Beruflich Dokumente
Kultur Dokumente
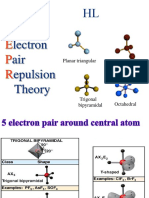
VSEPR Practice Problems
Hochgeladen von
rajaijahOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
VSEPR Practice Problems
Hochgeladen von
rajaijahCopyright:
Verfügbare Formate
VSEPR practice problems
ANSWER KEY
Draw the 2-D LEWIS structure below the molecular formula.
Determine both electron-domain (ED) and molecular geometry.
Determine whether bond angles are ideal (90 o, 109.5o, 120o, 180o) or
distorted due to lone pair bonding pair repulsion.
From the overall molecular geometry and the presence and arrangement
of polar bonds (if any), determine if a molecule is polar. (Polarity does not
apply to polyatomic ions.)
1) PF3 ED geometry: tetrahedral
Molecular geometry: trigonal pyramidal
Bond angles: < 109.5
Angles distorted? yes
Is the molecule polar? yes
2) PF5 ED geometry: trigonal bipyramidal
Molecular geometry: trigonal bipyramidal
Bond angles: 90, 120
Angles distorted? no
Is the molecule polar? no
3) SF4 ED geometry: trigonal bipyramidal
Molecular geometry: see-saw
Bond angles: < 90, < 120
Angles distorted? yes
Is the molecule polar? yes
4) SF6 ED geometry: octahedral
Molecular geometry: octahedral
Bond angles: 90
Angles distorted? no
Is the molecule polar? no
5) CH3+ ED geometry: trigonal planar
Molecular geometry: trigonal planar
Bond angles: 120
Angles distorted? no
Is the molecule polar? n/a
6) ClO- ED geometry: linear
Molecular geometry: linear
Bond angles: n/a
Angles distorted? n/a
Is the molecule polar? n/a
7) ClO2- ED geometry: tetrahedral
Molecular geometry: bent
Bond angles: < 109.5
Angles distorted? yes
Is the molecule polar? n/a
8) ClO3- ED geometry: tetrahedral
Molecular geometry: trigonal pyramidal
Bond angles: < 109.5
Angles distorted? yes
Is the molecule polar? n/a
9) ClO4- ED geometry: tetrahedral
Molecular geometry: tetrahedral
Bond angles: 109.5
Angles distorted? no
Is the molecule polar? n/a
10) KrF2 ED geometry: trigonal bipyramidal
Molecular geometry: linear
Bond angles: 180
Angles distorted? no
Is the molecule polar? no
11) XeF4 ED geometry: octahedral
Molecular geometry: square planar
Bond angles: 90
Angles distorted? no
Is the molecule polar? no
12) XeF6 ED geometry: pentagonal bipyramidal
Molecular geometry: fluctuating, distorted octahedral
You can skip this one. Bond angles: 90
Angles distorted? yes
Is the molecule polar? no
13) XeO3 ED geometry: tetrahedral
Molecular geometry: trigonal pyramidal
Bond angles: < 109.5
Angles distorted? yes
Is the molecule polar? yes
14) XeO2F2 ED geometry: trigonal bipyramidal
Molecular geometry: seesaw
Bond angles: < 90, < 120
Angles distorted? yes
Is the molecule polar? yes
15) CS2 ED geometry: linear
Molecular geometry: linear
Bond angles: 180
Angles distorted? no
Is the molecule polar? no
16) NO3- ED geometry: trigonal planar
Molecular geometry: trigonal planar
Bond angles: 120
Angles distorted? no
Is the molecule polar? n/a
17) CO32- ED geometry: trigonal planar
Molecular geometry: trigonal planar
Bond angles: 120
Angles distorted? no
Is the molecule polar? n/a
18) BH3 ED geometry: trigonal planar
Molecular geometry: trigonal planar
Bond angles: 120
Angles distorted? no
Is the molecule polar? no
19) XeF3+ ED geometry: trigonal bipyramidal
Molecular geometry: T-shaped
Bond angles: < 90, < 180
Angles distorted? yes
Is the molecule polar? n/a
20) BrF3 ED geometry: trigonal bipyramidal
Molecular geometry: T-shaped
Bond angles: < 90, < 180
Angles distorted? yes
Is the molecule polar? yes
21) IF5 ED geometry: octahedral
Molecular geometry: square planar
Bond angles: < 90
Angles distorted? yes
Is the molecule polar? yes
22) HCN ED geometry: linear
Molecular geometry: linear
Bond angles: 180
Angles distorted? no
Is the molecule polar? yes
23) NH3Cl+ ED geometry: tetrahedral
Molecular geometry: tetrahedral
Bond angles: 109.5
Angles distorted? yes
Is the molecule polar? n/a
24) I3 - ED geometry: trigonal bipyramidal
Molecular geometry: linear
Bond angles: 180
Angles distorted? no
Is the molecule polar? n/a
25) N3- ED geometry: linear
Molecular geometry: linear
Bond angles: 180
Angles distorted? no
Is the molecule polar? n/a
26) BHCl2 ED geometry: trigonal planar
Molecular geometry: trigonal planar
Bond angles: 120
Angles distorted? yes
Is the molecule polar? yes
Das könnte Ihnen auch gefallen
- Vsepr-HlDokument25 SeitenVsepr-HlRyan BoukaaNoch keine Bewertungen
- Chapter 4-Structure of AtomDokument49 SeitenChapter 4-Structure of AtomDn Zack0% (1)
- Chemistry Cheat SheetDokument1 SeiteChemistry Cheat SheetAnis FatemaNoch keine Bewertungen
- Chp-4, VSEPR Powerpoint (Autosaved)Dokument37 SeitenChp-4, VSEPR Powerpoint (Autosaved)Ju KaNoch keine Bewertungen
- Worksheet 15 VSEPR PDFDokument5 SeitenWorksheet 15 VSEPR PDFAbdur RehmanNoch keine Bewertungen
- 4.chemical Bonding - Molecular Structure AK 2 (2018-19)Dokument12 Seiten4.chemical Bonding - Molecular Structure AK 2 (2018-19)Tarun RaoNoch keine Bewertungen
- CH 3 StoichiometryDokument30 SeitenCH 3 StoichiometrymedinoNoch keine Bewertungen
- Atomic StructureDokument89 SeitenAtomic StructureDr. Rajni GargNoch keine Bewertungen
- Chem12 SM 01 4Dokument4 SeitenChem12 SM 01 4Firdausia Rahma PutriNoch keine Bewertungen
- Bonding Notes General Chemistry 1Dokument48 SeitenBonding Notes General Chemistry 1JL VANoch keine Bewertungen
- 1st Sec WorkbookDokument109 Seiten1st Sec WorkbookTarekKhouzam100% (1)
- Chemistry 12: Solutions Manual Part ADokument44 SeitenChemistry 12: Solutions Manual Part ADerrick JamesNoch keine Bewertungen
- Topic 10 Organic ChemistryDokument12 SeitenTopic 10 Organic ChemistrySiddharth JainNoch keine Bewertungen
- ASOE Chemistry 2020 SsDokument33 SeitenASOE Chemistry 2020 Ssnavraj singhNoch keine Bewertungen
- Applied Inorganic ChemistryDokument238 SeitenApplied Inorganic ChemistryZemen JM100% (1)
- Unit 2 Notes - Molecular & Ionic Compound Structure & PropertiesDokument18 SeitenUnit 2 Notes - Molecular & Ionic Compound Structure & PropertiesDragonbariumNoch keine Bewertungen
- Chemistry Solutes Solvents Solubility Solutions VCBCCTDokument17 SeitenChemistry Solutes Solvents Solubility Solutions VCBCCTDIONYSUS100% (1)
- Characteristics of Chemical EquilibriumDokument43 SeitenCharacteristics of Chemical Equilibriumpimpin1Noch keine Bewertungen
- 2020 Chemical Energetics Part 1 TutorialDokument13 Seiten2020 Chemical Energetics Part 1 TutorialSalman ShethNoch keine Bewertungen
- 3 Chemical Bonding (CB)Dokument11 Seiten3 Chemical Bonding (CB)Gadde Gopala KrishnaNoch keine Bewertungen
- 11 Chemistry Redox Reactions Test Paper 01Dokument1 Seite11 Chemistry Redox Reactions Test Paper 01mohapatramugdha99Noch keine Bewertungen
- Worksheet - Concentration Calculations - HonorsDokument3 SeitenWorksheet - Concentration Calculations - HonorsJulia Manaog0% (1)
- Chemistry Final Study Guide: Identify The Choice That Best Completes The Statement or Answers The QuestionDokument22 SeitenChemistry Final Study Guide: Identify The Choice That Best Completes The Statement or Answers The Questionsrahimi@verizon.netNoch keine Bewertungen
- Unit 3 Exam-SolutionsDokument8 SeitenUnit 3 Exam-SolutionsbrunosipodNoch keine Bewertungen
- Limiting Reagent ProblemsDokument7 SeitenLimiting Reagent ProblemsKaiRisNoch keine Bewertungen
- Empirical Versus Molecular FormulasDokument5 SeitenEmpirical Versus Molecular FormulasJaz SantosNoch keine Bewertungen
- Solutions Manual Chapter8 PDFDokument54 SeitenSolutions Manual Chapter8 PDFKwan-Soo ParkNoch keine Bewertungen
- Chemistry 12: Solutions Manual Part ADokument38 SeitenChemistry 12: Solutions Manual Part AhairtNoch keine Bewertungen
- Week12 Mole Student 2019Dokument35 SeitenWeek12 Mole Student 2019api-4915646430% (1)
- C F C CL C - BR: HalogenoalkanesDokument11 SeitenC F C CL C - BR: HalogenoalkanesMufaro MutotiNoch keine Bewertungen
- Stoichiometry Worksheet: L.M. PetrovichDokument9 SeitenStoichiometry Worksheet: L.M. PetrovichJomon ThomasNoch keine Bewertungen
- SCH4 Organic Unit TestDokument6 SeitenSCH4 Organic Unit TestMariiam CiiNoch keine Bewertungen
- Worksheet On Atoms, Molecules and IonsDokument7 SeitenWorksheet On Atoms, Molecules and IonsTariqNoch keine Bewertungen
- States of Matter Notes Class 11 Chemistry Chapter 5 Download in PDokument2 SeitenStates of Matter Notes Class 11 Chemistry Chapter 5 Download in PisaacNoch keine Bewertungen
- Chemistry Periodic Trends ActivityDokument6 SeitenChemistry Periodic Trends ActivityocNoch keine Bewertungen
- ACS Review: - Quick Refresher of Materials - Some Sample Questions and Short CutsDokument31 SeitenACS Review: - Quick Refresher of Materials - Some Sample Questions and Short Cutsjhhjjh100% (1)
- 1020-Lewis and Shapes PDFDokument4 Seiten1020-Lewis and Shapes PDFJaya Chitra Degala RamaluNoch keine Bewertungen
- Beginners Guide To StoichiometryDokument17 SeitenBeginners Guide To StoichiometryEric Moose Fortier100% (1)
- Lecture 02/unit II (Chemical Bonding) VSEPR TheoryDokument4 SeitenLecture 02/unit II (Chemical Bonding) VSEPR TheorySkyblueNoch keine Bewertungen
- Chapter 1. L1. Structure & BondingDokument35 SeitenChapter 1. L1. Structure & BondingMohammad Al-KhoderNoch keine Bewertungen
- Unit 5 The Mole Concept (S)Dokument24 SeitenUnit 5 The Mole Concept (S)Karm VeerNoch keine Bewertungen
- Alkene Practice QuestionDokument10 SeitenAlkene Practice Questionscsa31619Noch keine Bewertungen
- Unit 1 Periodic TableDokument44 SeitenUnit 1 Periodic TablePratik ParkaleNoch keine Bewertungen
- 15.2 Born-Haber Cycle: 15.2.2 Explain How The Relative Sizes and The Charges of IonsDokument16 Seiten15.2 Born-Haber Cycle: 15.2.2 Explain How The Relative Sizes and The Charges of IonsGiselle PeachNoch keine Bewertungen
- Day 2 - Introduction To Stoichiometry Guided Notes AssignmentDokument15 SeitenDay 2 - Introduction To Stoichiometry Guided Notes AssignmentDaveNoch keine Bewertungen
- Pearson Chemistry TeachingDokument51 SeitenPearson Chemistry TeachingZheng Joey100% (1)
- Electrochemistry 494 PDFDokument55 SeitenElectrochemistry 494 PDFHarsh SaxenaNoch keine Bewertungen
- 14 Lewis Structures and Molecuar Models S19Dokument14 Seiten14 Lewis Structures and Molecuar Models S19victorNoch keine Bewertungen
- SNC2D Chemistry Practice TestDokument8 SeitenSNC2D Chemistry Practice TestSteve M Hall0% (1)
- AP Chemistry 1998 Free ResponseDokument7 SeitenAP Chemistry 1998 Free Responsesabbate1994Noch keine Bewertungen
- Stoichiometry UnitDokument56 SeitenStoichiometry UnitCaiaphasNoch keine Bewertungen
- Mole Conversion ClassworkDokument4 SeitenMole Conversion ClassworkAdvanced PastryNoch keine Bewertungen
- U3 Oxidation and Reduction PPT WatermarkDokument45 SeitenU3 Oxidation and Reduction PPT Watermarkapi-125934329Noch keine Bewertungen
- Redox WKSHTDokument4 SeitenRedox WKSHTMarco ConopioNoch keine Bewertungen
- Regents Living Environment Practice Questions: New York Regents Living Environment Practice Questions with Detailed ExplanationsVon EverandRegents Living Environment Practice Questions: New York Regents Living Environment Practice Questions with Detailed ExplanationsNoch keine Bewertungen
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterVon EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterBewertung: 5 von 5 Sternen5/5 (1)
- Practice Makes Perfect in Chemistry: Chemical Bonding with AnswersVon EverandPractice Makes Perfect in Chemistry: Chemical Bonding with AnswersBewertung: 5 von 5 Sternen5/5 (1)
- Chapter 8 - 2019-2020 Molecular Geometry (General)Dokument64 SeitenChapter 8 - 2019-2020 Molecular Geometry (General)Patrick Alexander Putra CenggaNoch keine Bewertungen
- Isomerism Theory-MinDokument4 SeitenIsomerism Theory-MinrajaijahNoch keine Bewertungen
- Digestion and Absorption: Digestive System of HumanDokument4 SeitenDigestion and Absorption: Digestive System of HumanrajaijahNoch keine Bewertungen
- Cell MembraneDokument25 SeitenCell MembranerajaijahNoch keine Bewertungen
- PKT Bonding2 Student NotesDokument40 SeitenPKT Bonding2 Student NotesrajaijahNoch keine Bewertungen
- All Biology Values - CompressedDokument42 SeitenAll Biology Values - CompressedrajaijahNoch keine Bewertungen
- Lab 9 CHM 130LL Lewis Dot StructureDokument12 SeitenLab 9 CHM 130LL Lewis Dot StructurerajaijahNoch keine Bewertungen
- Nacl So Po MGBR Cao H O K O Cu-Zn Alloy O Cucl No Tio HF RB S Au-Ag Mixture Fe O C H ODokument1 SeiteNacl So Po MGBR Cao H O K O Cu-Zn Alloy O Cucl No Tio HF RB S Au-Ag Mixture Fe O C H OrajaijahNoch keine Bewertungen
- Ionic Bonds Packet 1 WeeblyDokument4 SeitenIonic Bonds Packet 1 WeeblyrajaijahNoch keine Bewertungen
- Ionic and Covalent Chemical Bonding WS enDokument4 SeitenIonic and Covalent Chemical Bonding WS enrajaijahNoch keine Bewertungen
- No N Non-Metal O Non-Metal Covalent Nacl So Po MGBR Cao H O K O Cu-Zn Alloy O Cucl No Tio HF RB S Au-Ag Mixture Fe O C H ODokument2 SeitenNo N Non-Metal O Non-Metal Covalent Nacl So Po MGBR Cao H O K O Cu-Zn Alloy O Cucl No Tio HF RB S Au-Ag Mixture Fe O C H OrajaijahNoch keine Bewertungen
- Ionic Bonds Packet 1 WeeblyDokument4 SeitenIonic Bonds Packet 1 WeeblyrajaijahNoch keine Bewertungen
- Come Together ChartDokument1 SeiteCome Together ChartrajaijahNoch keine Bewertungen
- EqDokument3 SeitenEqrajaijahNoch keine Bewertungen
- CH 6: Bonding Packet Study Guide: Name - Chemistry Mr. HarperDokument13 SeitenCH 6: Bonding Packet Study Guide: Name - Chemistry Mr. HarperrajaijahNoch keine Bewertungen
- Nacl So Po MGBR Cao H O K O Cu-Zn Alloy O Cucl No Tio HF RB S Au-Ag Mixture Fe O C H ODokument1 SeiteNacl So Po MGBR Cao H O K O Cu-Zn Alloy O Cucl No Tio HF RB S Au-Ag Mixture Fe O C H OrajaijahNoch keine Bewertungen
- Chemical Bonding WebquestDokument3 SeitenChemical Bonding Webquestrajaijah33% (3)
- Chemical Compounds Ionic and Covalent BondsDokument4 SeitenChemical Compounds Ionic and Covalent BondsrajaijahNoch keine Bewertungen
- .Ws Ionic Bonding Activity KeyDokument4 Seiten.Ws Ionic Bonding Activity KeyrajaijahNoch keine Bewertungen
- Ionic BondsDokument3 SeitenIonic BondsrajaijahNoch keine Bewertungen
- Organic Isomers Multiple Choice QuestionsDokument3 SeitenOrganic Isomers Multiple Choice QuestionsrajaijahNoch keine Bewertungen
- CFT IiDokument19 SeitenCFT IiTY OrganicNoch keine Bewertungen
- Color de Complejos de CromoDokument18 SeitenColor de Complejos de CromoFelipe VilchesNoch keine Bewertungen
- Organic Chemistry Structure and BondingDokument13 SeitenOrganic Chemistry Structure and BondingHossNoch keine Bewertungen
- The Electronic Structures of Homonuclear Diatomic MoleculesDokument13 SeitenThe Electronic Structures of Homonuclear Diatomic MoleculesSiskaWahyuniNoch keine Bewertungen
- Conjugation in Organic Group 14 Element Compounds: Design, Synthesis and Experimental EvaluationDokument72 SeitenConjugation in Organic Group 14 Element Compounds: Design, Synthesis and Experimental EvaluationYash TandonNoch keine Bewertungen
- Presentation Full CHM433 2015Dokument168 SeitenPresentation Full CHM433 2015Selvaraju ChellappanNoch keine Bewertungen
- Experiment Report: Spectrophotometric Analysis of Caffeine and Benzoic Acid in Soft DrinkDokument12 SeitenExperiment Report: Spectrophotometric Analysis of Caffeine and Benzoic Acid in Soft DrinkNitty MeYaNoch keine Bewertungen
- Lars Medice: Activity 1Dokument31 SeitenLars Medice: Activity 1Kate MagpayoNoch keine Bewertungen
- RAMAN Chapter 18 Part 11Dokument50 SeitenRAMAN Chapter 18 Part 11Nasroedien Fikry100% (1)
- Physical Science - Module 5 (Assignment)Dokument1 SeitePhysical Science - Module 5 (Assignment)CharlesNoch keine Bewertungen
- 407 ChemistryDokument2 Seiten407 ChemistrybholuNoch keine Bewertungen
- Atomic Spectroscopy: Theory of Atomic Absorption Spectrometry (AAS)Dokument20 SeitenAtomic Spectroscopy: Theory of Atomic Absorption Spectrometry (AAS)Nandini KapoorNoch keine Bewertungen
- Bonding BB3Dokument4 SeitenBonding BB3DeveshNoch keine Bewertungen
- inthinIN TH T12Dokument7 SeiteninthinIN TH T12ANTONIOSNoch keine Bewertungen
- 7.2 Molecular ShapeDokument13 Seiten7.2 Molecular ShapeNURBALQIS BINTI ZAILAN KMNSNoch keine Bewertungen
- Theoretical Study of Hydrogen Bond Formation in Trimethylene Glycol-Water ComplexDokument13 SeitenTheoretical Study of Hydrogen Bond Formation in Trimethylene Glycol-Water ComplexChern YuanNoch keine Bewertungen
- Modern Methods of Pharmaceutical AnalysisDokument12 SeitenModern Methods of Pharmaceutical AnalysisJafrineNoch keine Bewertungen
- Characterization of NanoparticlesDokument16 SeitenCharacterization of NanoparticlesPrasun SarkarNoch keine Bewertungen
- Chemical Bonding - Part 2Dokument2 SeitenChemical Bonding - Part 2Om TipsetwarNoch keine Bewertungen
- Chemistry The Molecular Nature of Matter and Change 7th Edition Silberberg Test BankDokument25 SeitenChemistry The Molecular Nature of Matter and Change 7th Edition Silberberg Test Bankjenniferrichardsonjrwfpzsdim100% (27)
- Chemsheets As 1009 (Electron Arrangement)Dokument31 SeitenChemsheets As 1009 (Electron Arrangement)Rishabh MathurNoch keine Bewertungen
- Physical and Chemical Properties of WaterDokument17 SeitenPhysical and Chemical Properties of Watershubham debNoch keine Bewertungen
- LC MsDokument24 SeitenLC MsVshn VardhnNoch keine Bewertungen
- KBRDokument81 SeitenKBRRobby FirdausNoch keine Bewertungen
- Chemistry PYQ With SyllabusDokument8 SeitenChemistry PYQ With Syllabusanushkasingh0dtoNoch keine Bewertungen
- General Chemistry (09-210-034) : Acids and Bases #1Dokument16 SeitenGeneral Chemistry (09-210-034) : Acids and Bases #1kms jodie lazuardi haickalNoch keine Bewertungen
- Factor Affecting Proton NMRDokument6 SeitenFactor Affecting Proton NMRPrashantNoch keine Bewertungen
- Chemistry: Proton (H) NMR SpectrosDokument16 SeitenChemistry: Proton (H) NMR SpectrosSatyaki MajumdarNoch keine Bewertungen
- As AnorganicaDokument234 SeitenAs AnorganicaLoredana OlariuNoch keine Bewertungen
- Formation of Ionic BondingDokument1 SeiteFormation of Ionic BondingRevely DomdomNoch keine Bewertungen