Beruflich Dokumente
Kultur Dokumente
Set 3f
Hochgeladen von
kjjkimkmk0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
38 Ansichten1 Seited
Copyright
© © All Rights Reserved
Verfügbare Formate
DOC, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldend
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOC, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
38 Ansichten1 SeiteSet 3f
Hochgeladen von
kjjkimkmkd
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOC, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 1
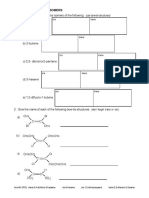
DK035
Semester III 2014/2015
Quiz 2/ SET 3
KOLEJ MATRIKULASI KEDAH
QUIZ 2 (THERMOCHEMISTRY)
___________________________________________________________________________
NAME:__________________________________ TUTORIAL:_________
1. Draw an energy diagram for an exothermic reaction for the reaction that has
H=-20kJ.
2. Refer to the diagram below;
enthalpy products
reactants
The heat of reaction is,
a) -100 kJ
b) -80 kJ
c) +80 kJ
d) +100 kJ
3. Classify each of the following process as endothermic or exothermic . in each case,
indicate which has higher greater heat content(enthalpy), reactants or products.
a) Combustion of natural gas
b) Condensation of water vapor
c) Solidification of melted wax
d) Formation of sodium chloride (NaCl) from it elements
e) Evaporation of alcohol
4. Dry ice is solid carbon dioxide. It does not melt, but instead it sublimes directly to the
gas state.
a) Is this change endothermic or exothermic?
b) What sign would the H have?
Das könnte Ihnen auch gefallen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Identifying Types of Reactions - KeyDokument3 SeitenIdentifying Types of Reactions - KeykjjkimkmkNoch keine Bewertungen
- 4CH0 1C ChemistryDokument1 Seite4CH0 1C ChemistrykjjkimkmkNoch keine Bewertungen
- Isi KandunganDokument1 SeiteIsi KandungankjjkimkmkNoch keine Bewertungen
- 4CH0 1C Rms ChemistryDokument32 Seiten4CH0 1C Rms ChemistryAlex Smith100% (1)
- Group Choice Activity - MoodleDocsDokument4 SeitenGroup Choice Activity - MoodleDocskjjkimkmkNoch keine Bewertungen
- Set 1Dokument1 SeiteSet 1kjjkimkmkNoch keine Bewertungen
- Set 2Dokument2 SeitenSet 2kjjkimkmkNoch keine Bewertungen
- Kolej Matrikulasi KedahDokument2 SeitenKolej Matrikulasi KedahkjjkimkmkNoch keine Bewertungen
- Kuliah K2S3Dokument4 SeitenKuliah K2S3kjjkimkmkNoch keine Bewertungen
- Carbocation KeyDokument1 SeiteCarbocation KeykjjkimkmkNoch keine Bewertungen
- Chapter 16: Benzene - Electrophilic Aromatic Substitution: Chem231 Study Notes On McmurryDokument20 SeitenChapter 16: Benzene - Electrophilic Aromatic Substitution: Chem231 Study Notes On McmurrykjjkimkmkNoch keine Bewertungen
- K1S3T4ADokument1 SeiteK1S3T4AkjjkimkmkNoch keine Bewertungen
- K2S2T3Dokument1 SeiteK2S2T3kjjkimkmkNoch keine Bewertungen
- Amalkebajikan Quiz Alkene OkDokument3 SeitenAmalkebajikan Quiz Alkene OkkjjkimkmkNoch keine Bewertungen
- Alkenes (Fasi)Dokument3 SeitenAlkenes (Fasi)kjjkimkmkNoch keine Bewertungen
- Alkene Reactions:: Doug Young CHE 118BDokument2 SeitenAlkene Reactions:: Doug Young CHE 118BkjjkimkmkNoch keine Bewertungen
- Carbo CationDokument1 SeiteCarbo CationkjjkimkmkNoch keine Bewertungen
- 24 - Organic Chemistry: Practice TestDokument2 Seiten24 - Organic Chemistry: Practice TestkjjkimkmkNoch keine Bewertungen
- Exercise AlkaneDokument2 SeitenExercise AlkanekjjkimkmkNoch keine Bewertungen
- Ws 10.8 Geometric Isomers: (Use Bow-Tie Structures)Dokument1 SeiteWs 10.8 Geometric Isomers: (Use Bow-Tie Structures)kjjkimkmkNoch keine Bewertungen
- Alkanes Who Am IDokument2 SeitenAlkanes Who Am IkjjkimkmkNoch keine Bewertungen
- Self-Study Worksheet III Isomerism ANSWERSDokument2 SeitenSelf-Study Worksheet III Isomerism ANSWERSkjjkimkmkNoch keine Bewertungen
- Studyon Alkane NameDokument3 SeitenStudyon Alkane NamekjjkimkmkNoch keine Bewertungen
- Al KanesDokument4 SeitenAl KaneskjjkimkmkNoch keine Bewertungen
- 1hr AlkaneskmppDokument12 Seiten1hr AlkaneskmppkjjkimkmkNoch keine Bewertungen
- Hydrocarbons: at The End of The Lesson Students Should Be Able ToDokument45 SeitenHydrocarbons: at The End of The Lesson Students Should Be Able TokjjkimkmkNoch keine Bewertungen
- Alkane'S: - Alkanes Based On Molecular Weight - Isomeric Alkanes - Alkanes and CycloalkanesDokument1 SeiteAlkane'S: - Alkanes Based On Molecular Weight - Isomeric Alkanes - Alkanes and CycloalkaneskjjkimkmkNoch keine Bewertungen
- Sk027 / Chapter 5: Hydrocarbon / Amalkebajikan01 / Nomenclature AlkaneDokument7 SeitenSk027 / Chapter 5: Hydrocarbon / Amalkebajikan01 / Nomenclature AlkanekjjkimkmkNoch keine Bewertungen
- 3rd AlkanekmppDokument18 Seiten3rd AlkanekmppkjjkimkmkNoch keine Bewertungen
- Class 13834Dokument10 SeitenClass 13834adewunmi olufemiNoch keine Bewertungen
- AMS165 Tridol C6 ATF C3 3 AR AFFFDokument8 SeitenAMS165 Tridol C6 ATF C3 3 AR AFFFChacón C JohnyNoch keine Bewertungen
- Lecture 11Dokument6 SeitenLecture 11RajeevNoch keine Bewertungen
- Accepted Manuscript: Moringa StenopetalaDokument50 SeitenAccepted Manuscript: Moringa Stenopetalaimran shaukatNoch keine Bewertungen
- Ps Kalsi SpectrosDokument5 SeitenPs Kalsi SpectrosNawab AliNoch keine Bewertungen
- Lecture 3 Enthalpy and Heat CapacityDokument46 SeitenLecture 3 Enthalpy and Heat CapacitylisaNoch keine Bewertungen
- Atomic Structure Electron Configuration Valences and Ions PDFDokument21 SeitenAtomic Structure Electron Configuration Valences and Ions PDFJosh CharisNoch keine Bewertungen
- Technical Specification SampleDokument122 SeitenTechnical Specification SampleRajaramNoch keine Bewertungen
- Trinity Touch PVC Channel Price List Wef 07-06-2023Dokument4 SeitenTrinity Touch PVC Channel Price List Wef 07-06-2023M/S.TEJEET ELECTRICAL & ENGG. CORP.Noch keine Bewertungen
- Formula SprayDokument3 SeitenFormula SprayW MegaNoch keine Bewertungen
- Protecting GroupsDokument36 SeitenProtecting Groupssantosh kumar sahoo100% (1)
- Adsorption of Congo Red Dye From Aqueous Solutions Using Tunics of TheDokument6 SeitenAdsorption of Congo Red Dye From Aqueous Solutions Using Tunics of TheIana RilaNoch keine Bewertungen
- Frac Code List 2023 - FinalDokument18 SeitenFrac Code List 2023 - FinalFir NurNoch keine Bewertungen
- NEET Phase 2 Solutions PDFDokument65 SeitenNEET Phase 2 Solutions PDFsubhakpatel patelNoch keine Bewertungen
- ASNT NDT Level II ExaminationDokument11 SeitenASNT NDT Level II ExaminationPardie YanNoch keine Bewertungen
- Metallographic Printing MethodsDokument3 SeitenMetallographic Printing MethodsAnup Tigga100% (1)
- Msds-Alkyd PrimerDokument5 SeitenMsds-Alkyd Primerrian wijayaNoch keine Bewertungen
- 351 352 ManualDokument7 Seiten351 352 ManualsakornthiemNoch keine Bewertungen
- Paper - A Review For Characterization of Silicafume & Its Effect On ConcreteDokument8 SeitenPaper - A Review For Characterization of Silicafume & Its Effect On ConcreteAnto Destianto100% (1)
- Atoms Ions & Isotopes Study Guide AnswersDokument2 SeitenAtoms Ions & Isotopes Study Guide AnswersNevaeh DouglasNoch keine Bewertungen
- Conveyor Idler Rollers - Waminco - Industrial Rubber & Polymer Products - Perth WADokument1 SeiteConveyor Idler Rollers - Waminco - Industrial Rubber & Polymer Products - Perth WACarlos Ediver Arias RestrepoNoch keine Bewertungen
- GlazingDokument3 SeitenGlazingchuckNoch keine Bewertungen
- Optimization of Ultrasound Extraction Flavonoids CelastrusDokument9 SeitenOptimization of Ultrasound Extraction Flavonoids CelastrusBat DanNoch keine Bewertungen
- PHARMA SterilizationDokument36 SeitenPHARMA Sterilizationassistantedt3Noch keine Bewertungen
- Chemistry (P-Block Group 13 - 14) Answer Key: Single Choice Questions Solution 1: (D)Dokument11 SeitenChemistry (P-Block Group 13 - 14) Answer Key: Single Choice Questions Solution 1: (D)Dhruv KuchhalNoch keine Bewertungen
- Ultratech Cement 7 Days Test Report Week 37Dokument3 SeitenUltratech Cement 7 Days Test Report Week 37Venkatesh VictoryNoch keine Bewertungen
- Sustainable Recovery of Metals From Spent Lithium-Ion Batteries - A Green ProcessDokument11 SeitenSustainable Recovery of Metals From Spent Lithium-Ion Batteries - A Green ProcessErma Ro'ichatul JannahNoch keine Bewertungen
- StoichiometryDokument10 SeitenStoichiometryArella TondobalaNoch keine Bewertungen
- Practical No. 2: Solution: - Code For Part ADokument8 SeitenPractical No. 2: Solution: - Code For Part AYuvraj ArdekarNoch keine Bewertungen
- Ointments 1Dokument41 SeitenOintments 1Hossain Arfan Rion 2111217642Noch keine Bewertungen