Beruflich Dokumente
Kultur Dokumente
COCII
Hochgeladen von
ARIF AHAMMED PCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
COCII
Hochgeladen von
ARIF AHAMMED PCopyright:
Verfügbare Formate
400/800
Cocaine II
Order information
COBAS INTEGRA 200 Tests Cat. No. 03800130 190 Indicates analyzer(s) on which cobas c pack can be used
ONLINE DAT Cocaine II System-ID 07 6723 9
Preciset DAT Plus I 6 5 mL Cat. No. 03304671 190
CAL 1-6
C.f.a.s. DAT Qualitative Plus 6 5 mL Cat. No. 03304698 190
C.f.a.s. DAT Qualitative Plus Clinical 3 5 mL Cat. No. 04590856 190
Control Set DAT I Cat. No. 03312950 190
PreciPos DAT Set I 2 10 mL
PreciNeg DAT Set I 2 10 mL
Control Set DAT III Cat. No. 03312976 190
PreciPos DAT Set III 2 10 mL
PreciNeg DAT Set III 2 10 mL
Control Set DAT Clinical Cat. No. 04500873 190
PreciPos DAT Clinical 2 10 mL
PreciNeg DAT Clinical 2 10 mL
System information and paranoia.3,4 Users may revert to other drugs at this time
COBAS INTEGRA ONLINE DAT Cocaine II (COCII) to relieve the depressive effects of the crash.2
Test CO1S2, test-ID 0-126 for semiquantitative assay, 150 ng/mL Cocaine is traditionally administered intranasally or smoked in
Test CO3S2, test-ID 0-127 for semiquantitative assay, 300 ng/mL its purer, free-base form; oral ingestion is ineffective, as cocaine
Test CO1Q2, test-ID 0-015 for qualitative assay, 150 ng/mL is broken down in the gastrointestinal tract. It is absorbed
Test CO3Q2, test-ID 0-016 for qualitative assay, 300 ng/mL readily across the mucous membranes of the nose and lungs into
Test CO3QC, test-ID 0-115 for qualitative assay, 300 ng/mL; the circulation. Its effects are intense but short-lived. Cocaine
using C.f.a.s. DAT Qualitative Plus Clinical is rapidly inactivated by hydrolysis of its ester linkages.1,5,6
Blood cholinesterases hydrolyze cocaine to ecgonine methyl
Intended use
ester, while hydrolysis of the parent drug to benzoylecgonine is
Cocaine II (COCII) is an in vitro diagnostic test intended for the
thought to be non-enzymatic; both of these metabolites may
semiquantitative and qualitative detection of benzoylecgonine,
be further hydrolyzed to ecgonine. Unmetabolized cocaine has
the primary metabolite of cocaine, in human urine at cutoff
an affinity for fatty tissue and rapidly enters the brain; cocaine
concentrations of 150 ng/mL and 300 ng/mL on COBAS
metabolites, however, are more water soluble and are readily
INTEGRA systems. Semiquantitative test results may be
excreted in the urine along with some portion of unchanged
obtained that permit laboratories to assess assay performance
drug.5,7 The prominent benzoylecgonine metabolite is the
as part of a quality control program. Semiquantitative assays
primary urinary marker for detecting cocaine use.1,5
are intended to determine an appropriate dilution of the
Tolerance has been observed with some chronic, high-dose
specimen for confirmation by a confirmatory method such as
users.8 Physical dependence does not appear to occur in abusers,
gas chromatography/mass spectrometry (GC/MS).
although the development of strong psychological dependence
Cocaine II provides only a preliminary analytical test result. A
is well known. Cessation of drug use may result in depression,
more specific alternate chemical method must be used in order
hallucinations, and in extreme cases, psychosis.2
to obtain a confirmed analytical result. GC/MS is the preferred
confirmatory method.1 Clinical consideration and professional Test principle
judgment should be applied to any drug of abuse test result, Kinetic interaction of microparticles in a solution (KIMS)9,10
particularly when preliminary positive results are used. as measured by changes in light transmissions.
In the absence of sample drug, soluble drug conjugates bind
Summary
to antibody-bound microparticles, causing the formation of
Cocaine, a natural product found in the leaves of the coca
particle aggregates. As the aggregation reaction proceeds in the
plant, is a potent central nervous system (CNS) stimulant and
absence of sample drug, the absorbance increases.
a local anesthetic. Its pharmacological effects are identical to
When a urine sample contains the drug in question, this
those of amphetamines (also CNS stimulants), though cocaine
drug competes with the drug derivative conjugate for
has a shorter duration of action.2 Cocaine induces euphoria,
microparticle-bound antibody. Antibody bound to sample
confidence, and a sense of increased energy in the user; these
drug is no longer available to promote particle aggregation,
psychological effects are accompanied by increased heart rate,
and subsequent particle lattice formation is inhibited. The
dilation of pupils, fever, tremors, and sweating. The crash
presence of sample drug diminishes the increasing absorbance
following a cocaine high is profound, ranging from irritability,
in proportion to the concentration of drug in the sample.
lassitude, and the desire for more drug, to anxiety, hallucinations,
Sample drug content is determined relative to the value obtained
for a known cutoff concentration of drug.11
2012-10, V 6 EN 1/6 COCII
Drug Abuse Testing
4 0 0 /8 0 0
Reagents - working solutions Application for urine
R1 Conjugate Reagent COBAS INTEGRA 400/400 plus test definition
Conjugated benzoylecgonine derivative in buffer with
150 and 300 ng/mL cutoffs
BSA and 0.09 % sodium azide.
Semiquantitative Qualitative
SR Antibody/Microparticle Reagent
Measuring mode Absorbance Absorbance
Microparticles attached to benzoylecgonine antibody
Abs. calculation mode Endpoint Endpoint
(mouse monoclonal) in buffer with BSA and 0.09 %
Reaction mode R1-S-SR R1-S-SR
sodium azide.
Reaction direction Increase Increase
R1 is in position B and SR is in position C. Reaction start SR SR
Wavelength A 629 nm 629 nm
Precautions and warnings Test range 0-5000 ng/mL 0-9000 CO1Q2
Pay attention to all precautions and warnings listed in 0-4000 CO3Q2,
Section 1 / Introduction of this Method Manual. CO3QC
with postdilution 0-50000 ng/mL
Reagent handling Postdilution factor 10 recommendeda No
COBAS INTEGRA 400/400 plus analyzers Calc. first/last 35/46 35/46
All new (not punctured) cobas c packs must be mixed for Unit ng/mL
1 minute using the Cassette Mixer before placing on-board the
instrument. All in-use cobas c packs must also be mixed in the a) For use when estimating concentration in preparation for GC/MS analysis.
same manner at the beginning of each week (once a week). Pipetting parameters
COBAS INTEGRA 800 analyzer
Diluent (H2O)
The reagent is automatically mixed for 1 minute after cobas c
R1 90 L 5 L
pack puncture and for half a minute during Begin of Day.
Sample 8 L 3 L
Storage and stability SR 35 L 3 L
Total volume 144 L
Shelf life at 2-8 C See expiration date on
cobas c pack label
COBAS INTEGRA 400/400 plus analyzers COBAS INTEGRA 800 test definition
On-board in use at 10-15 C 12 weeks 150 and 300 ng/mL cutoffs
COBAS INTEGRA 800 analyzer Semiquantitative Qualitative
On-board in use at 8 C 12 weeks Measuring mode Absorbance Absorbance
Abs. calculation mode Endpoint Endpoint
The on-board in use stability period begins at the time of
Reaction mode R1-S-SR R1-S-SR
cobas c pack puncture. Do not freeze reagents. Reagents
that have been frozen should be discarded. Reaction direction Increase Increase
Reaction start SR SR
Specimen collection and preparation Wavelength A 629 nm 629 nm
Only the specimens listed below were tested and found acceptable. Test range 0-5000 ng/mL 0-9000 CO1Q2
Urine: Collect urine samples in clean glass or plastic containers. 0-4000 CO3Q2,
Fresh urine samples do not require special handling or CO3QC
pretreatment, but an effort should be made to keep pipetted with postdilution 0-50000 ng/mL
samples free of gross debris. Samples should be within the normal Postdilution factor 10 recommendedb No
physiological pH range of 5-8. No additives or preservatives Calc. first/last 45/78 45/78
are required. It is recommended that urine specimens be
Unit ng/mL
stored at 2-8 C and tested within 5 days of collection.12 For
prolonged storage, freezing of samples is recommended. b) For use when estimating concentration in preparation for GC/MS analysis.
Centrifuge highly turbid specimens before testing. Pipetting parameters
Adulteration or dilution of the sample can cause erroneous
Diluent (H2O)
results. If adulteration is suspected, another sample should
R1 90 L 5 L
be collected. Specimen validity testing is required for
specimens collected under the Mandatory Guidelines for Sample 8 L 3 L
Federal Workplace Drug Testing Programs.13 SR 35 L 3 L
Total volume 144 L
Caution: Specimen dilutions should only be used as an estimation
for GC/MS and are not intended for patient values. Dilution
procedures, when used, should be validated.
Materials provided
See Reagents - working solutions section for reagents.
Assay
For optimum performance of the assay follow the
directions given in this document for the analyzer
concerned. Refer to the appropriate operators manual for
analyzer-specific assay instructions.
COCII 2/6 2012-10, V 6 EN
Drug Abuse Testing
4 0 0 /8 0 0
Calibration Quality control
Calibrators Semiquantitative applications Quality control 150 ng/mL cutoff
CO1S2, 0-126; Preciset DAT Plus I calibrators, CAL 1-6 Control Set DAT I
CO3S2, 0-127 0, 75, 150, 300, 1000, 5000 ng/mL PreciPos DAT Set I
benzoylecgonine (DAT1P, system-ID 07 6753 0)
(DATS2, system-ID 07 6764 6) PreciNeg DAT Set I
Qualitative applications (DAT1N, system-ID 07 6754 9)
CO1Q2, 0-015 Preciset DAT Plus I calibrators, CAL 1 300 ng/mL cutoff
0 ng/mL or deionized water Control Set DAT III
and PreciPos DAT Set III
Preciset DAT Plus I calibrators, CAL 3c or (DAT3P, system-ID 07 6773 5)
C.f.a.s. DAT Qualitative Plus PreciNeg DAT Set III
150 ng/mL (DAT3N, system-ID 07 6774 3)
(150 cutoff, DATQ1, system-ID 07 6744 1) or
For qualitative applications, the cutoff of Control Set DAT Clinical
150 ng/mL is assigned a value of 1000. PreciPos DAT Clinical
(DATCP, system-ID 07 6879 0)
CO3Q2, 0-016 Preciset DAT Plus I calibrators, CAL 1
0 ng/mL or deionized water PreciNeg DAT Clinical
(DATCN, system-ID 07 6878 2)
and
Preciset DAT Plus I calibrators, CAL 4 Control sequence User defined
300 ng/mL Control after calibration Recommended
(300 cutoff, DATQ2, system-ID 07 6768 9) For quality control, use control materials as listed in the
CO3QC, 0-115 Preciset DAT Plus I or IId calibrators, CAL 1 Order information section. In addition, other suitable
0 ng/mL or deionized water control material can be used.
and Drug concentrations of Control Set DAT I, III, and Clinical
C.f.a.s. DAT Qualitative Plus Clinical have been verified by GC/MS.
300 ng/mL The control intervals and limits should be adapted to
(300 cutoff, DATQ5, system-ID 07 6880 4) each laboratorys individual requirements. Values obtained
should fall within the defined limits.
For qualitative applications, the cutoff of
Each laboratory should establish corrective measures to be
300 ng/mL is assigned a value of 1000.
taken if values fall outside the defined limits.
c) Do not use Preciset DAT Plus I, CAL 3 if calibrating the Opiates 300/2000 Follow the applicable government regulations and local
qualitative 2000 ng/mL assay (test OP2QL, test-ID 0-410). guidelines for quality control.
d) Preciset DAT Plus II, CAL 1, while generally not required for the
calibration of Cocaine II, may be used as an alternative 0 ng/mL Results
level for DATQ5, system-ID 07 6880 4.
COBAS INTEGRA systems report results with the
Calibration mode Semiquantitative applications following test flags.
Logit/Log 4 Semiquantitative result reporting
Qualitative applications CO1S2 (150 ng/mL cutoff)
Linear regression Flag COBAS INTEGRA Value range
Calibration replicate Duplicate recommended No flag Negative < 150 ng/mL
Calibration interval COBAS INTEGRA 400/400 plus analyzers: <TEST RNG Negative < 0 ng/mL
Each lot, every 4 weeks, and as required >TEST RNG Positive > 5000 ng/mL
following quality control procedures. POS 150 Positive 150 ng/mL
COBAS INTEGRA 800 analyzer: Value ranges listed above are based on a cutoff value of 150 ng/mL.
Each lot, every 4 weeks, and as required CO3S2 (300 ng/mL cutoff)
following quality control procedures.
Flag COBAS INTEGRA Value range
A calibration curve is generated using the calibrators. Calibrators No flag Negative < 300 ng/mL
must be placed from the highest concentration first to the lowest
<TEST RNG Negative < 0 ng/mL
last on the CAL/QC rack. This curve is retained in memory by
>TEST RNG Positive > 5000 ng/mL
the COBAS INTEGRA system and recalled for later use.
POS 300 Positive 300 ng/mL
Traceability: This method has been standardized against
a primary reference method (GC/MS). Value ranges listed above are based on a cutoff value of 300 ng/mL.
Qualitative result reporting
CO1Q2 (150 ng/mL cutoff)
Flag COBAS INTEGRA Value range
No flag Negative < 1000
<TEST RNG Negative <0
>TEST RNG Positive > 9000
POS 1000 Positive 1000
Value ranges above are based on assigning the cutoff
of 150 ng/mL a value of 1000.
2012-10, V 6 EN 3/6 COCII
Drug Abuse Testing
4 0 0 /8 0 0
CO3Q2, CO3QC (300 ng/mL cutoff) Interfering substances were added to drug free urine at twice the
concentration listed below. These samples were then spiked to
Flag COBAS INTEGRA Value range
300 ng/mL using a 600 ng/mL benzoylecgonine stock solution.
No flag Negative < 1000
Samples were tested and the following results were obtained:
<TEST RNG Negative <0
>TEST RNG Positive > 4000 % Cocaine
POS 1000 Positive 1000 Concentration Metabolite
Substance Tested Recovery
Value ranges above are based on assigning the cutoff
Acetone 1% 99
of 300 ng/mL a value of 1000.
Ascorbic Acid 1.5 % 112
Semiquantitative result reporting Bilirubin 0.25 mg/mL 102
The semiquantitation of preliminary positive results should only Creatinine 5 mg/mL 104
be used by laboratories to determine an appropriate dilution of
Ethanol 1% 99
the specimen for confirmation by a confirmatory method such
Glucose 2% 104
as GC/MS. It also permits the laboratory to establish quality
control procedures and assess control performance. Hemoglobin 0.1 g/L 104
Hemoglobin 1 g/L 104
Note: When using the post-dilution function (1:10 dilution), to
ensure the sample was not over-diluted, the diluted result must Hemoglobin 7.5 g/L 104
be at least half the analyte cutoff value times 10. If the diluted Human Albumin 0.025 % 105
result falls below half the analyte cutoff value times 10, repeat Human Albumin 0.05 % 103
the sample with a smaller dilution. A dilution that produces a Human Albumin 0.5 % 106
result closest to the analyte cutoff is the most accurate estimation. Oxalic Acid 2 mg/mL 102
To estimate the preliminary positive samples concentration, Sodium Chloride 0.5 M 96
multiply the result by the appropriate dilution factor. Dilutions Sodium Chloride 1M 95
should only be used as an estimation for GC/MS.
Urea 6% 101
Limitations - interference
See the Specific performance data section of this document ACTION REQUIRED
for information on substances tested with this assay. There Special wash programming: The use of special wash steps is
is the possibility that other substances and/or factors mandatory when certain test combinations are run together
may interfere with the test and cause erroneous results on COBAS INTEGRA analyzers. Refer to the Method Manual,
(e.g., technical or procedural errors). Introduction, Extra Wash Cycles for further instructions.
A preliminary positive result with this assay indicates Where required, special wash/carry-over evasion programming
the presence of cocaine metabolite in urine. It does not must be implemented prior to reporting results with this test.
measure the level of intoxication. Specific performance data
Interfering substances were added to drug free urine at twice the Representative performance data on the COBAS INTEGRA
concentration listed below. These samples were then spiked to analyzers are given below. Results obtained in individual
150 ng/mL using a 300 ng/mL benzoylecgonine stock solution. laboratories may differ.
Samples were tested and the following results were obtained:
Precision
% Cocaine Precision was determined in an internal protocol by using
Concentration Metabolite a series of benzoylecgonine calibrator and controls in
Substance Tested Recovery replicates of 20, once a day, for 5 days.
Acetone 1% 96 The following results were obtained on a COBAS
Ascorbic Acid 1.5 % 113 INTEGRA 700 analyzer:
Bilirubin 0.25 mg/mL 106 Semiquantitative precision (150 ng/mL cutoff)
Creatinine 5 mg/mL 107 Repeatabilitye Mean SD CV
Ethanol 1% 96 ng/mL ng/mL %
Glucose 2% 109 Level 1 (113 ng/mL) 118 5 4.4
Hemoglobin 0.1 g/L 101 Level 2 (150 ng/mL) 161 6 3.8
Hemoglobin 1 g/L 99 Level 3 (188 ng/mL) 184 5 2.8
Hemoglobin 7.5 g/L 106
Intermediate Mean SD CV
Human Albumin 0.025 % 104
precisionf ng/mL ng/mL %
Human Albumin 0.05 % 107
Level 1 (113 ng/mL) 119 6 5.3
Human Albumin 0.5 % 104
Level 2 (150 ng/mL) 155 7 4.5
Oxalic Acid 2 mg/mL 106
Level 3 (188 ng/mL) 188 7 3.7
Sodium Chloride 0.5 M 94
Sodium Chloride 1M 91
Urea 6% 110
COCII 4/6 2012-10, V 6 EN
Drug Abuse Testing
4 0 0 /8 0 0
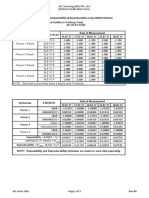
Semiquantitative precision (300 ng/mL cutoff) Cocaine II Clinical Correlation (Cutoff = 150 ng/mL)
e Mean SD CV
Repeatability GC/MS values (ng/mL)
ng/mL ng/mL % Negative
Level 1 (225 ng/mL) 199 5 2.7 COBAS samples Near Cutoff 344 -
296 7 2.3 INTEGRA 113 188 106072
Level 2 (300 ng/mL)
Level 3 (375 ng/mL) 355 7 2.0 700 + 0 0 10 50
Intermediate Mean SD CV - 100 10 0 0
precisionf ng/mL ng/mL %
Cocaine II Clinical Correlation (Cutoff = 300 ng/mL)
Level 1 (225 ng/mL) 208 8 3.9
Level 2 (300 ng/mL) 294 10 3.4 GC/MS values (ng/mL)
Negative
Level 3 (375 ng/mL) 341 12 3.5 Near Cutoff 428 -
COBAS samples
e) repeatability = within-run precision INTEGRA 225 309-402 106072
f) intermediate precision = total precision / between-run precision /
between-day precision
700 + 0 0 11 49
Qualitative precision - 100 10 0 0
150 ng/mL cutoff
Analytical specificity
300 ng/mL cutoff
The specificity of the COBAS INTEGRA Cocaine II assay for
Number Correct cocaine and its metabolites was determined by generating
Cutoff (x) tested results Confidence level inhibition curves for each of the compounds listed and
0.75x 100 100 > 95 % negative reading determining the approximate quantity of each compound
1.25x 100 100 > 95 % positive reading that is equivalent in assay reactivity to the 150 ng/mL and
300 ng/mL benzoylecgonine assay cutoffs.
Lower detection limit of the test
7 ng/mL (150 ng/mL and 300 ng/mL cutoff assays) Approximate
The lower detection limit represents the lowest measurable ng/mL equivalent Approximate
analyte level that can be distinguished from zero. It is calculated to 150 ng/mL of percent
Compound
as the value lying 2 standard deviations above that of the zero benzoylecgonine cross-reactivity
calibrator (zero calibrator + 2 SD, repeatability, n = 21). Cocaine 7112 2.1
Cocaethylene 37260 0.4
Accuracy
100 urine samples, obtained from a clinical laboratory where they
screened negative in a drug test panel by another technology, Approximate
were evaluated for cocaine metabolite on a COBAS INTEGRA ng/mL equivalent Approximate
700 analyzer. All 100 clinical samples were negative relative to 300 ng/mL of percent
Compound
to the 150 ng/mL and 300 ng/mL cutoffs. benzoylecgonine cross-reactivity
50 urine samples, obtained from clinical laboratories where they Cocaine 18508 1.6
screened preliminary positive by a commercially available enzyme Cocaethylene 70108 0.4
immunoassay and confirmed positive for cocaine metabolite
by GC/MS, were also evaluated on a COBAS INTEGRA 700 Additionally, the following compounds were tested at a
analyzer. All 50 samples were positive with the COBAS INTEGRA concentration of 100000 ng/mL in pooled human urine and
Cocaine II assay relative to the 150 ng/mL and 300 ng/mL cutoffs. shown to have cross-reactivity values of less than 0.05 %:
In addition, 10 samples were diluted to a benzoylecgonine Ecgonine Ecgonine methyl ester Norcocaine
concentration of 75-100 % of the cutoff concentration
for each cutoff; and 10 samples were diluted to a
benzoylecgonine concentration of 100-125 % of the cutoff
concentration for each cutoff.
Data from the accuracy studies described above that fell within
the near cutoff value ranges were combined with data generated
from the diluted positive urine samples. The following results
were obtained with Cocaine II on a COBAS INTEGRA 700
analyzer relative to the GC/MS values.
2012-10, V 6 EN 5/6 COCII
Drug Abuse Testing
4 0 0 /8 0 0
Drug interference References
1. Karch SB ed. Drug Abuse Handbook. Boca Raton,
The following compounds were added to aliquots of pooled
FL: CRC Press LLC 1998.
normal human urine at a concentration of 100000 ng/mL. None
2. Blum K. Handbook of Abusable Drugs. 1st ed. New
of these compounds gave values in the assay that were equal to or
York, NY: Gardner Press Inc 1984.
greater than 0.05 % (50 ng/mL) cross reactivity.
3. Wilson MC, Bedford JA, Buelke J, et al. Acute pharmacological
Acetaminophen EDDP Nordoxepin activity of intravenous cocaine in the rhesus monkey.
Acetylsalicylic acid EMDP Norethindrone Psychopharmacol Comm 1976;2:251.
Aminopyrine Erythromycin l-Norpseudoephedrine 4. Rappolt RT Sr, Gray GR, Inaba DS. Propranolol in
Amitriptyline Estriol Nortriptyline the treatment of cardiopressor effects of cocaine.
Amobarbital Fenoprofen Orphenadrine N Engl J Med 1976;295:448.
d-Amphetamine Fluconazole Oxazepam 5. Fish F, Wilson WD. Excretion of cocaine and its metabolites
in man. J Pharm Pharmacol 1969;21:135S.
l-Amphetamine Fluoxetine Oxycodone
6. Hamilton HE, Wallace JE, Shimek EL Jr, et al.
Ampicillin Furosemide Penicillin G
Cocaine and benzoylecgonine excretion in humans.
Ascorbic acid Gentisic acid Pentobarbital J Forensic Sci 1977;22:697.
Aspartame Glutethimide Perphenazine 7. Stewart DJ, Inaba T, Lucassen M, et al. Cocaine metabolism:
Atropine Guaiacol glycerol ether -Phenethylamine Cocaine and norcocaine hydrolysis by liver and serum
Benzocaine Haloperidol Phencyclidine esterases. Clin Pharmacol Ther 1979;25:464.
Benzphetamine Hydrochlorothiazide Phenobarbital 8. Connell PH. Clinical manifestations and treatment of
Butabarbital Hydroxymethadone Phenothiazine amphetamine-type dependence. JAMA 1966.
9. Armbruster DA, Schwarzhoff RH, Pierce BL, et al. Method
Caffeine Ibuprofen Phentermine
comparison of EMIT II and ONLINE with RIA for drug
Calcium hypochlorite Imipramine Phenylbutazone
screening. J Forensic Sci 1993;38:1326-1341.
Cannabidiol Isoproterenol Phenylpropanolamine 10. Armbruster DA, Schwarzhoff RH, Hubster EC, et al.
Carbamazepine Ketamine d-Phenylpropanol- Enzyme immunoassay, kinetic microparticle immunoassay,
Chlordiazepoxide LAAM amine radioimmunoassay, and fluorescence polarization
Chloroquine Lidocaine Phendimetrazine immunoassay compared for drugs-of-abuse screening.
Chlorpheniramine LSD Procaine Clin Chem 1993;39:2137-2146.
Chlorpromazine Maprotiline Promazine 11. Bates M, Brandle J, Casaretto E, et al. An Abuscreen
Chlorprothixene MDA Promethazine immunoassay for opiates in urine on the COBAS
MIRA automated analyzer. Amer Acad Forensic
Clomipramine MDMA Propoxyphene
Sci. Abstract 1991;37(6):1000.
Codeine Melanin Protriptyline
12. Toxicology and Drug Testing in the Clinical Laboratory;
Cotinine Meperidine d-Pseudoephedrine Approved Guideline. 2nd ed. (C52-A2). Clinical and
Cyclobenzaprine Methadol l-Pseudoephedrine Laboratory Standards Institute 2007;27:33.
Cyproheptadine Methadone Quinidine 13. Mandatory Guidelines for Federal Workplace Drug Testing
Desipramine d-Methamphetamine Quinine Programs. Fed Regist 2010;73:71858-71907.
Dextromethorphan l-Methamphetamine Secobarbital
A point (period/stop) is always used in this Method Sheet
Dextropropoxyphene Methaqualone Sulindac as the decimal separator to mark the border between the
Diazepam Methotrimeprazine Tetracycline integral and the fractional parts of a decimal numeral.
Diphenhydramine Methylphenidate 9 THC-9-carboxylic Separators for thousands are not used.
Diphenylhydantoin Methyprylon acid
FOR US CUSTOMERS ONLY: LIMITED WARRANTY
Dopamine Mianserin Tetrahydrozoline Roche Diagnostics warrants that this product will meet the
Disopyramide Morphine sulfate Thioridazine specifications stated in the labeling when used in accordance
Doxepin Naloxone Thiothixene with such labeling and will be free from defects in material and
workmanship until the expiration date printed on the label.
Doxylamine Naltrexone Trifluoperazine
THIS LIMITED WARRANTY IS IN LIEU OF ANY OTHER
d-Ephedrine Naproxen Trimipramine WARRANTY, EXPRESS OR IMPLIED, INCLUDING ANY
d,l-Ephedrine Niacinamide Tyramine IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS
l-Ephedrine Nicotine Verapamil FOR PARTICULAR PURPOSE. IN NO EVENT SHALL ROCHE
Zomepirac DIAGNOSTICS BE LIABLE FOR INCIDENTAL, INDIRECT,
Epinephrine Nordiazepam
SPECIAL OR CONSEQUENTIAL DAMAGES.
Any modification of the instrument as set forth in this
COBAS INTEGRA, COBAS C, ONLINE DAT, PRECISET, ABUSCREEN, and COBAS
labeling requires validation by the laboratory. MIRA are trademarks of Roche.
All other product names and trademarks are the property of their respective owners.
Significant additions or changes are indicated by a change bar in the margin.
2012, Roche Diagnostics
Roche Diagnostics GmbH, Sandhofer Strasse 116, D-68305 Mannheim
www.roche.com
Distribution in USA by:
Roche Diagnostics, Indianapolis, IN
US Customer Technical Support 1-800-428-2336
COCII 6/6 2012-10, V 6 EN
Das könnte Ihnen auch gefallen
- Sysmex XW - 100: Instructions For Use ManualDokument32 SeitenSysmex XW - 100: Instructions For Use ManualNahom BalchaNoch keine Bewertungen
- Quintus 5-Part Hematology Analyzer: In-Depth, Quality-Controlled 26-Parameter ResultsDokument6 SeitenQuintus 5-Part Hematology Analyzer: In-Depth, Quality-Controlled 26-Parameter ResultsSubhanullah JalalNoch keine Bewertungen
- Broschyr GEM5000Dokument12 SeitenBroschyr GEM5000Oo Kenx OoNoch keine Bewertungen
- GLLUDokument4 SeitenGLLUARIF AHAMMED PNoch keine Bewertungen
- CYSC2Dokument4 SeitenCYSC2ARIF AHAMMED PNoch keine Bewertungen
- Elecsys BRAHMS PCT: ProcalcitoninDokument5 SeitenElecsys BRAHMS PCT: ProcalcitoninMilagrosLcNoch keine Bewertungen
- ETOHDokument4 SeitenETOHARIF AHAMMED PNoch keine Bewertungen
- ALTDokument10 SeitenALTLiviu Athos Tamas0% (1)
- Insert - Elecsys Syphilis - Ms 07802960190.V3.EnDokument5 SeitenInsert - Elecsys Syphilis - Ms 07802960190.V3.EnGuneyden GuneydenNoch keine Bewertungen
- CL - 1000i Immunofluorescence Analyzer.Dokument6 SeitenCL - 1000i Immunofluorescence Analyzer.bikouvoNoch keine Bewertungen
- c111 Operator Manual PDFDokument502 Seitenc111 Operator Manual PDFSuponoNoch keine Bewertungen
- TotalBhCG ARCDokument7 SeitenTotalBhCG ARCLau GómezNoch keine Bewertungen
- CREJ2Dokument4 SeitenCREJ2ARIF AHAMMED PNoch keine Bewertungen
- COBAS Elecsys HBsAgDokument45 SeitenCOBAS Elecsys HBsAgmeghnaNoch keine Bewertungen
- Brochure PDFDokument72 SeitenBrochure PDFkisa guyNoch keine Bewertungen
- Elecsys Reference RangesDokument95 SeitenElecsys Reference Rangescandy jmzNoch keine Bewertungen
- T4 IflashDokument4 SeitenT4 IflashNIGHT tubeNoch keine Bewertungen
- Insert TRIGL 0020767107322COIN V11 enDokument4 SeitenInsert TRIGL 0020767107322COIN V11 entechlabNoch keine Bewertungen
- HydraulicsDokument101 SeitenHydraulicsHuseyn aliyevNoch keine Bewertungen
- Elecsys Hbsag Ii: A) Tris (2,2'-Bipyridyl) Ruthenium (Ii) - Complex (Ru (Bpy) )Dokument5 SeitenElecsys Hbsag Ii: A) Tris (2,2'-Bipyridyl) Ruthenium (Ii) - Complex (Ru (Bpy) )Brian SamanyaNoch keine Bewertungen
- 0812 in Vitro Blood Gas Analyzers GuideDokument9 Seiten0812 in Vitro Blood Gas Analyzers GuidedjebrutNoch keine Bewertungen
- Trouble Shooting Elecsys2 010v53Dokument142 SeitenTrouble Shooting Elecsys2 010v53Hồ Thế NguyênNoch keine Bewertungen
- Ichroma II Test Panels 210331 104829Dokument2 SeitenIchroma II Test Panels 210331 104829Sinergy DiagnosticNoch keine Bewertungen
- Totalt4 ArcDokument6 SeitenTotalt4 Arctesteste testeNoch keine Bewertungen
- Brochure PocH 100i MKT 10 1025Dokument4 SeitenBrochure PocH 100i MKT 10 1025Klayte TayongNoch keine Bewertungen
- ACTHDokument4 SeitenACTHHassan GillNoch keine Bewertungen
- Control de Calidad 1039Dokument106 SeitenControl de Calidad 1039MARITZA MUÑOZ100% (1)
- Cholesterol Specimen Collection & Handling GuideDokument4 SeitenCholesterol Specimen Collection & Handling GuideCriiiiisl100% (1)
- Method Verification of TestDokument2 SeitenMethod Verification of TestObak PrithibiNoch keine Bewertungen
- ADD-00058823-R1 - ARCHITECT Specifications PDFDokument6 SeitenADD-00058823-R1 - ARCHITECT Specifications PDFMauri SadaniowskiNoch keine Bewertungen
- BS-240 and BS-230 Clinical Chemistry Analyzer: Harry HuDokument30 SeitenBS-240 and BS-230 Clinical Chemistry Analyzer: Harry Hutest testerNoch keine Bewertungen
- CA-BA-BS-120 Product IntroductionDokument36 SeitenCA-BA-BS-120 Product IntroductionsilentNoch keine Bewertungen
- Medica EasyLyte BrochureDokument6 SeitenMedica EasyLyte BrochureaoxoxzNoch keine Bewertungen
- Method Report for GPT- GISAN AssayDokument1 SeiteMethod Report for GPT- GISAN AssayHussein N. FarhatNoch keine Bewertungen
- H-046-003250-00 CEA KIT (CLIA) Muti LaguageDokument14 SeitenH-046-003250-00 CEA KIT (CLIA) Muti LaguageSinari Alfat100% (1)
- BC5000¡ BC5150 Service Training Material1.0Dokument180 SeitenBC5000¡ BC5150 Service Training Material1.0Jaime EspinosaNoch keine Bewertungen
- 1975ec-2026ec-2028ec 2023-04Dokument57 Seiten1975ec-2026ec-2028ec 2023-04Sujit KushwahaNoch keine Bewertungen
- Lyphochek Immunoassay Plus Control Levels 1, 2 and 3Dokument26 SeitenLyphochek Immunoassay Plus Control Levels 1, 2 and 3Aniket dubeyNoch keine Bewertungen
- Budi Altgpt - Doc NewDokument3 SeitenBudi Altgpt - Doc NewIrvanda ENVIOUSNoch keine Bewertungen
- Balio Ax-200 Brochure enDokument4 SeitenBalio Ax-200 Brochure enphyo waiNoch keine Bewertungen
- 1066UE 2023-01 RANDOX Level 3Dokument92 Seiten1066UE 2023-01 RANDOX Level 3Dharmesh PatelNoch keine Bewertungen
- XN-L - Reference Interval From General Information 2017Dokument4 SeitenXN-L - Reference Interval From General Information 2017widiawaty100% (1)
- ACL TOP Family SOP Manual 2016-10-19 enDokument238 SeitenACL TOP Family SOP Manual 2016-10-19 enAndra Radulescu100% (1)
- Ureal: GLDH 4 2Dokument8 SeitenUreal: GLDH 4 2CarinaVillasantiNoch keine Bewertungen
- Calset TSHDokument2 SeitenCalset TSHJimboreanu György Paula100% (1)
- En EA Cobas 4000 Analyzer SeriesDokument20 SeitenEn EA Cobas 4000 Analyzer Seriesatiqa aslamNoch keine Bewertungen
- Centrifugeuse Br4iDokument36 SeitenCentrifugeuse Br4iKada HaliliNoch keine Bewertungen
- BS-200 Brochura ENDokument3 SeitenBS-200 Brochura ENmdkNoch keine Bewertungen
- Catalog (ENG) May Sinh Hoa Tu Dong BS-600Dokument4 SeitenCatalog (ENG) May Sinh Hoa Tu Dong BS-600anhhp8xNoch keine Bewertungen
- Access 2 Immunoassay System BrochureDokument4 SeitenAccess 2 Immunoassay System BrochureSenyum SehatNoch keine Bewertungen
- Competitive Evaluation of The GEM Premier 3000 With PDFDokument8 SeitenCompetitive Evaluation of The GEM Premier 3000 With PDFEllya Latifah IlyasNoch keine Bewertungen
- PF Folder Sepsis 0819 WebDokument6 SeitenPF Folder Sepsis 0819 WebWilliams Alejandro Choroco VillegasNoch keine Bewertungen
- Rheumatoid Factors: InstrumentsDokument1 SeiteRheumatoid Factors: InstrumentsEnrique DuarteNoch keine Bewertungen
- Assay SolutionsDokument1 SeiteAssay SolutionsAlex RamirezNoch keine Bewertungen
- Anti-Tpo 2017-07 v5Dokument4 SeitenAnti-Tpo 2017-07 v5Ismael CulquiNoch keine Bewertungen
- Multistix Reagent StripDokument3 SeitenMultistix Reagent StripFrancis TorresNoch keine Bewertungen
- G7 Service ManualDokument314 SeitenG7 Service Manualzhigang yangNoch keine Bewertungen
- Co2 LDokument3 SeitenCo2 LARIF AHAMMED P100% (1)
- 110748-GC1 RP500 Quick Reference Guide FINAL 94552391 1Dokument1 Seite110748-GC1 RP500 Quick Reference Guide FINAL 94552391 1Neamtu2Noch keine Bewertungen
- Liquid Chromatographic Determination of Cocaine, Benzoylecgonine, and Cocaethylene in Whole Blood and Serum Samples With Diode-Array DetectionDokument8 SeitenLiquid Chromatographic Determination of Cocaine, Benzoylecgonine, and Cocaethylene in Whole Blood and Serum Samples With Diode-Array DetectionAngie Diaz TorresNoch keine Bewertungen
- Nafi QuizDokument14 SeitenNafi QuizARIF AHAMMED PNoch keine Bewertungen
- Notebook Work: Wonders in The Water: GEMS United Indian School - LP - Rev 2: April 2018Dokument3 SeitenNotebook Work: Wonders in The Water: GEMS United Indian School - LP - Rev 2: April 2018ARIF AHAMMED PNoch keine Bewertungen
- Validation SOP ChecklistDokument5 SeitenValidation SOP ChecklistARIF AHAMMED PNoch keine Bewertungen
- July 2018 A1cDokument1 SeiteJuly 2018 A1cARIF AHAMMED PNoch keine Bewertungen
- Ckklst-Qual Meth VLDDokument3 SeitenCkklst-Qual Meth VLDARIF AHAMMED PNoch keine Bewertungen
- RangeeeDokument43 SeitenRangeeeARIF AHAMMED PNoch keine Bewertungen
- Estab QC RNG - Use of CVDokument6 SeitenEstab QC RNG - Use of CVARIF AHAMMED PNoch keine Bewertungen
- Plants and Their UsesDokument3 SeitenPlants and Their UsesARIF AHAMMED PNoch keine Bewertungen
- Arabic Letters ةَّيب َرَعلا فرحلأا: j th t b aDokument10 Seiten Arabic Letters ةَّيب َرَعلا فرحلأا: j th t b aARIF AHAMMED P100% (1)
- ElecsyyysDokument45 SeitenElecsyyysARIF AHAMMED PNoch keine Bewertungen
- CHKLST Intr QCDokument3 SeitenCHKLST Intr QCARIF AHAMMED PNoch keine Bewertungen
- Smile Linearity Worksheet: Acceptable Acceptable Acceptable Acceptable Acceptable AcceptableDokument3 SeitenSmile Linearity Worksheet: Acceptable Acceptable Acceptable Acceptable Acceptable AcceptableARIF AHAMMED PNoch keine Bewertungen
- Cap CompDokument12 SeitenCap CompARIF AHAMMED PNoch keine Bewertungen
- IcDokument1 SeiteIcARIF AHAMMED PNoch keine Bewertungen
- Top Laboratory Deficiencies Across Accreditation Agencies PDFDokument7 SeitenTop Laboratory Deficiencies Across Accreditation Agencies PDFARIF AHAMMED PNoch keine Bewertungen
- When Direct and Indirect Ion Selective Electrode Results Conflict PDFDokument3 SeitenWhen Direct and Indirect Ion Selective Electrode Results Conflict PDFARIF AHAMMED PNoch keine Bewertungen
- Joint Commission Top 10 Deficiencies in 2016 PDFDokument1 SeiteJoint Commission Top 10 Deficiencies in 2016 PDFARIF AHAMMED PNoch keine Bewertungen
- Stand by Bottle ThresholdDokument1 SeiteStand by Bottle ThresholdARIF AHAMMED PNoch keine Bewertungen
- PreciControl HbA1c Norm.05975115001.V4.EnDokument2 SeitenPreciControl HbA1c Norm.05975115001.V4.EnARIF AHAMMED PNoch keine Bewertungen
- PreciControl HbA1c Path.05854237001.V4.EnDokument2 SeitenPreciControl HbA1c Path.05854237001.V4.EnARIF AHAMMED PNoch keine Bewertungen
- TPLA Control Set.04955188001.V4.EnDokument2 SeitenTPLA Control Set.04955188001.V4.EnARIF AHAMMED PNoch keine Bewertungen
- PreciControl ClinChem Multi 2.05117224001.V4.EnDokument2 SeitenPreciControl ClinChem Multi 2.05117224001.V4.EnARIF AHAMMED P29% (7)
- STFR Control Set.12178206001.V6.EnDokument2 SeitenSTFR Control Set.12178206001.V6.EnARIF AHAMMED PNoch keine Bewertungen
- Total MPA Controls.05885442001.V4.EnDokument2 SeitenTotal MPA Controls.05885442001.V4.EnARIF AHAMMED PNoch keine Bewertungen
- TDM Control Set.04714768001.V6.EnDokument2 SeitenTDM Control Set.04714768001.V6.EnARIF AHAMMED PNoch keine Bewertungen
- Precipath CK-MB.04362349001.V6.en PDFDokument2 SeitenPrecipath CK-MB.04362349001.V6.en PDFARIF AHAMMED PNoch keine Bewertungen
- Precipath U Plus.12173697001.V12.EnDokument2 SeitenPrecipath U Plus.12173697001.V12.EnARIF AHAMMED PNoch keine Bewertungen
- RF Control Set.03005526001.V6.enDokument2 SeitenRF Control Set.03005526001.V6.enARIF AHAMMED PNoch keine Bewertungen
- RPR Control Set.04955196001.V4.EnDokument2 SeitenRPR Control Set.04955196001.V4.EnARIF AHAMMED PNoch keine Bewertungen
- Precipath L.12174685001.V8.en PDFDokument2 SeitenPrecipath L.12174685001.V8.en PDFARIF AHAMMED PNoch keine Bewertungen
- SOP For Raw Material ReleaseDokument4 SeitenSOP For Raw Material ReleaseSolomonNoch keine Bewertungen
- AIA 360 T111 (Final Edition)Dokument4 SeitenAIA 360 T111 (Final Edition)Rogger RuffiniNoch keine Bewertungen
- Microwave BCADokument10 SeitenMicrowave BCAarchivo_lcNoch keine Bewertungen
- MDCG 2021-22 enDokument6 SeitenMDCG 2021-22 eneliNoch keine Bewertungen
- ACCURUN 190 Anti-Trypanosoma Cruzi (Chagas) Positive ControlDokument2 SeitenACCURUN 190 Anti-Trypanosoma Cruzi (Chagas) Positive ControlClauss RubiNoch keine Bewertungen
- Assessment of The Target-Capture PCR Hepatitis B Virus (HBV) DNA Quantitative Assay and Comparison With Commercial HBV DNA Quantitative AssaysDokument6 SeitenAssessment of The Target-Capture PCR Hepatitis B Virus (HBV) DNA Quantitative Assay and Comparison With Commercial HBV DNA Quantitative AssaysdwimarufNoch keine Bewertungen
- Alkaline Phosphatase 40 EngDokument4 SeitenAlkaline Phosphatase 40 EngRakib Hossain 3A-159Noch keine Bewertungen
- Johnson Dam Prospect Positive Drilling Results1684 - 230517 - 212154Dokument15 SeitenJohnson Dam Prospect Positive Drilling Results1684 - 230517 - 212154ChrisNoch keine Bewertungen
- Test Results 7.10. Interpretation of Requirements: Rounding RulesDokument2 SeitenTest Results 7.10. Interpretation of Requirements: Rounding RulesClint MuscatNoch keine Bewertungen
- F748 16Dokument7 SeitenF748 16masoudNoch keine Bewertungen
- (hCG) Test System: β-Human Chorionic GonadotropinDokument2 Seiten(hCG) Test System: β-Human Chorionic GonadotropinJoão José Damian SalazarNoch keine Bewertungen
- Clinical Research Scientist in Washington DC Resume Catherine KibirigeDokument2 SeitenClinical Research Scientist in Washington DC Resume Catherine KibirigeCatherineKibirigeNoch keine Bewertungen
- API II Vol 1 PDFDokument335 SeitenAPI II Vol 1 PDFTerryNoch keine Bewertungen
- Analytical Chemistry Role in Pharma IndustryDokument39 SeitenAnalytical Chemistry Role in Pharma Industrypawan kumar gupta0% (1)
- FAO DeltametrineDokument101 SeitenFAO DeltametrinemercuriusNoch keine Bewertungen
- ADD - 00002921 - Architect - Immunoassay - Menu - en - Valor de ReferenciaDokument8 SeitenADD - 00002921 - Architect - Immunoassay - Menu - en - Valor de ReferenciaHasley FlidmanNoch keine Bewertungen
- Clinica Chimica Acta: Alberto Dolci, Mauro PanteghiniDokument6 SeitenClinica Chimica Acta: Alberto Dolci, Mauro PanteghiniJoseAbdalaNoch keine Bewertungen
- List The Factors Influencing The Interpretation of TDM ReportDokument3 SeitenList The Factors Influencing The Interpretation of TDM ReportOdyNoch keine Bewertungen
- Parenteral Process ValidationDokument30 SeitenParenteral Process Validationravindra82% (11)
- Cell Cytotoxicity AssaysDokument19 SeitenCell Cytotoxicity AssaysKhyati VedNoch keine Bewertungen
- D0023985 PDFDokument55 SeitenD0023985 PDFEliceCrysVenelleNoch keine Bewertungen
- Experience San Francisco, CA Senior Quality Control MicrobiologistDokument5 SeitenExperience San Francisco, CA Senior Quality Control MicrobiologistJamal Abdel SiamNoch keine Bewertungen
- Microprotein: (Pyrogallol Red Method)Dokument2 SeitenMicroprotein: (Pyrogallol Red Method)Ranjit PathakNoch keine Bewertungen
- 44 - 75 - Detection of Autoantibodies Against Platelet Glycoproteins in ... HeDokument21 Seiten44 - 75 - Detection of Autoantibodies Against Platelet Glycoproteins in ... HevishnupgiNoch keine Bewertungen
- Service Manual: Immulite 2000 / Immulite 2000 Xpi / Immulite 2500 Immunoassay SystemsDokument463 SeitenService Manual: Immulite 2000 / Immulite 2000 Xpi / Immulite 2500 Immunoassay SystemsIvan100% (2)
- PreciControl Tumor Marker - Ms - 11776452122.V20.EnDokument2 SeitenPreciControl Tumor Marker - Ms - 11776452122.V20.EnARIF AHAMMED PNoch keine Bewertungen
- Bioprospecting - Success, Potential and ConstraintsDokument310 SeitenBioprospecting - Success, Potential and ConstraintsPriyanka Lale100% (1)
- CC3 Lab ExpDokument44 SeitenCC3 Lab ExpDia, Mark Kenneth A.Noch keine Bewertungen
- PH Eur - Botulinum Toxin Type A For Injection 2113eDokument3 SeitenPH Eur - Botulinum Toxin Type A For Injection 2113eErna Von Der WaldeNoch keine Bewertungen
- Narrative Report CataagDokument6 SeitenNarrative Report CataagCarlo CataagNoch keine Bewertungen