contexts. Ratios closer to 0.5 indicate that mice were unable to
distinguish between the novel and training environments, as they would have frozen equally in both
contexts.
Velocity index To compare shock reactivity between the chimeric mice and control littermates,
we calculated the velocity index before and immediately after the CS, using the Anymaze tracking
program.
Barnes Maze
The Barnes maze was used to study hippocampus dependent spatial learning (Asrar et al.,
2011; O'Leary et al., 2011). In the Barnes maze, the animals are trained to use spatial clues mice to
find a small dark escape chamber under the platform called the escape box. The maze consists of a
white circular platform, 1 meter in diameter with 20 evenly spaced holes at the edges. The platform is
elevated 1 meter from the ground to prevent animals from jumping off. The escape tunnel is retained at
the same position relative to the room, while the platform is rotated with each trial to prevent any
possible scent trails (Dawood et al., 2004).
Animals were first acclimated to the maze in a cylindrical black start chamber placed in the
center of the maze for 30 sec. Following the adaptation period, mice are allowed to explore the maze
for 3 minutes. If the mouse failed to find the escape tunnel, by the end of the 3 minute period it was
Han et al., Supplementary Information page 15
guided back. The mouse was left in the escape tunnel for 1 minute and gently guided back if it decided
to leave within the 1 min period. Animals were trained in 4 sessions daily with an inter-trial interval of 15
minutes for 4 days. The endpoints of the analysis were latency and the number of errors made finding
the escape hole (Harrison et al., 2006)
On the fifth day, the animals were exposed to a probe trial in which the escape box was closed.
Mice were allowed to explore the maze for 90s. The latency and amount of errors of the mouse to find
the location of the previous escape hole were noted (Patil et al., 2009). All sessions were recorded by a
camera above the chamber and scored by a blinded observer.
Object Location Memory Task
To evaluate hippocampal-dependent spatial learning, mice underwent two day object location
memory task. A chamber (25 x 18 x 17 inches) made of non-transparent polyvinyl chloride (PVC) was
used (Ennaceur and Delacour, 1988) On the first day animals were habituated to the chamber for 10
minutes. After the 10 minute habituation period, animals were exposed to the 5 objects. Objects were
place in a pentagon shape around the animals close to the walls. No objects were placed in the center
of field to minimize basal levels of anxiety. On the second day were again exposed to the empty
chamber for 6 minutes. This was followed up with 3 additional 6 minutes sessions with the 5 objects.
After the last session two objects had their objects reversed. The mouse was then reintroduced to the
chamber and allowed to interact with the objects for another 6 minutes. All sessions were recorded by a
camera above the chamber and scored by a blinded observer. A location index was calculated based
on the equation: (Time with objects in novel location) * 100/ (Time with all object)(Assini et al., 2009;
Murai et al., 2007).
Crawleys Social Interaction Task:
To address potential social interaction deficits, Crawleys Sociability test was utilized (Li et al.,
2007; Tanda et al., 2009). This three-chamber tests allows for the quantification of a subjects desire to
both interact with another mouse and the subjects desire to interact with a novel mouse providing
crucial evidence on the health of the subjects social health. Unlike traditional one chamber social
interaction tests, the three chamber test has the advantage of preventing physical contact between the
mice, allowing the experimental mouse to terminate or engage in interaction with little interference from
the other animals. Furthermore the 3-chamber tests allows for accurate analysis of social interactions
that is relatively independent of the locomotive activity of the subjects.
Thermal and mechanical sensitivity
To determine pain threshold of animals, both the Plantar Test (Ugo Basile) and the Von Frey
Filaments test (Ugo Basile) were used. Pain tolerance for mechanical stimulus was tested using
Han et al., Supplementary Information page 16
repeated stimulation with a Von Frey filament exerting 0.02 g of force onto the plantar surface. The
percentage of negative responses over a total of 10 trials was calculated (Goldman et al., 2010; Liedtke
and Friedman, 2003). Pain tolerance for thermal stimulus was tested using the Hargreaves Apparatus
(Plantar test) (Goldman et al., 2010; Hargreaves et al., 1988). A mobile radiant heat source was
focused on the hind paw of the animal. Paw withdrawal latency was defined as the time it took the
mouse to remove its paw from the heat source. This was repeated three times for each animal and an
average was obtained. To avoid conditioning to stimulation, animals were given a 5-minute rest period
between each trial.
Statistics
All histograms were presented as means SEM. The Steel-Dwass test was used to assess the
relative diameters of cells, input resistances, and Ca2+ velocities, all variables for which normality of the
data could not be assumed. For other electrophysiological data, either Students t-test for two groups,
or two-way ANOVA with Bonferroni post hoc t-tests, were used. For behavioral data, Students t test or
two-way repeated measures ANOVA with Bonferroni post hoc test were used. Normality of the data
was assessed by the Shapiro-Wilk test. P values <0.05 were considered significant.
Han et al., Supplementary Information page 17
References for Supplementary Methods and Materials
Anagnostaras, S.G., Josselyn, S.A., Frankland, P.W., and Silva, A.J. (2000). Computer-assisted
behavioral assessment of Pavlovian fear conditioning in mice. Learn Mem 7, 58-72.
Asrar, S., Kaneko, K., Takao, K., Negandhi, J., Matsui, M., Shibasaki, K., Miyakawa, T., Harrison, R.V.,
Jia, Z., Salter, M.W., et al. (2011). DIP/WISH deficiency enhances synaptic function and
performance in the Barnes maze. Mol Brain 4, 39.
Assini, F.L., Duzzioni, M., and Takahashi, R.N. (2009). Object location memory in mice:
pharmacological validation and further evidence of hippocampal CA1 participation. Behav Brain
Res 204, 206-211.
Colombo, J.A. (1996). Interlaminar astroglial processes in the cerebral cortex of adult monkeys but not
of adult rats. Acta Anat (Basel) 155, 57-62.
Dawood, M.Y., Lumley, L.A., Robison, C.L., Saviolakis, G.A., and Meyerhoff, J.L. (2004). Accelerated
Barnes maze test in mice for assessment of stress effects on memory. Ann N Y Acad Sci 1032,
304-307.
Ennaceur, A., and Delacour, J. (1988). A new one-trial test for neurobiological studies of memory in
rats. 1: Behavioral data. Behav Brain Res 31, 47-59.
Goldman, N., Chen, M., Fujita, T., Xu, Q., Peng, W., Liu, W., Jensen, T.K., Pei, Y., Wang, F., Han, X.,
et al. (2010). Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat
Neurosci 13, 883-888.
Hammond, E.R., Kaplin, A.I., and Kerr, D.A. (2007). Thalidomide for acute treatment of
neurosarcoidosis. Spinal Cord 45, 802-803.
Hargreaves, K., Dubner, R., Brown, F., Flores, C., and Joris, J. (1988). A new and sensitive method for
measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77-88.
Harrison, F.E., Reiserer, R.S., Tomarken, A.J., and McDonald, M.P. (2006). Spatial and nonspatial
escape strategies in the Barnes maze. Learn Mem 13, 809-819.
Li, W., Zhou, Y., Jentsch, J.D., Brown, R.A., Tian, X., Ehninger, D., Hennah, W., Peltonen, L.,
Lonnqvist, J., Huttunen, M.O., et al. (2007). Specific developmental disruption of disrupted-in-
schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proc Natl Acad
Sci U S A 104, 18280-18285.
Liedtke, W., and Friedman, J.M. (2003). Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad
Sci U S A 100, 13698-13703.
Lovatt, D., Xu, Q., Liu, W., Takano, T., Smith, N.A., Schnermann, J., Tieu, K., and Nedergaard, M.
(2012). Neuronal adenosine release, and not astrocytic ATP release, mediates feedback
inhibition of excitatory activity. Proc Natl Acad Sci U S A.
McHugh, T.J., Jones, M.W., Quinn, J.J., Balthasar, N., Coppari, R., Elmquist, J.K., Lowell, B.B.,
Fanselow, M.S., Wilson, M.A., and Tonegawa, S. (2007). Dentate gyrus NMDA receptors
mediate rapid pattern separation in the hippocampal network. Science 317, 94-99.
Murai, T., Okuda, S., Tanaka, T., and Ohta, H. (2007). Characteristics of object location memory in
mice: Behavioral and pharmacological studies. Physiol Behav 90, 116-124.
Nagy, G.G., Al-Ayyan, M., Andrew, D., Fukaya, M., Watanabe, M., and Todd, A.J. (2004). Widespread
expression of the AMPA receptor GluR2 subunit at glutamatergic synapses in the rat spinal cord
and phosphorylation of GluR1 in response to noxious stimulation revealed with an antigen-
unmasking method. J Neurosci 24, 5766-5777.
Nunes, M.C., Roy, N.S., Keyoung, H.M., Goodman, R.R., McKhann, G., 2nd, Jiang, L., Kang, J.,
Nedergaard, M., and Goldman, S.A. (2003). Identification and isolation of multipotential neural
progenitor cells from the subcortical white matter of the adult human brain. Nat Med 9, 439-447.
O'Leary, T.P., Savoie, V., and Brown, R.E. (2011). Learning, memory and search strategies of inbred
mouse strains with different visual abilities in the Barnes maze. Behav Brain Res 216, 531-542.
Oberheim, N.A., Takano, T., Han, X., He, W., Lin, J.H., Wang, F., Xu, Q., Wyatt, J.D., Pilcher, W.,
Ojemann, J.G., et al. (2009). Uniquely hominid features of adult human astrocytes. J Neurosci
29, 3276-3287.
Han et al., Supplementary Information page 18
Patil, S.S., Sunyer, B., Hoger, H., and Lubec, G. (2009). Evaluation of spatial memory of C57BL/6J and
CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze. Behav Brain
Res 198, 58-68.
Ryu, J.K., and McLarnon, J.G. (2008). Thalidomide inhibition of perturbed vasculature and glial-derived
tumor necrosis factor-alpha in an animal model of inflamed Alzheimer's disease brain. Neurobiol
Dis 29, 254-266.
Sim, F.J., McClain, C.R., Schanz, S.J., Protack, T.L., Windrem, M.S., and Goldman, S.A. (2011).
CD140a identifies a population of highly myelinogenic, migration-competent and efficiently
engrafting human oligodendrocyte progenitor cells. Nat Biotechnol 29, 934-941.
Tanda, K., Nishi, A., Matsuo, N., Nakanishi, K., Yamasaki, N., Sugimoto, T., Toyama, K., Takao, K.,
and Miyakawa, T. (2009). Abnormal social behavior, hyperactivity, impaired remote spatial
memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase
knockout mice. Molecular brain 2, 19.
Torres, A., Wang, F., Xu, Q., Fujita, T., Dobrowolski, R., Willecke, K., Takano, T., and Nedergaard, M.
(2012). Extracellular Ca(2)(+) acts as a mediator of communication from neurons to glia. Sci
Signal 5, ra8.
Tsenova, L., Bergtold, A., Freedman, V.H., Young, R.A., and Kaplan, G. (1999). Tumor necrosis factor
alpha is a determinant of pathogenesis and disease progression in mycobacterial infection in
the central nervous system. Proc Natl Acad Sci U S A 96, 5657-5662.
Tweedie, D., Sambamurti, K., and Greig, N.H. (2007). TNF-alpha inhibition as a treatment strategy for
neurodegenerative disorders: new drug candidates and targets. Curr Alzheimer Res 4, 378-385.
Wiltgen, B.J., and Silva, A.J. (2007). Memory for context becomes less specific with time. Learn Mem
14, 313-317.
Windrem, M.S., Nunes, M.C., Rashbaum, W.K., Schwartz, T.H., Goodman, R.A., McKhann, G., 2nd,
Roy, N.S., and Goldman, S.A. (2004). Fetal and adult human oligodendrocyte progenitor cell
isolates myelinate the congenitally dysmyelinated brain. Nat Med 10, 93-97.
Windrem, M.S., Schanz, S.J., Guo, M., Tian, G.F., Washco, V., Stanwood, N., Rasband, M., Roy, N.S.,
Nedergaard, M., Havton, L.A., et al. (2008). Neonatal chimerization with human glial progenitor
cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse.
Cell Stem Cell 2, 553-565.
Han et al., Supplementary Information page 19
Das könnte Ihnen auch gefallen
- PCR Lab ReportDokument3 SeitenPCR Lab Reportapi-462451258Noch keine Bewertungen
- Articulo Neuro 1Dokument12 SeitenArticulo Neuro 1Victor CuellarNoch keine Bewertungen
- 1 - Immunofluorescent StainingDokument5 Seiten1 - Immunofluorescent StainingNini CioconNoch keine Bewertungen
- Esearch Rticle: Requirement of Hippocampal Neurogenesis For The Behavioral Effects of AntidepressantsDokument5 SeitenEsearch Rticle: Requirement of Hippocampal Neurogenesis For The Behavioral Effects of AntidepressantsMariana JuncoNoch keine Bewertungen
- Stem Cell ReportsDokument20 SeitenStem Cell ReportschristopheNoch keine Bewertungen
- Generation of Humanized Aß Mouse For Alzheimer DiseaseDokument16 SeitenGeneration of Humanized Aß Mouse For Alzheimer Diseaseya minNoch keine Bewertungen
- Villeda, 2011 PDFDokument7 SeitenVilleda, 2011 PDFDaniel TorresNoch keine Bewertungen
- Deepak A. Lamba, Mike O. Karl, Carol B. Ware and Thomas A. Reh - Efficient Generation of Retinal Progenitor Cells From Human Embryonic Stem CellsDokument6 SeitenDeepak A. Lamba, Mike O. Karl, Carol B. Ware and Thomas A. Reh - Efficient Generation of Retinal Progenitor Cells From Human Embryonic Stem CellsHutsDMNoch keine Bewertungen
- Jin Wan, Hua Zheng, Hong-Lei Xiao, Zhen-Jue She and Guo-Min Zhou - Sonic Hedgehog Promotes Stem-Cell Potential of Muller Glia in The Mammalian RetinaDokument8 SeitenJin Wan, Hua Zheng, Hong-Lei Xiao, Zhen-Jue She and Guo-Min Zhou - Sonic Hedgehog Promotes Stem-Cell Potential of Muller Glia in The Mammalian RetinaHutsDMNoch keine Bewertungen
- Adult Hippocampal Neurogenesis Is Regulated by The Mi - 2015 - Biological PsychiDokument3 SeitenAdult Hippocampal Neurogenesis Is Regulated by The Mi - 2015 - Biological PsychiNoor AliNoch keine Bewertungen
- Paneth Cells in The Developing Gut When Do They Arise andDokument5 SeitenPaneth Cells in The Developing Gut When Do They Arise andRichard QiuNoch keine Bewertungen
- Michael C O'DonovanDokument3 SeitenMichael C O'DonovanFahrunnisa NurdinNoch keine Bewertungen
- Fluoxetine Enhances Cell Proliferation and Prevents Apoptosis in Dentate Gyrus of Maternally Separated RatsDokument4 SeitenFluoxetine Enhances Cell Proliferation and Prevents Apoptosis in Dentate Gyrus of Maternally Separated RatsVitor PereiraNoch keine Bewertungen
- tmp5C3D TMPDokument10 Seitentmp5C3D TMPFrontiersNoch keine Bewertungen
- Calorie RestrictionDokument18 SeitenCalorie RestrictionJuan Felipe QuinteroNoch keine Bewertungen
- Variation Exercises: Exercise 1 - Human Population Genetics and Phenotype DataDokument6 SeitenVariation Exercises: Exercise 1 - Human Population Genetics and Phenotype Dataquique ddmNoch keine Bewertungen
- Zhang2020 InteractionDokument18 SeitenZhang2020 InteractionJocilene Dantas Torres NascimentoNoch keine Bewertungen
- PNAS 2013 Marazziti 16486 91Dokument6 SeitenPNAS 2013 Marazziti 16486 91Princess ViliranNoch keine Bewertungen
- J Exp Med-1978-Cheung-1539-49Dokument11 SeitenJ Exp Med-1978-Cheung-1539-49nanopearlNoch keine Bewertungen
- Genetic Analysis of Down Syndrome Facilitated by Mouse Chromosome EngineeringDokument5 SeitenGenetic Analysis of Down Syndrome Facilitated by Mouse Chromosome EngineeringRubashni SubramaniamNoch keine Bewertungen
- Novel Peptide SemaxDokument1 SeiteNovel Peptide SemaxAsh PachelliNoch keine Bewertungen
- TMP FDE0Dokument5 SeitenTMP FDE0FrontiersNoch keine Bewertungen
- Zns 2449Dokument14 SeitenZns 2449Bogdan A. GireadăNoch keine Bewertungen
- Haemophilus Influenzae Isolates From Children With AcuteDokument4 SeitenHaemophilus Influenzae Isolates From Children With AcuteNatalia IhalauwNoch keine Bewertungen
- Full PDFDokument6 SeitenFull PDFLateecka R KulkarniNoch keine Bewertungen
- Brain Research: Jingjing Tai, Weizhen Liu, Yanwei Li, Lin Li, Christian HölscherDokument11 SeitenBrain Research: Jingjing Tai, Weizhen Liu, Yanwei Li, Lin Li, Christian HölscherNAM LÊ THANHNoch keine Bewertungen
- FJ 05-4058comDokument8 SeitenFJ 05-4058comSwathi sampathkumarNoch keine Bewertungen
- Meta-Analysis Shows Strong Positive AssociationDokument8 SeitenMeta-Analysis Shows Strong Positive AssociationFahrunnisa NurdinNoch keine Bewertungen
- Correcting Mir92A-Vgat-Mediated Gabaergic Dysfunctions Rescues Human Tau-Induced Anxiety in MiceDokument13 SeitenCorrecting Mir92A-Vgat-Mediated Gabaergic Dysfunctions Rescues Human Tau-Induced Anxiety in MicegabrielledireitoNoch keine Bewertungen
- tmp42AA TMPDokument8 Seitentmp42AA TMPFrontiersNoch keine Bewertungen
- Delivery of DNA Vaccine For Alzheimer Disease by Electroporation Vs Gene Gun Generates Potent and Similar Immune ResponseDokument5 SeitenDelivery of DNA Vaccine For Alzheimer Disease by Electroporation Vs Gene Gun Generates Potent and Similar Immune ResponseSkzAznNoch keine Bewertungen
- Ref 5Dokument11 SeitenRef 5Tiago BaraNoch keine Bewertungen
- Practical Clinical Epidemiology for the VeterinarianVon EverandPractical Clinical Epidemiology for the VeterinarianNoch keine Bewertungen
- Biocompatibility Evaluation of Tramadol Loaded Nanoparticulate SystemsDokument2 SeitenBiocompatibility Evaluation of Tramadol Loaded Nanoparticulate SystemsVeronica NedelcuNoch keine Bewertungen
- Peran M Pada TikusDokument8 SeitenPeran M Pada TikusShampuy ShampuyNoch keine Bewertungen
- Garb Ett 2012Dokument8 SeitenGarb Ett 2012Amir HayatNoch keine Bewertungen
- Neurobiology of Disease: E.N. Mangano, S. Peters, D. Litteljohn, R. So, C. Bethune, J. Bobyn, M. Clarke, S. HayleyDokument14 SeitenNeurobiology of Disease: E.N. Mangano, S. Peters, D. Litteljohn, R. So, C. Bethune, J. Bobyn, M. Clarke, S. HayleyShawn HayleyNoch keine Bewertungen
- TP 2014138Dokument9 SeitenTP 2014138ancuta.lupaescuNoch keine Bewertungen
- Functional Differences and Similarities in Activated Peripheral Blood Mononuclear Cells by Lipopolysaccharide or Phytohemagglutinin Stimulation Between Human and Cynomolgus Monkeys PDFDokument25 SeitenFunctional Differences and Similarities in Activated Peripheral Blood Mononuclear Cells by Lipopolysaccharide or Phytohemagglutinin Stimulation Between Human and Cynomolgus Monkeys PDFLluis GomezNoch keine Bewertungen
- Alzheimer e Exercício Ratos 2016aDokument11 SeitenAlzheimer e Exercício Ratos 2016aRonaldo BorgesNoch keine Bewertungen
- BAI1 Regulates Spatial Learning and Synaptic Plasticity in The HippocampusDokument12 SeitenBAI1 Regulates Spatial Learning and Synaptic Plasticity in The HippocampusPeter TuNoch keine Bewertungen
- Kaur 2016Dokument9 SeitenKaur 2016Lucio Bolaños vindasNoch keine Bewertungen
- Restraint Stress Affects Hippocampal CelDokument4 SeitenRestraint Stress Affects Hippocampal CelJean Pierre Chastre LuzaNoch keine Bewertungen
- Interictal, Circulating Sphingolipids in Women With Episodic MigraineDokument11 SeitenInterictal, Circulating Sphingolipids in Women With Episodic MigraineAnonymous bx7YQvNoch keine Bewertungen
- PNAS 2012 Son 1201191109Dokument6 SeitenPNAS 2012 Son 1201191109Ranox Rizzy'star RockpunkNoch keine Bewertungen
- 2010 Ramocki AmJHumGenetDokument9 Seiten2010 Ramocki AmJHumGenetEAPNoch keine Bewertungen
- Role of Dexamethasone in Neonatal Meningitis 2012Dokument6 SeitenRole of Dexamethasone in Neonatal Meningitis 2012jose alberto gamboaNoch keine Bewertungen
- Altered Neurogenesis in Mouse Models of Alzheimer DiseaseDokument10 SeitenAltered Neurogenesis in Mouse Models of Alzheimer Diseaseapi-682804894Noch keine Bewertungen
- AAV9 - s41591 019 0674 1Dokument39 SeitenAAV9 - s41591 019 0674 1Felipe CourtNoch keine Bewertungen
- Antibody Appears To Attack Cancer Cells, Leaving Other Cells UnscathedDokument13 SeitenAntibody Appears To Attack Cancer Cells, Leaving Other Cells UnscathedAnonymous EdgflnjcNoch keine Bewertungen
- Cultures Were Determined With The Use of The 2Dokument11 SeitenCultures Were Determined With The Use of The 2Gio Vano NaihonamNoch keine Bewertungen
- Sex and Congenital Diaphragmatic Hernia: OriginalarticleDokument5 SeitenSex and Congenital Diaphragmatic Hernia: OriginalarticleBlank SpaceNoch keine Bewertungen
- Scolioza 1Dokument11 SeitenScolioza 1Sava MihaelaNoch keine Bewertungen
- Maternal Care and Hipoc Weaver and Meaney PDFDokument6 SeitenMaternal Care and Hipoc Weaver and Meaney PDFmarielaNoch keine Bewertungen
- The Beta2-Adrenoceptor Agonist Salb PDFDokument1 SeiteThe Beta2-Adrenoceptor Agonist Salb PDFKeelan DayeNoch keine Bewertungen
- Nerve Growth Factor-Inducing Activity of Hericium Erinaceus in 1321N1 Human Astrocytoma CellsDokument6 SeitenNerve Growth Factor-Inducing Activity of Hericium Erinaceus in 1321N1 Human Astrocytoma Cellschad_woodsonNoch keine Bewertungen
- Ramesh 2018Dokument11 SeitenRamesh 2018Ricardo MedinaNoch keine Bewertungen
- Li2021 - Article - PETCTImagingOf89Zr N Sucdf PemDokument10 SeitenLi2021 - Article - PETCTImagingOf89Zr N Sucdf PemWSNoch keine Bewertungen
- A New Approach To Immunological Sexing of Sperm Blecher 1999Dokument13 SeitenA New Approach To Immunological Sexing of Sperm Blecher 1999Sergio L.Noch keine Bewertungen
- JC4 - 41467 - 2018 - Article - 6783Dokument11 SeitenJC4 - 41467 - 2018 - Article - 6783Roaster AppsNoch keine Bewertungen
- Molecular and Cellular NeuroscienceDokument8 SeitenMolecular and Cellular NeuroscienceDefi DestyawenyNoch keine Bewertungen
- Form A & B ThesisDokument2 SeitenForm A & B ThesisBaoz PingNoch keine Bewertungen
- Statement of Original Authorship FormDokument2 SeitenStatement of Original Authorship FormBaoz PingNoch keine Bewertungen
- Batt Rawden2013Dokument7 SeitenBatt Rawden2013Baoz PingNoch keine Bewertungen
- Form Thesis Proposal EvaluationDokument3 SeitenForm Thesis Proposal EvaluationBaoz PingNoch keine Bewertungen
- Form Notice of Thesis SubmissionDokument2 SeitenForm Notice of Thesis SubmissionBaoz PingNoch keine Bewertungen
- Request For Extension To Submission of Thesis FormDokument2 SeitenRequest For Extension To Submission of Thesis FormBaoz PingNoch keine Bewertungen
- SunU REC Cover Sheet 2019 - StudentDokument1 SeiteSunU REC Cover Sheet 2019 - StudentBaoz PingNoch keine Bewertungen
- Letters To The Editor: Change in Empathy in Medical SchoolDokument3 SeitenLetters To The Editor: Change in Empathy in Medical SchoolBaoz PingNoch keine Bewertungen
- The Importance of Empathy-As I Have Studied and Experienced ItDokument3 SeitenThe Importance of Empathy-As I Have Studied and Experienced ItBaoz PingNoch keine Bewertungen
- ContentServer PDFDokument3 SeitenContentServer PDFBaoz PingNoch keine Bewertungen
- Content ServerDokument7 SeitenContent ServerBaoz PingNoch keine Bewertungen
- ContentServer PDFDokument3 SeitenContentServer PDFBaoz PingNoch keine Bewertungen
- Empathy Score Among Medical Students in Mashhad, Iran: Study of The Jefferson Scale of Physician EmpathyDokument7 SeitenEmpathy Score Among Medical Students in Mashhad, Iran: Study of The Jefferson Scale of Physician EmpathyBaoz PingNoch keine Bewertungen
- Empathy in Medicine: Neuroscience, Education and Challenges: Eve Ekman and Michael KrasnerDokument11 SeitenEmpathy in Medicine: Neuroscience, Education and Challenges: Eve Ekman and Michael KrasnerBaoz PingNoch keine Bewertungen
- Advan 00043 2006 PDFDokument3 SeitenAdvan 00043 2006 PDFBaoz PingNoch keine Bewertungen
- Hooker - Understanding Empathy: Why Phenomenology and Hermeneutics Can Help Medical Education and PracticeDokument12 SeitenHooker - Understanding Empathy: Why Phenomenology and Hermeneutics Can Help Medical Education and PracticeCamille TobilloNoch keine Bewertungen
- Narrative Medicine: A Model For Empathy, Reflection, Profession, and TrustDokument7 SeitenNarrative Medicine: A Model For Empathy, Reflection, Profession, and TrustBaoz PingNoch keine Bewertungen
- Content ServerDokument10 SeitenContent ServerBaoz PingNoch keine Bewertungen
- Teacher Education's Challenge of Changing Research Relationships With SchoolsDokument24 SeitenTeacher Education's Challenge of Changing Research Relationships With SchoolsBaoz PingNoch keine Bewertungen
- Content ServerDokument8 SeitenContent ServerBaoz PingNoch keine Bewertungen
- Content ServerDokument11 SeitenContent ServerBaoz PingNoch keine Bewertungen
- Making A Difference in Theory in Sweden and The UKDokument18 SeitenMaking A Difference in Theory in Sweden and The UKBaoz PingNoch keine Bewertungen
- Advan 00043 2006 PDFDokument3 SeitenAdvan 00043 2006 PDFBaoz PingNoch keine Bewertungen
- Teacher Resistance Against School Reform Reflecting An Inconvenient TruthDokument16 SeitenTeacher Resistance Against School Reform Reflecting An Inconvenient TruthBaoz PingNoch keine Bewertungen
- The Development of A Scale To Measure Personal Reflection in Medical Practice and EducationDokument6 SeitenThe Development of A Scale To Measure Personal Reflection in Medical Practice and EducationBaoz PingNoch keine Bewertungen
- Traumatic Brain Injury Cause Non-Traumatic Brain Injury CausesDokument10 SeitenTraumatic Brain Injury Cause Non-Traumatic Brain Injury CausesBaoz PingNoch keine Bewertungen
- Soemantri2018 Article MeasuringMedicalStudentsReflec PDFDokument10 SeitenSoemantri2018 Article MeasuringMedicalStudentsReflec PDFBaoz PingNoch keine Bewertungen
- Soemantri2018 Article MeasuringMedicalStudentsReflec PDFDokument10 SeitenSoemantri2018 Article MeasuringMedicalStudentsReflec PDFBaoz PingNoch keine Bewertungen
- Acquired Hypopigmentation Disorders Other Than Vitiligo - UpToDateDokument53 SeitenAcquired Hypopigmentation Disorders Other Than Vitiligo - UpToDateBaoz PingNoch keine Bewertungen
- PHD Course Guide 2019 - Gsls Reduced File SizeDokument84 SeitenPHD Course Guide 2019 - Gsls Reduced File SizeBaoz PingNoch keine Bewertungen
- Chase Et Al 1993 Phylogenetics of Seed PlantsDokument54 SeitenChase Et Al 1993 Phylogenetics of Seed PlantsAndrés FrankowNoch keine Bewertungen
- Science and Technology EssayDokument18 SeitenScience and Technology EssayDean Kurt SaroopNoch keine Bewertungen
- CH 6 Test Bank For Essential Cell Biology 3rd Edition AlbertsDokument17 SeitenCH 6 Test Bank For Essential Cell Biology 3rd Edition AlbertsRokia GhariebNoch keine Bewertungen
- Molecular Diagnostics Proposal: International University-Vietnam National University School of BiotechnologyDokument27 SeitenMolecular Diagnostics Proposal: International University-Vietnam National University School of BiotechnologyGia HoàngNoch keine Bewertungen
- Polymerase Chain Reaction ProtocolDokument14 SeitenPolymerase Chain Reaction ProtocolDespoina ChatziNoch keine Bewertungen
- PresntionDokument30 SeitenPresntionMustafaNoch keine Bewertungen
- MBV OieDokument13 SeitenMBV OieMiftahuddin MadjidNoch keine Bewertungen
- Genome SequencingDokument16 SeitenGenome SequencingOhhh OkayNoch keine Bewertungen
- PCR PDFDokument5 SeitenPCR PDFrejin rejinrNoch keine Bewertungen
- Practice Questions For Dna Replication and RepairDokument11 SeitenPractice Questions For Dna Replication and RepairdhruviniNoch keine Bewertungen
- Jurnal CFP10Dokument5 SeitenJurnal CFP10Firda DamiruNoch keine Bewertungen
- Principles of (PCR) Polymerase Chain Reaction and Expression AnalysisDokument30 SeitenPrinciples of (PCR) Polymerase Chain Reaction and Expression AnalysisSandeep ChapagainNoch keine Bewertungen
- MA 7.2 DNA Replication Lab Answer KeyDokument5 SeitenMA 7.2 DNA Replication Lab Answer KeyddNoch keine Bewertungen
- Agricultural ScienceDokument266 SeitenAgricultural ScienceAnonymous XXENB3eONoch keine Bewertungen
- Alu LabDokument32 SeitenAlu LabSusan HuynhNoch keine Bewertungen
- (Directions Modified From Sarah Deel's Bio 125 Directions) : Week 7: Sequence AnalysisDokument7 Seiten(Directions Modified From Sarah Deel's Bio 125 Directions) : Week 7: Sequence Analysisjj1064Noch keine Bewertungen
- DNA, RNA and Protein Synthesis (Part 1) : Lesson 6Dokument69 SeitenDNA, RNA and Protein Synthesis (Part 1) : Lesson 6Febbie IbatuanNoch keine Bewertungen
- Pathogenic Waterborne Free-Living Amoebae: An Update From Selected Southeast Asian CountriesDokument17 SeitenPathogenic Waterborne Free-Living Amoebae: An Update From Selected Southeast Asian CountriesMehul JainNoch keine Bewertungen
- Genetics Characterization, Nutritional and Phytochemicals Potential of Gedi Leaves (Abelmoschus Manihot (L.) Medik) Growing in The North Sulawesi of Indonesia As A Candidate of Poultry FeedDokument11 SeitenGenetics Characterization, Nutritional and Phytochemicals Potential of Gedi Leaves (Abelmoschus Manihot (L.) Medik) Growing in The North Sulawesi of Indonesia As A Candidate of Poultry FeedresearchinbiologyNoch keine Bewertungen
- Teknik ARMS PCRDokument4 SeitenTeknik ARMS PCRliyanaNoch keine Bewertungen
- Real Time PCR Handbook PDFDokument73 SeitenReal Time PCR Handbook PDFRodney SalazarNoch keine Bewertungen
- Protocol ATAC SeqDokument8 SeitenProtocol ATAC Seqcnoutsos316Noch keine Bewertungen
- Schoch Et Al., 2012. OOOO ITS Universal DNA Barcode FungiDokument14 SeitenSchoch Et Al., 2012. OOOO ITS Universal DNA Barcode Funginiver_27Noch keine Bewertungen
- Agricultural ScienceDokument268 SeitenAgricultural ScienceGorana RašićNoch keine Bewertungen
- Laboratory Activity No. 7: DNA Extraction and AmplificationDokument4 SeitenLaboratory Activity No. 7: DNA Extraction and AmplificationStephanie CorpuzNoch keine Bewertungen
- Heartwater: OIE Terrestrial Manual 2008 217Dokument14 SeitenHeartwater: OIE Terrestrial Manual 2008 217WormInchNoch keine Bewertungen
- Agronomy 11 01017 v2Dokument14 SeitenAgronomy 11 01017 v2Jose LuisNoch keine Bewertungen
- QIAGEN PCR Cloning HandbookDokument32 SeitenQIAGEN PCR Cloning HandbookpkhkhawarNoch keine Bewertungen
- ISSR Studies On Small and Large Seed Varieties of Glycine Max PDFDokument5 SeitenISSR Studies On Small and Large Seed Varieties of Glycine Max PDFjagdishchandra monparaNoch keine Bewertungen