Beruflich Dokumente
Kultur Dokumente
Practice Emission Spectra
Hochgeladen von
api-270967967Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Practice Emission Spectra
Hochgeladen von
api-270967967Copyright:
Verfügbare Formate
Lab Methods: Emission Spectra and Energy Levels Practice
Name: _______________________________
Period: _____ Date: _____
Discussion:
One convenient method of exciting atoms of an element is to
pass an electric current through a gas sample of the element.
This is the principle behind the spectrum tubes in the
demonstration. A spectrum tube contains a small sample of an
element in the vapor phase. An electric discharge through the
tube will cause the vapor to glow brightly. The glow is
produced when excited electrons emit visible light energy as
they return to their original levels.
When visible light energy from a spectrum tube is passed through a
diffraction grating, a bright line spectrum, or line-emission spectrum is
produced. Each element has its own unique emission spectrum by which it distinctive
can be identified, analogous to a fingerprint. Such a spectrum consists of a energy
series of bright lines of definite wavelength. Each wavelength can be levels in
mathematically related to a definite quantity of energy produced by the an atom.
movement of an electron from one discrete energy level to another. Thus,
emission spectra are experimental proof that electrons exist in definite,
Questions:
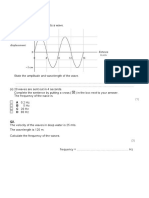
1. Look at the spectrum and determine the colors of the following four lines described by their
wavelength. These are the four emission lines in the visible part of the spectrum for
hydrogen.
410 432 486 656
nm__________ nm__________ nm__________ nm__________
2. Which color of visible light has
a. the shortest wavelength?
b. the longest wavelength?
c. the least amount of energy?
d. the greatest amount of energy?
3. The color of the emitted light is dependent on the _________________________________ of the
light.
4. The brightness of each line is dependent on ____________________________________________.
5. According to the modern theory of the atom, how are the atoms arranged around the
nucleus?
6. How do electrons become excited?
7. What form of energy accompanies the return of excited electrons to the ground state? Is this
energy always visible to the human eye? Why or why not?
8. The quantum level occupied by an electron in an atom depends on the energy of the
electron. Changes in quantum level are related to absorption or emission of energy. The
figure below represents the four lowest energy levels of an atom. (n = 1 to 4). The six
lettered arrows represent changes in the energy level of an electron.
2
C D E F
"And anyone who thinks they
1 can talk about quantum theory
A B without feeling dizzy hasn't
yet understood the first thing
about it."
9. Why do these energy levels mean that the atom will show an emission spectrum of discrete
lines rather than a continuous spectrum of emitted light?
10.Which three of the lettered energy changes involve absorption of energy by the atom?
11.Which three of the lettered energy changes involve emission of light energy by the atom?
12.How does a hydrogen atom, which has only one electron, have so many lines in its
spectrum?
Das könnte Ihnen auch gefallen
- Atomic Structure - Exam QuestionsDokument5 SeitenAtomic Structure - Exam QuestionsIman WafaNoch keine Bewertungen
- Aes WorksheetDokument1 SeiteAes Worksheetapi-270967967Noch keine Bewertungen
- Food Web Analysis Set 1: Name - Date: - PeriodDokument2 SeitenFood Web Analysis Set 1: Name - Date: - PeriodRose ThornsNoch keine Bewertungen
- Wave On A String Phet LabDokument5 SeitenWave On A String Phet LabNicholas BarresiNoch keine Bewertungen
- Sugar Yeast FermentationDokument4 SeitenSugar Yeast FermentationLauraNoch keine Bewertungen
- Periodic Table Trends WorksheetDokument4 SeitenPeriodic Table Trends WorksheetSHEILA MAE VILLANTESNoch keine Bewertungen
- SPM Practice Chap3 F4Dokument7 SeitenSPM Practice Chap3 F4Jonathan LingNoch keine Bewertungen
- Revision Worksheet (Unit # 11) - Class VIIIDokument7 SeitenRevision Worksheet (Unit # 11) - Class VIIISitwat BatoolNoch keine Bewertungen
- Reaction Kinetics WSDokument44 SeitenReaction Kinetics WSMustufa FerozNoch keine Bewertungen
- Emission SpectraDokument4 SeitenEmission SpectraKarla Jara Hidalgo GalarionNoch keine Bewertungen
- Periodic Table Unit Test PDFDokument4 SeitenPeriodic Table Unit Test PDFTon TonNoch keine Bewertungen
- Electronic Structure Que 2Dokument13 SeitenElectronic Structure Que 2Rainidah Mangotara Ismael-DericoNoch keine Bewertungen
- Introduction to the Theory of Atomic Spectra: International Series of Monographs in Natural PhilosophyVon EverandIntroduction to the Theory of Atomic Spectra: International Series of Monographs in Natural PhilosophyBewertung: 5 von 5 Sternen5/5 (1)
- Wave QuestionsDokument13 SeitenWave QuestionsShivank MenonNoch keine Bewertungen
- Mass Spectroscopy WorksheetDokument2 SeitenMass Spectroscopy Worksheetapi-270967967Noch keine Bewertungen
- Atomic Structure and Bonding Past QuestionsDokument9 SeitenAtomic Structure and Bonding Past Questionsinvictorium100% (2)
- Chapter 23 - Alcohols and Carboxylic AcidsDokument6 SeitenChapter 23 - Alcohols and Carboxylic AcidsJERVINLIMNoch keine Bewertungen
- Physics Chapter WaveDokument22 SeitenPhysics Chapter Wavesaffiya imanNoch keine Bewertungen
- Energy Pyramaid LabDokument2 SeitenEnergy Pyramaid LabQuinn Jackson100% (1)
- 3.1 Classifying Matter NotesDokument5 Seiten3.1 Classifying Matter NotesJam Uly GastyNoch keine Bewertungen
- Chapter 1 Chemical Reactions and Equations Class 1 - 220502 - 062545Dokument5 SeitenChapter 1 Chemical Reactions and Equations Class 1 - 220502 - 062545GarimaNoch keine Bewertungen
- International Symposium on Selective Ion-Sensitive Electrodes: International Union of Pure and Applied ChemistryVon EverandInternational Symposium on Selective Ion-Sensitive Electrodes: International Union of Pure and Applied ChemistryG. J. MoodyNoch keine Bewertungen
- Chemistry Periodic Trends ActivityDokument6 SeitenChemistry Periodic Trends ActivityocNoch keine Bewertungen
- Biophysical Chemistry: Thermodynamics, Electrostatics, and the Biological Significance of the Properties of MatterVon EverandBiophysical Chemistry: Thermodynamics, Electrostatics, and the Biological Significance of the Properties of MatterNoch keine Bewertungen
- Regents Living Environment Practice Questions: New York Regents Living Environment Practice Questions with Detailed ExplanationsVon EverandRegents Living Environment Practice Questions: New York Regents Living Environment Practice Questions with Detailed ExplanationsNoch keine Bewertungen
- Everything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksVon EverandEverything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksNoch keine Bewertungen
- Atoms and Atomic Theory: Essential Questions: How Can We Describe TH e Molecular Motion of TH e States of Matter?Dokument29 SeitenAtoms and Atomic Theory: Essential Questions: How Can We Describe TH e Molecular Motion of TH e States of Matter?Anonymous eMOb79RNt5Noch keine Bewertungen
- 1.1 Introduction To The Particulate Nature of Matter and Chemical ChangeDokument19 Seiten1.1 Introduction To The Particulate Nature of Matter and Chemical ChangeJuan Fernando Velasco ForeroNoch keine Bewertungen
- Practical MYP 4 Speed of SoundDokument6 SeitenPractical MYP 4 Speed of SoundPrasanna PatilNoch keine Bewertungen
- Alkanes Nomenclature, Conformational Analysis, and An Introduction To SynthesisDokument41 SeitenAlkanes Nomenclature, Conformational Analysis, and An Introduction To SynthesisDenisse BadiolaNoch keine Bewertungen
- CH 3 StoichiometryDokument30 SeitenCH 3 StoichiometrymedinoNoch keine Bewertungen
- As Chemistry Unit 1 NotesDokument8 SeitenAs Chemistry Unit 1 NoteshamzazzNoch keine Bewertungen
- Valence and Core ElectronsDokument19 SeitenValence and Core Electronsapi-233187566Noch keine Bewertungen
- Intro To Energy WorksheetDokument2 SeitenIntro To Energy WorksheetMelecia SeniorNoch keine Bewertungen
- Writing & Naming Binary Ionic Compounds WorksheetDokument2 SeitenWriting & Naming Binary Ionic Compounds WorksheetAmber100% (1)
- Notes and Questions: Aqa GcseDokument12 SeitenNotes and Questions: Aqa Gcseapi-422428700Noch keine Bewertungen
- Physics 1922 – 1941: Including Presentation Speeches and Laureates' BiographiesVon EverandPhysics 1922 – 1941: Including Presentation Speeches and Laureates' BiographiesNoch keine Bewertungen
- Chemistry Edexcel As Keywords Unit 1Dokument4 SeitenChemistry Edexcel As Keywords Unit 1Ashan BopitiyaNoch keine Bewertungen
- Argumentation in Chemistry Education: Research, Policy and PracticeVon EverandArgumentation in Chemistry Education: Research, Policy and PracticeNoch keine Bewertungen
- Chemistry MYP 3 Section 3Dokument15 SeitenChemistry MYP 3 Section 3Hadeel IbrahimNoch keine Bewertungen
- Ions Worksheet 1Dokument4 SeitenIons Worksheet 1Vasto L OrdeNoch keine Bewertungen
- In-Class Worksheet AnswersDokument6 SeitenIn-Class Worksheet AnswersalgonzNoch keine Bewertungen
- Year 8 Science Unit Outline For EnergyDokument3 SeitenYear 8 Science Unit Outline For Energyapi-297560946Noch keine Bewertungen
- Light Scattering by Particles in Water: Theoretical and Experimental FoundationsVon EverandLight Scattering by Particles in Water: Theoretical and Experimental FoundationsNoch keine Bewertungen
- Chemistry in Action Note PackageDokument21 SeitenChemistry in Action Note Packageapi-235471411Noch keine Bewertungen
- H2 Biology - Notes On Genetics of BacteriaDokument10 SeitenH2 Biology - Notes On Genetics of BacteriaSefLRho0% (1)
- Cell StructureDokument6 SeitenCell StructureL.y. Chong100% (1)
- Chromatography Worksheet - (C) 2002 Ian Guch - All RightsDokument2 SeitenChromatography Worksheet - (C) 2002 Ian Guch - All RightsJulian Alberti100% (1)
- Class Test 01Dokument2 SeitenClass Test 01sachinkurhekarNoch keine Bewertungen
- AP Biology - Ecological Pyramids - Worksheet PDFDokument6 SeitenAP Biology - Ecological Pyramids - Worksheet PDFVictoria LowmanNoch keine Bewertungen
- Electricity Worksheet Page 2Dokument1 SeiteElectricity Worksheet Page 2Laura LucumiNoch keine Bewertungen
- Edexcel A2 Chemistry Paper 5Dokument386 SeitenEdexcel A2 Chemistry Paper 5AbdulRahman Mustafa100% (1)
- Mass Spectroscopy WorksheetDokument2 SeitenMass Spectroscopy Worksheetapi-270967967Noch keine Bewertungen
- Acid and Base WorksheetDokument4 SeitenAcid and Base Worksheetapi-270967967Noch keine Bewertungen
- Isotope and Mass Spec WorksheetDokument2 SeitenIsotope and Mass Spec Worksheetapi-270967967Noch keine Bewertungen
- Oxidation State WorksheetDokument1 SeiteOxidation State Worksheetapi-270967967Noch keine Bewertungen
- Redox Reactions WorksheetsDokument2 SeitenRedox Reactions Worksheetsapi-270967967Noch keine Bewertungen
- Year 12 BiologyDokument2 SeitenYear 12 Biologyapi-270967967Noch keine Bewertungen
- AtomstructurewkstDokument1 SeiteAtomstructurewkstapi-270967967Noch keine Bewertungen
- Buffer WorksheetDokument1 SeiteBuffer Worksheetapi-270967967Noch keine Bewertungen
- Atomic Structure ks4 F HWKDokument1 SeiteAtomic Structure ks4 F HWKapi-270967967Noch keine Bewertungen
- Atomic Absorption Spectrum WorksheetDokument1 SeiteAtomic Absorption Spectrum Worksheetapi-270967967100% (1)
- FlowersDokument9 SeitenFlowersapi-270967967Noch keine Bewertungen
- Electrophoresis WorksheetDokument1 SeiteElectrophoresis Worksheetapi-270967967Noch keine Bewertungen
- Essay Group 8 - NMRDokument10 SeitenEssay Group 8 - NMRMiranti SjahriNoch keine Bewertungen
- Welcomes You To A Presentation On Photoluminescence Applications and InstrumentationDokument35 SeitenWelcomes You To A Presentation On Photoluminescence Applications and InstrumentationMai PhuNoch keine Bewertungen
- Use of Isosbestic Points For Determination of Quantum Efficiency in Transient AbsorptionDokument7 SeitenUse of Isosbestic Points For Determination of Quantum Efficiency in Transient AbsorptionalkimiaNoch keine Bewertungen
- Important Concepts in UV-Vis and IR SpectrosDokument53 SeitenImportant Concepts in UV-Vis and IR SpectrosmarkkkkkNoch keine Bewertungen
- Instruments For Optical SpectrometryDokument2 SeitenInstruments For Optical SpectrometrySean CollinsNoch keine Bewertungen
- Schripsema - 2019 - Similarity and Differential NMR Spectroscopy in Metabolomics Application To The Analysis of Vegetable Oils With 1H ADokument12 SeitenSchripsema - 2019 - Similarity and Differential NMR Spectroscopy in Metabolomics Application To The Analysis of Vegetable Oils With 1H Ayannick brunatoNoch keine Bewertungen
- University of Gour Banga: M.Sc. in Physics Two Years (Four Semesters) SyllabusDokument23 SeitenUniversity of Gour Banga: M.Sc. in Physics Two Years (Four Semesters) SyllabusBaishali BanerjeeNoch keine Bewertungen
- Application Handbook Food Release PDFDokument279 SeitenApplication Handbook Food Release PDFu_komyo187Noch keine Bewertungen
- Gem Webinar - 4Dokument36 SeitenGem Webinar - 4venu r sNoch keine Bewertungen
- On The Anomalous Adsorption of (PD (Edta) ) at The Water/Goethite Interface: Spectroscopic Evidence For Two Types of Surface ComplexesDokument7 SeitenOn The Anomalous Adsorption of (PD (Edta) ) at The Water/Goethite Interface: Spectroscopic Evidence For Two Types of Surface ComplexesPaolo BrosioNoch keine Bewertungen
- Rotational Spectra of N: An Advanced Undergraduate Laboratory in Atomic and Molecular SpectrosDokument17 SeitenRotational Spectra of N: An Advanced Undergraduate Laboratory in Atomic and Molecular SpectrosGuilherme MartinsNoch keine Bewertungen
- ASHWIN RAM (Cology Dept) MPAT SEMINAR 1Dokument33 SeitenASHWIN RAM (Cology Dept) MPAT SEMINAR 1ASHWIN RAMNoch keine Bewertungen
- Analytical Chemistry 4 Spectroscopy - 1Dokument43 SeitenAnalytical Chemistry 4 Spectroscopy - 1PERPETUAL TAKYINoch keine Bewertungen
- RamanDokument29 SeitenRamanapi-3723327100% (8)
- Lab Report Exp 1 CHM260 (Nurul Shuhadah)Dokument7 SeitenLab Report Exp 1 CHM260 (Nurul Shuhadah)wnayNoch keine Bewertungen
- Forensic Syllabus@Dokument6 SeitenForensic Syllabus@YocobSamandrewsNoch keine Bewertungen
- Chapter 8 SolutionsDokument9 SeitenChapter 8 SolutionsARSYIAN RIZKI PRATAMANoch keine Bewertungen
- Algerian Bee Pollen Classification by Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy Atr Ftir Combined With Chemometrics AnalysisDokument22 SeitenAlgerian Bee Pollen Classification by Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy Atr Ftir Combined With Chemometrics AnalysisIoNoch keine Bewertungen
- Pulse Program Catalog 1 (1D and 2D Experiments) PDFDokument251 SeitenPulse Program Catalog 1 (1D and 2D Experiments) PDFEdmilson RodriguesNoch keine Bewertungen
- List Chemical BooksDokument14 SeitenList Chemical BooksmcclaytonNoch keine Bewertungen
- Report UV-VIS YayaDokument13 SeitenReport UV-VIS YayaInfra Nadya67% (3)
- Periyar Dde Bsc-PhysicsDokument23 SeitenPeriyar Dde Bsc-PhysicsPaul DaduNoch keine Bewertungen
- Spectral Line ShapeDokument4 SeitenSpectral Line ShapeLeo HuangNoch keine Bewertungen
- University of KeralaDokument11 SeitenUniversity of Keralamaneesh sNoch keine Bewertungen
- Atomic Absorption SpectrosDokument9 SeitenAtomic Absorption SpectrosKishore CivilNoch keine Bewertungen
- The Application of Quantum Mechanics: SpectrosDokument7 SeitenThe Application of Quantum Mechanics: SpectrosRa saNoch keine Bewertungen
- Atom Spectroscopy in Brief From Unit 28, Fundamental of Analytical Chemistry, SkoogDokument11 SeitenAtom Spectroscopy in Brief From Unit 28, Fundamental of Analytical Chemistry, SkooggommentNoch keine Bewertungen
- Atomic Fluoresence SpektrokopiDokument15 SeitenAtomic Fluoresence Spektrokopinora santiNoch keine Bewertungen
- Chris Busby Curriculum VitaeDokument28 SeitenChris Busby Curriculum VitaeAkmens Raimonds - RAYSTARNoch keine Bewertungen
- Syllabus M.Tech. (Nanotechnology)Dokument11 SeitenSyllabus M.Tech. (Nanotechnology)selva.natarajNoch keine Bewertungen