Beruflich Dokumente
Kultur Dokumente
CH 321 Schedule 04fall
Hochgeladen von
Nadherdaman Alshamary0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
20 Ansichten1 Seitechemistry
Originaltitel
CH 321 Schedule 04Fall
Copyright
© © All Rights Reserved
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenchemistry
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
20 Ansichten1 SeiteCH 321 Schedule 04fall
Hochgeladen von
Nadherdaman Alshamarychemistry
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 1
Analytical Chemistry Lab Schedule
CH 321, Fall 2004, Dr. Schmidt
8/19 Check-in, etc.
Check-in, syllabus, schedule, etc.
Wash Glassware
Read Chapter 2 in textbook
8/26 Calibration of Glassware
Perform Experiment 1
Calibrate all Volumetric Glassware in Your Drawer
9/2 Penny Statistics
Turn-in Analysis Report for Experiment 1
Perform Experiment 4 Penny Statistics
Start Experiment 2 Gravimetric Determination of Ca as CaC2O4H2O
9/9 Gravimetric Determination of Calcium as CaC2O4H2O
Turn-in Analysis Report for Experiment 4
Complete Experiment 2 Gravimetric Determination of Ca as CaC2O4H2O
9/16 Gravimetric Determination of Fe as Fe2O3
Turn-in Analysis Report for Experiment 2
Start Experiment 3 Gravimetric Determination of Fe as Fe2O3
9/23 Gravimetric Determination of Fe as Fe2O3
Start Experiment 3 Gravimetric Determination of Fe as Fe2O3
Start Acid / Base Determinations (see list 9/30)
9/30 Acid / Base Determinations
Turn-in Analysis Report for Experiment 3
Acid / Base Determinations to be completed by 10/14 are
Standardize 0.1 M HCl and 0.1 M NaOH (Experiment 6)
Determine the % composition of a Sodium carbonate or Potassium hydrogenphthalate unknown (Exp. 6)
Determine the molar mass and pKa(s) for an Unknown acid or base by potentiometric titration (Experiment 7)
Analyze a Mixture of Carbonate and Bicarbonate (Experiment 8)
10/7 Acid / Base Determinations
Continue work on Acid / Base Determinations
10/14 Acid / Base Determinations
Complete work on Acid / Base Determinations
10/21 Fall Break -- No Class
10/28 EDTA titration of Ca2+ and Mg2+ in Natural Waters
Turn-in Analysis Report for Experiments 6, 7, and 8 (three separate reports)
Start Experiment 11
Students will analyze a sample of the water provided as well as a sample of an environmental water sample.
Students are to collect their own environmental sample for analysis. Be sure to document the collection of the
sample.
11/4 EDTA titration of Ca2+ and Mg2+ in Natural Waters
Complete Experiment 11
11/11 Spectrophotometric Determination of Iron
Turn-in Analysis Report for Experiment 11
Start Experiments 19 and 20
Students are to supply their own food for analysis. Each student must analyze a different food.
11/18 Spectrophotometric Determination of Iron
Complete Experiments 19 and 20
11/25 Thanksgiving Break -- No Class
12/2 Cleanup day
Turn-in Analysis Report for Experiments 19 and 20 (two separate reports)
Clean-up and Check-out
Das könnte Ihnen auch gefallen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5795)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1091)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Voice Feminization Therapy and Quality of Life in Transgender Women A Critical Review and Case StudyDokument8 SeitenVoice Feminization Therapy and Quality of Life in Transgender Women A Critical Review and Case Studyjreljosh1994Noch keine Bewertungen
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Alexander Technique A Systematic Review of Controled TrialsDokument11 SeitenAlexander Technique A Systematic Review of Controled TrialsAna Rita PintoNoch keine Bewertungen
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Business Analytics: Methods, Models, and Decisions: Descriptive StatisticsDokument100 SeitenBusiness Analytics: Methods, Models, and Decisions: Descriptive Statisticsbekas bagusNoch keine Bewertungen
- E10-Q3 Melc 3 Las-2Dokument7 SeitenE10-Q3 Melc 3 Las-2Leo EvidorNoch keine Bewertungen
- Market Research and Competitor Analysis of Online Food PortalDokument67 SeitenMarket Research and Competitor Analysis of Online Food Portalgaurav dadhich40% (10)
- Chapter Three - Structure-Property Relationships at The NanoscaleDokument10 SeitenChapter Three - Structure-Property Relationships at The NanoscaleNadherdaman AlshamaryNoch keine Bewertungen
- Experiment (11) Determination of Calcium As Oxalate: TheoryDokument3 SeitenExperiment (11) Determination of Calcium As Oxalate: TheoryNadherdaman AlshamaryNoch keine Bewertungen
- Synthesis of A New Three Dimensional Network Co Polymer and Studying The Ability of Drug Delivery SystemDokument7 SeitenSynthesis of A New Three Dimensional Network Co Polymer and Studying The Ability of Drug Delivery SystemNadherdaman AlshamaryNoch keine Bewertungen
- Double Headed Arrows Indicates Reversible Reactions. 2-The Angle Between SP Hybrid Orbitals Is 109.5Dokument1 SeiteDouble Headed Arrows Indicates Reversible Reactions. 2-The Angle Between SP Hybrid Orbitals Is 109.5Nadherdaman AlshamaryNoch keine Bewertungen
- Answer Sheet (Chapter 3) : The Islamic University Student Name: KEY Chemistry Department CHEMA1301 Student No.Dokument1 SeiteAnswer Sheet (Chapter 3) : The Islamic University Student Name: KEY Chemistry Department CHEMA1301 Student No.Nadherdaman AlshamaryNoch keine Bewertungen
- MCQ ChemistryDokument7 SeitenMCQ ChemistryNadherdaman Alshamary0% (1)
- Lec No 1 Electrochemistry An IntroductionDokument7 SeitenLec No 1 Electrochemistry An IntroductionNadherdaman AlshamaryNoch keine Bewertungen
- 11 Chemistry Exemplar Chapter 13 AnswerDokument5 Seiten11 Chemistry Exemplar Chapter 13 AnswerNadherdaman AlshamaryNoch keine Bewertungen
- Applications of Kohlrausch LawDokument6 SeitenApplications of Kohlrausch LawNadherdaman Alshamary100% (4)
- Expt 1 (Solubility of Organic Compounds) PDFDokument3 SeitenExpt 1 (Solubility of Organic Compounds) PDFanon_253019003Noch keine Bewertungen
- Identity and Purity of Solid Organic CompoundsDokument8 SeitenIdentity and Purity of Solid Organic CompoundsNadherdaman AlshamaryNoch keine Bewertungen
- Practical Organic Chemistry Lab: Name: Class: Group: Name of Experiment: Date: The Purpose of This ExperimentDokument3 SeitenPractical Organic Chemistry Lab: Name: Class: Group: Name of Experiment: Date: The Purpose of This ExperimentNadherdaman AlshamaryNoch keine Bewertungen
- Determination of Melting PointsDokument3 SeitenDetermination of Melting PointsNadherdaman AlshamaryNoch keine Bewertungen
- 201 Bonding For C N ODokument1 Seite201 Bonding For C N ONadherdaman AlshamaryNoch keine Bewertungen
- Figure 5a. Vapor Pressure-Temperature Diagram. Figure 5b. Temperature-Composition DiagramDokument1 SeiteFigure 5a. Vapor Pressure-Temperature Diagram. Figure 5b. Temperature-Composition DiagramNadherdaman AlshamaryNoch keine Bewertungen
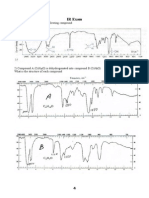
- IR Exam: 1) Find The Structure of The Following CompoundDokument1 SeiteIR Exam: 1) Find The Structure of The Following CompoundNadherdaman AlshamaryNoch keine Bewertungen
- Thesis EgosDokument48 SeitenThesis EgosJamie HaravataNoch keine Bewertungen
- GlossaryDokument31 SeitenGlossaryzubairulhassanNoch keine Bewertungen
- VE For Green BuildingDokument11 SeitenVE For Green Buildinganthony csNoch keine Bewertungen
- Competitive Intelligence Systems: Qualitative DSS For Strategic Decision MakingDokument15 SeitenCompetitive Intelligence Systems: Qualitative DSS For Strategic Decision Makingمهنوش جوادی پورفرNoch keine Bewertungen
- Working Papers: Policy Failure, Political Constraints and Political Resources: Basic Education in Pakistan Haris GazdarDokument57 SeitenWorking Papers: Policy Failure, Political Constraints and Political Resources: Basic Education in Pakistan Haris Gazdarcooll7553950Noch keine Bewertungen
- Continuous Random VariableDokument19 SeitenContinuous Random VariableMark Niño JavierNoch keine Bewertungen
- BSBA Enhancement of Façade Using Brand Management PrincipleDokument63 SeitenBSBA Enhancement of Façade Using Brand Management PrincipleFEPC StudentNoch keine Bewertungen
- Bba 218 Business ResearchDokument351 SeitenBba 218 Business ResearchHamza KhalidNoch keine Bewertungen
- 1413 3555 Rbfis Nahead - 0562013Dokument12 Seiten1413 3555 Rbfis Nahead - 0562013Isabella DanteNoch keine Bewertungen
- Ch. 10 Principal Components Analysis (PCA)Dokument17 SeitenCh. 10 Principal Components Analysis (PCA)José António PereiraNoch keine Bewertungen
- Chapter 1 QuizDokument3 SeitenChapter 1 QuizbuilugawNoch keine Bewertungen
- Ball Mill Inspection - Chap 2Dokument5 SeitenBall Mill Inspection - Chap 2Min MCLNoch keine Bewertungen
- 5) Information LiteracyDokument90 Seiten5) Information LiteracyanisNoch keine Bewertungen
- Probability & Statistics Syllabus GTU 3rd Sem CEDokument2 SeitenProbability & Statistics Syllabus GTU 3rd Sem CEPinak Vadher100% (1)
- Evaluating The Usability of Educational Websites Based On Students' Preferences of Design CharacteristicsDokument16 SeitenEvaluating The Usability of Educational Websites Based On Students' Preferences of Design CharacteristicsszarNoch keine Bewertungen
- Case Study On Working From HomeDokument10 SeitenCase Study On Working From HomeHimanshu Bhatia100% (1)
- Model LinearDokument33 SeitenModel LinearIvan AliagaNoch keine Bewertungen
- A Map of Threats To Validity of Systematic Literature Reviews in Software EngineeringDokument8 SeitenA Map of Threats To Validity of Systematic Literature Reviews in Software EngineeringJonnathan RiquelmoNoch keine Bewertungen
- Quarter K-12 Learning Competencies MelcsDokument4 SeitenQuarter K-12 Learning Competencies MelcstitserscradleNoch keine Bewertungen
- Review Jurnal Internasional Restoran Ayam Penyet RiaDokument3 SeitenReview Jurnal Internasional Restoran Ayam Penyet RiaZain RivaldhyNoch keine Bewertungen
- Ogilvy's 360 Digital Influence's Conversation Impact Model For Social Media MeasurementDokument8 SeitenOgilvy's 360 Digital Influence's Conversation Impact Model For Social Media MeasurementirfankamalNoch keine Bewertungen
- Communication Patterns in Massively Open Online CoursesDokument9 SeitenCommunication Patterns in Massively Open Online CoursesTung NghiaNoch keine Bewertungen
- A Comparative Review of Road Safety Audit GuidelinDokument8 SeitenA Comparative Review of Road Safety Audit GuidelinSawaluddin SawalNoch keine Bewertungen
- 2amanat Chaudhry - Gas Well Testing Handbook-Gulf Professional Publishing (2003) - 2Dokument6 Seiten2amanat Chaudhry - Gas Well Testing Handbook-Gulf Professional Publishing (2003) - 2TuralNoch keine Bewertungen
- 011 RFP Data Collection in Jordan 1Dokument15 Seiten011 RFP Data Collection in Jordan 1julianNoch keine Bewertungen