Beruflich Dokumente
Kultur Dokumente
Revues - Voluntad 2
Hochgeladen von
Victor CarrenoOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Revues - Voluntad 2
Hochgeladen von
Victor CarrenoCopyright:
Verfügbare Formate
Neuron
Perspective
Context Processing and the Neurobiology
of Post-Traumatic Stress Disorder
Israel Liberzon1,2,* and James L. Abelson1
1University of Michigan, Department of Psychiatry, Ann Arbor, MI 48109-2700, USA
2Mental Health Service, Veterans Affairs Ann Arbor Health System, Ann Arbor, MI 48105, USA
*Correspondence: liberzon@umich.edu
http://dx.doi.org/10.1016/j.neuron.2016.09.039
Progress in clinical and affective neuroscience is redefining psychiatric illness as symptomatic expression of
cellular/molecular dysfunctions in specific brain circuits. Post-traumatic stress disorder (PTSD) has been an
exemplar of this progress, with improved understanding of neurobiological systems subserving fear learning,
salience detection, and emotion regulation explaining much of its phenomenology and neurobiology. How-
ever, many features remain unexplained and a parsimonious model that more fully accounts for symptoms
and the core neurobiology remains elusive. Contextual processing is a key modulatory function of hippocam-
pal-prefrontal-thalamic circuitry, allowing organisms to disambiguate cues and derive situation-specific
meaning from the world. We propose that dysregulation within this context-processing circuit is at the
core of PTSD pathophysiology, accounting for much of its phenomenology and most of its biological find-
ings. Understanding core mechanisms like this, and their underlying neural circuits, will sharpen diagnostic
precision and understanding of risk factors, enhancing our ability to develop preventive and personalized
interventions.
Introduction intensively in recent decades; but despite important break-
Historical Perspective on the Evolution of the PTSD throughs, we still lack a comprehensive, integrated model that
Concept coherently and parsimoniously explains both its phenomenology
The past two decades have witnessed a fundamental transfor- and its neurobiology. In this Perspective, we will describe PTSD
mation in our understanding and conceptualization of psycho- as a clinical entity and touch on its well-established neurobiolog-
pathology. Cultural, clinical, and scientific perspectives increas- ical findings. We will then try to organize emerging findings within
ingly converge on a view of psychiatric disorders as dysfunction existing conceptual frameworks, highlighting the value and limi-
within specific components of the CNS, rather than functional tations of those frameworks. Finally, we will describe a new
disorders unrelated to neurobiological substrates. This shift has framework, focused on the neural circuitry of context process-
forced theoretical approaches to grapple with the biological un- ing, which adds additional explanatory power and can potentially
derpinning of psychological processes, grounding our models of better integrate more of what we currently know about this
illness in growing understanding of neural circuits, cellular mech- important disorder.
anisms and molecular processes. This conceptual shift has been PTSD as a Clinical Phenomenon
challenging but also transformational in the study of post-trau- In the absence of scientific understanding of the pathophysio-
matic stress disorder (PTSD), which has seemed like a quintes- logic processes involved in PTSD development, the gold stan-
sentially psychological illness because it develops in reaction dard for defining it has been expert consensus as reflected in
to specific environmental events. DSM criteria. These criteria have changed as clinical perspec-
The deleterious effects of severe trauma on the human psyche tives and scientific understanding have evolved, but they have
have been documented for centuries, generally depicted as remained phenomenological at their core since they are not yet
normative responses to extraordinary circumstances. During grounded in biological processes and CNS neuro-circuitry.
the 19th and early 20th century, with emergence of psychiatry Despite periodic and sometimes substantial changes, there
and psychology as clinical disciplines, the more debilitating has been consistent agreement on three sets of symptom clus-
consequences of trauma were re-conceptualized as clinical ters considered characteristic of PTSDthe intrusive, avoidant,
conditions, amenable to scientific inquiry and treatment (Jones, and hyperarousal clusters. These encompass unwanted and
2006). Conceptual challenges persisted, however, in some- repeatedly re-experienced memories, sensations, or dreams
times controversial efforts to differentiate true psychopathology associated with the trauma (intrusive cluster); behavioral avoid-
from normative responses to extreme circumstances. Resolu- ance of trauma reminders; and excessive physiological arousal
tion of these controversies awaits empirical documentation of both in response to trauma cues or independently (e.g., sleep
causative neurobiological pathways, which will facilitate an problems). These core clusters have been embraced in DSM
understanding of how brain-based vulnerabilities lead to dysre- III, IV, and 5, but other symptoms or criteria have been added
gulated psychological responses, and emergence of disorder- (negative affect or reckless behavior in DSM-5), deleted (trauma
specific neurobiological abnormalities. PTSD has been studied outside of usual human experience), or included descriptively
14 Neuron 92, October 5, 2016 Published by Elsevier Inc.
Neuron
Perspective
(shame/guilt, dissociation). Shifting criteria create scientific chal- endocannabinoids (Wilker et al., 2016), and endogenous opioids
lenges to sample homogeneity across time and undermine clin- (van der Kolk et al., 1989). Genetic work is rapidly expanding and
ical efficacy when targets for intervention are imprecise and has identified PTSD-linked polymorphisms in genes encoding
continuously moving. As clinician scientists, we will do better elements within the HPA axis (FKBP5 [Binder et al., 2008] and
when we can target precisely defined dysregulations within spe- CRHR1 [Amstadter et al., 2011; White et al., 2013; Etkin et al.,
cific neural circuits sub-serving particular, mechanistically rele- 2015]), and the sympatho-adrenal system (ADRB2 [Liberzon
vant brain functions. As we identify underlying mechanisms et al., 2014] and COMT [Boscarino et al., 2011]). These require
rooted in specific neural circuits, diagnostic precision will confirmation in large-scale GWAS studies but are consistent
sharpen and our ability to personalize interventions will be with the peripheral findings and suggest that PTSD is character-
enhanced. For PTSD, we will also be able to go beyond treat- ized by ANS hypersensitivity, associated with or leading to
ment and apply preventive interventions before or immediately hyperadrenergic states, and a hypersensitive glucocorticoid re-
after trauma exposure. ceptor system. However, potential linkages between these ab-
PTSD PathophysiologyEarly Studies normalities and the central mechanisms that drive them remain
Prior to development of in vivo functional neuroimaging and unknown.
valid animal models, allowing direct examination of CNS struc- PTSD Neurocircuits
ture and function, biological study of PTSD focused on identifi- The development of in vivo neuroimaging methodologies and
cation of abnormal peripheral markers. The best replicated of emergence of affective neuroscience have shifted the focus of
these have been psychophysiological indices of autonomic ner- contemporary neuropsychiatric inquiry to specific neural circuits
vous system (ANS) function, and altered blood, saliva, and urine that might govern the development of PTSD. Converging evi-
concentration of stress hormones and catecholamines. Norad- dence from human imaging studies and animal models of com-
renergic hyper-reactivity was the most reliably replicated finding plex behaviors like fear-associated learning has generated
in PTSD (Pitman et al., 2012), along with altered function of the powerful, neurocircuitry-based models of its pathophysiology.
hypothalamic-pituitary adrenal (HPA) axis, where hypersensitiv- In the following sections, we selectively review the evolving pic-
ity of glucocorticoid receptors was accompanied by unaltered or ture, organizing it within dominant emergent models, identified
decreased levels of circulating cortisol (Yehuda, 2001). as (1) an abnormal fear learning model (FL), which encompasses
ANS abnormalities have been seen at rest and in reaction to altered fear conditioning, fear extinction, and fear generalization
trauma cue exposure (Agorastos et al., 2013; Cohen et al., hypotheses; (2) an exaggerated threat detection model (TD),
2000; Lee and Theus, 2012; Minassian et al., 2014; Shah et al., which postulates altered attention, anticipation, or alarm func-
2013) (for meta-analysis, see Pole, 2007). Heart rate variability tions; and (3) a diminished emotional regulation/executive func-
(HRV) is a sensitive index of ANS function (Bilchick and Berger, tion (ER/EF) model that postulates deficient regulatory capacity
2006; Malik and Camm, 1995) and reductions in itwhich sug- of cognitive/executive function regions over emotion-generating
gest arousal dysregulation and autonomic inflexibility due to limbic structures. These models are distinct in their focus on
sympathetic overdrive and/or parasympathetic insufficiency different aspects of PTSD psychopathology and different brain
(Shah et al., 2013; Cohen et al., 2000)predict PTSD develop- circuits and may be driven by different cellular and molecular
ment whether measured prior to (Minassian et al., 2015) or imme- processes. However, they are not necessarily mutually exclu-
diately after trauma exposure (Shaikh al arab et al., 2012). PTSD sive. Each may explain different aspects of the disorder and a
is also associated with exaggerated catecholamine responses to different subset of its biological abnormalities. We then identify
trauma cues (Liberzon et al., 1999a) and elevated levels of NE important aspects of PTSD that are not explained by these
secretion in 24 hr urine collections (Wingenfeld et al., 2015). models. Together, they can explain much, but they invoke at
PTSD may thus reflect a hyper-adrenergic state (Krystal and least three independent pathophysiological processes while
Neumeister, 2009). Dysregulation is also evident in HPA axis leaving some key symptoms and findings unexplained. This is
function, but this appears more complex. The more consistent not very parsimonious or conceptually satisfying. It is possible,
abnormalities involve enhanced dexamethasone suppression or even likely, that PTSD is not a single homogeneous disorder
of cortisol (Yehuda et al., 1993), often attributed to glucocorticoid but rather is a syndrome that houses within it a number of
(GC) receptor hyper-sensitivity (Yehuda, 2001). This is supported sub-categories with somewhat different presentations, endo-
by in vivo assay evidence of GC receptor hyper-sensitivity on phenotypes, and pathophysiological mechanisms. Neverthe-
lymphocytes (Yehuda et al., 1995a). Measurement of cortisol less, the fact that important parts remain unexplained suggests
levels in PTSD has generally suggested normal (Baker et al., a need for additional, alternative, or more mechanistically expan-
1999) or reduced levels (Yehuda et al., 1990, 1995b, 1996), sive models, which might parsimoniously explain both the com-
though there are some inconsistent reports (Meewisse et al., plex symptomatology of PTSD and its existing neurobiological
2007; Lindley et al., 2004; Pitman and Orr, 1990). Reduced levels findings. Following discussion of the FL, TD, and ER/EF models,
would be consistent with GC receptor hypersensitivity and early we present a novel model that is both more general and more
HPA axis shutdown. parsimonious, explaining multiple, not previously explained
Additional peripheral biological abnormalities linked to PTSD symptoms, and integrating neurobiological findings within the
require replication but are consistent with the changes reported framework of a focal pathophysiologic processaltered contex-
above. These include alterations in serotonin systems (South- tual processing (CP). The CP model focuses our attention on
wick et al., 1999), DHEA (Yehuda et al., 2006), neuropeptide dysfunction within specific brain circuits governing the critical
Y (Rasmusson et al., 2000), neurosteroids (Morris et al., 2012), adaptive function of contextualizationinvolving hippocampus
Neuron 92, October 5, 2016 15
Neuron
Perspective
framework, these processes and regions have been seen
through the prism of their impact on amygdala function and
output, rather than as key psychological/pathophysiologic pro-
cesses shaping PTSD development. Other conceptual models
(Threat Detection, Emotional Regulation, and Contextual Pro-
cessing) focus on these extra-amygdala processes as key con-
tributors to PTSD pathophysiology, and they are discussed in
subsequent sections.
Fear Conditioning/Acquisition. The basic circuits of fear con-
ditioning/extinction have been well-worked out. They involve
perceptual inputs to thalamus, then to amygdala and to effector
systems (LeDoux et al., 1990). Within the amygdala, the baso-
lateral complex (BLC), composed of the lateral (LA), basolateral
(BLA), and accessory basal (AB) nuclei, provides the main input
to the central nucleus (CE), which constitutes the final relay in
coordinated outputs to physiological and behavioral effector
systems that express fear (e.g., Campeau and Davis, 1995).
The BLC is the region where associative fear learning, extinc-
tion learning, consolidation, and expression are thought to
take place (Davis et al., 1994; Fanselow and LeDoux, 1999; Da-
vis, 1992). The cellular substrates for associative learning are
long-term potentiation (LTP) and long-term depression (LTD)
(Eichenbaum, 1996; Matynia et al., 2002), whereby associative
LTP at BLC principal neurons (Blair et al., 2001; Goosens and
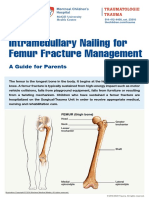
Figure 1. Neural Circuits within the Basolateral Complex of the
Amygdala
Maren, 2002) creates a neural link between an unconditioned
Altered functioning within basolateral complex (BLC) circuits has been impli- stimulus (US) and a conditioned stimulus (CS). The phenome-
cated in the Abnormal Fear Learning model of PTSD (see text). Interrupted nology of PTSD readily suggests disruption in this process,
lines represent distal inputs to and outputs from BLC. since so many CSs (e.g., a backfiring car) seem to be inap-
propriately linked to the sense of threat carried by traumatic
(Hpc) prefrontal cortex (PFC), thalamic circuits that modulate ac- memories. However, BLC sub-networks also have specific
tivity in amygdala, and other limbic and cortical regions. Prior connections with prefrontal cortical regions that modulate fear
work has identified clear abnormalities in PTSD in the Hpc, expression, including prelimbic and infralimbic areas. Specific
PFC, and amygdala, and we suggest that dysfunction within BLC neurons can activate prelimbic PFC during fear learning,
this circuit may be a key pathophysiologic process underlying while others target infralimbic PFC during extinction (Senn
expression of various PTSD symptoms, in a way that may be et al., 2014). The BLC can thus provide higher level processors
able to integrate the contributions of the altered noradrenergic with information about what is dangerous and what is safe,
and glucocorticoid functions that have been so consistently potentially for use in future situations that are ambiguous but
documented in this disorder. carry threat potential.
Though intuitively appealing, evidence that PTSD is a disorder
of fear conditioning itself is sparse. Trauma exposure itself is
Models of Pathophysiology readily thought of as an explicit conditioning episode (Elzinga
Abnormal Fear Learning and Bremner, 2002; Jovanovic and Ressler, 2010; VanElzakker
Learning what is threatening and what is safe is a critical, highly et al., 2014), but evidence for abnormalities in fear acquisition
conserved function that has been extensively studied neuro- in PTSD is conflicting. In typical differential fear conditioning par-
biologically using fear associated learning paradigms and fear adigms, where subjects are presented with a US-predicting CS
conditioning, fear extinction, and fear renewal, processes. (CS+) and a non-US-predicting CS (CS ), which acts as a safety
PTSD has been conceptualized as heightened fear reactivity signal (Lissek et al., 2005), PTSD patients have difficulty differen-
that develops in response to exposure to a threatening event, tiating safety from threat (Grillon et al., 1998; Grillon, 2002), but
so abnormal fear learning was a logical initial place to look in they generally do not show abnormal acquisition of the condi-
searching for its neurobiology. Fear and safety learning studies tioned response (to CS+) itself (Garfinkel et al., 2014; Milad
have focused mechanistically on a key structure governing these et al., 2009). Enhanced fear potentiated startle or skin conduc-
processesthe amygdalaand the complex interplay within tance responses have been reported during fear learning by
the amygdaloid complex (Figure 1) between various nuclei some (Morgan et al., 1995; Orr et al., 2000; Peri et al., 2000),
and cell types. Additional processes clearly participate in fear/ but not others (Blechert et al., 2007; Milad et al., 2008; Milad
safety learning, like memory consolidation, fear generalization, et al., 2009). Trauma that can cause PTSD is much more severe
and extinction retention, and these involve additional brain than the simple US threats used in laboratory studies, but
mechanisms and regions (e.g., sensory cortex, thalamus, hippo- these studies do suggest that the basic ability to acquire new
campus, vmPFC). However, within the fear-associated learning fear conditioned responses is not inherently abnormal in PTSD.
16 Neuron 92, October 5, 2016
Neuron
Perspective
Fear Extinction. Alternatively, it has been proposed that fear intensity of the US impacts the breadth of generalization beyond
associative learning deficits in PTSD stem from abnormalities the CS+ (Baldi et al., 2004; Ghosh and Chattarji, 2015). A very
in extinction or safety learning rather than fear conditioning. A strong US produces broad generalization. During PTSD-genera-
key feature of PTSD is inability to learn that cues once associated tive traumatic exposure, the US (e.g., threat to life) may be suffi-
with trauma are no longer dangerousa process akin to fear cient to lead to broad generalization, undermining discrimination
extinction in animal models. Dysregulation of extinction, under- between dangerous and safe cues, contributing to high reactivity
mining ability to learn that something once dangerous is now to reminders that bear any resemblance to trauma cues.
safe, may contribute to safety learning deficits in PTSD (Jova- Neurobiological studies of generalization in fear conditioning
novic et al., 2012). In general, extinction is less robust than con- paradigms have been sparse (Likhtik and Paz, 2015). Typical
ditioning and does not erase an established fear memory. fear conditioning work uses easily discriminable stimuli to ensure
Rather, it creates a safety memory trace that overlays the strong CS+ versus CS differentiation, avoiding potential for
fear memory trace. It is context specific, decays with time, and generalization (Genud-Gabai et al., 2013). Generalization studies
involves learned inhibition of fear expression. It is mediated by demonstrate neuronally instantiated tuning curves that differ-
distinct and dedicated pathways within BLC (Jasnow et al., entiate tones of differing frequencies within auditory cortex,
2013). Dysregulation in the topographically organized BLC auditory thalamus, and amygdala (Bordi and LeDoux, 1992,
output systems may induce imbalances within the larger fear- 1994; Resnik and Paz, 2015). Multiple amygdalar regions are
on and extinction circuits, leading to extinction deficits and involved, including CE (Ciocchi et al., 2010), other subnuclei
persistence of fear memories. Indeed, a number of fear extinc- (Shaban et al., 2006), and LA (Ghosh and Chattarji, 2015). The
tion studies report heightened autonomic reactions during amygdala appears to play a role in generating graded generaliza-
extinction learning in PTSD, including increased skin conduc- tion curves (Schechtman et al., 2010; Resnik et al., 2011; Laufer
tance and heart rate and sustained fear-potentiated startle (Orr and Paz, 2012), and amygdala neurons show tuning properties
et al., 2000; Peri et al., 2000; Blechert et al., 2007; Norrholm that could account for behavioral generalization (Resnik and
et al., 2011). Paz, 2015), perhaps contributing to the broad fear generalization
Problems with safety signal learning and inability to modulate gradients suggested for PTSD. However, amygdala changes in
fear responses with safety cues (Jovanovic et al., 2009, 2012) these studies could be due to inputs coming from other brain re-
also suggest impairment in inhibition of fear pathways. However, gions. For example, there is conflicting evidence about the role of
other work suggests intact extinction learning in PTSD but auditory cortex in stimulus discrimination and in overgeneraliza-
impaired recall of fear extinction memories 24 hr later (Garfinkel tion (Aizenberg and Geffen, 2013; Letzkus et al., 2011). Complex
et al., 2014; Milad et al., 2009). Intact extinction recall, however, interconnections make it difficult to isolate specific behavioral
requires cortical inputs from vmPFC to amygdala (Quirk and Mu- dysfunction, like overgeneralization, to disruptions at highly spe-
eller, 2008), and intact safety learning after fear conditioning in- cific locations, and the complete circuit that mediates general-
volves inputs from vmPFC and hippocampus (Corcoran and ization of more complex real-life stimuli might involve interac-
Quirk, 2007). Prefrontal regions have well-traced inputs to the tions between microcircuits within the amygdala (Duvarci and
amygdala through which they can amplify (prelimibic) or inhibit Pare, 2014), the prefrontal-cortex (Likhtik et al., 2014; Klavir
(infralimbic) fear expression (Sierra-Mercado et al., 2011). The et al., 2013; Chavez et al., 2009; Dunsmoor et al., 2011; Courtin
human homolog of IL (vmPFC) is linked to extinction recall and et al., 2014), hippocampus (Xu and Sudhof, 2013; Bergado-
is smaller in volume and hypo-responsive in PTSD patients Acosta et al., 2008; Kaouane et al., 2012), and other regions.
(Bremner et al., 2008). Reduced activation of this area is Neuroimaging work has begun to examine the human neuro-
associated with impaired fear inhibition (Jovanovic et al., circuitry of fear generalization, revealing that activity in the amyg-
2012), raising the possibility that core pathophysiologic pro- dala and insula correlate selectively with generalized SCRs and
cesses contributing to deficits in extinction retention may be that functional connectivity between the amygdala and extra-
located in regions outside amygdala and involve neuro-behav- striate visual cortex was selectively enhanced for a perceptually
ioral functions beyond basic fear-associated learning processes. similar cue of high emotional intensity that was never paired with
Fear Generalization. Fear generalization is another aspect of US (Dunsmoor et al., 2011). This suggests that amygdala-
fear learning implicated in PTSD. Fear generalization occurs cortical communication might be active during generalization.
when, following conditioning, stimuli other than the CS, but Lissek et al. (2014) reported that gradients of fMRI activity are
which share some similarities with CS, elicit a fear-related present in insula and dmPFC, with activity levels declining as
response. This is an adaptive process that facilitates protective similarity to CS+ declined, with a reverse gradient in vmPFC
responses to situations that are similar to but not identical with and hippocampus, where activity is greatest to the safety cue.
situations previously learned to be dangerous (Shepard, 1987). Clinically, some evidence shows that PTSD patients show a fail-
Disruption in generalization (e.g., over-generalization) could ure to discriminate CS+ (threat) from CS (safety), perhaps due
contribute to excessive fear expression in PTSD (Lissek and to overgeneralization (Lissek et al., 2005; Jovanovic et al., 2012).
van Meurs, 2015). Generalization is demonstrated by stimulus However, there is very little experimental evidence specifically
response gradientsif during fear conditioning, the CS+ and demonstrating overly broad generalization gradients in PTSD
CS are chosen to represent two ends along a continuum, (Lissek and van Meurs, 2015). It is often assumed that patients
fear responses occur to the CS+ and to stimuli close to it on with PTSD would exhibit broad generalization gradients based
the continuum, with a declining response gradient as the cues on their symptom profiles, but empirical documentation is still
approach the CS end (Lissek et al., 2008). Importantly, the needed to substantiate this model.
Neuron 92, October 5, 2016 17
Neuron
Perspective
Figure 2. Brain Regions of the Salience
Network
Abnormal function within Salience Network re-
gions is implicated in the Exaggerated Threat
Detection model of PTSD (see text).
1996) levels. Insula appears to be hyper-
active in PTSD during anticipation (La-
nius et al., 2007; Lindauer et al., 2008;
Aupperle et al., 2012); and increased
activation by threat cues is seen in the
amygdala (Liberzon et al., 1999b; Shin
et al., 2004; Bryant et al., 2008). There
is evidence that the SN might be hyper-
sensitive to threat in PTSD patients
even before trauma exposure, and this
Exaggerated Threat Detection sensitivity can predict subsequent symptom development (Ad-
Disruptions in Fear/Safety learning or fear generalization can in- mon et al., 2009). Increased dACC reactivity is also seen in
crease reactivity to potentially threatening stimuli. However, PTSD in response to conditioned fear cues (Rougemont-Buck-
threat hyper-reactivity can also stem from processes indepen- ing et al., 2011), demonstrating heightened sensitivity in all of
dent of fear learning. One alternative potential source is in threat the main nodes of the salience network. Excessive activity in
detection systems. An ability to detect salience in the environ- all three nodes (dACC, insula, and amygdala) predicted poor
ment, whether in the realm of threat or reward, is essential for response to treatment (van Rooij et al., 2016), suggesting that
survival, which requires a rapid detection system that can focus enhanced SN activity might be involved in symptom persis-
attention on salient cues, recruit multiple homeostatic systems tence. Resting-state connectivity studies have also demon-
to respond to them, and trigger automatic motor routines (Phelps strated hyper-connectivity within SN regions, involving amyg-
and LeDoux, 2005). Hypersensitive salience detection could dala, insula, and ACC (Sripada et al., 2012). Such connectivity
heighten vigilance and threat reactivity in PTSD and activate could enhance processing of threat cues. There thus may be
fear and protective responses disproportionate to actual threats. sensitivities at multiple levels within the SN that could contribute
Exaggerated threat detection has in fact been implicated in to enhanced fear behaviors.
PTSD pathophysiology by some investigators (for review, see On the cellular/molecular level there is little information on
Shin and Liberzon, 2010; Sripada et al., 2012). specific processes supporting exaggerated Salience or Threat
Functional neuroimaging and resting-state studies have iden- Detection (TD). Neuronal sensitization within the amygdala to
tified an interconnected network of brain regions, including repeated cue exposure is one potential pathway to amygdala hy-
amygdala, dorsal anterior cingulate cortex (dACC), and insula/ per-reactivity (Sandi and Richter-Levin, 2009; Li et al., 2010), but
operculum (Seeley et al., 2007), that activate when salience is this has not yet been directly linked to enhanced Threat Detec-
detected in the environment (Figure 2). The amygdalas role in tion or altered SN activity. A key challenge is the lack of animal
threat detection and fear expression (Ohman, 2005) is well es- models of SN hyperactivity, with amygdala reactivity models
tablished (see above), but human fMRI work has also demon- primarily focusing on conditioning and extinction processes.
strated amygdala reactivity to positive, rewarding, or social stim- Another potential pathway to enhanced threat detection in
uli (e.g., human faces; Javanbakht et al., 2015). Similarly, dACC PTSD patients could involve their peripheral ANS abnormalities.
is implicated in error and conflict detection, and in autonomic For example, low heart rate variability, which may reflect sympa-
arousal (Critchley, 2004); and anterior insula/operculum activity thetic-parasympathetic imbalance, is associated with increased
is associated with anticipation of meaningful events (Simmons threat detection (Melzig et al., 2009; Sevenster et al., 2015) and
et al., 2004), monitoring of internal states (Craig, 2009), and with risk for development of PTSD (Minassian et al., 2015). Ab-
pain perception (Craig, 2003). Functional connectivity between normalities in adrenergic tone or glucocorticoids could also
regions of this network creates a circuit, identified as a Salience contribute to enhanced Threat Detection, as noradrenergic hy-
Network (SN), which activates in response to emotionally peractivity is linked to increased amygdala reactivity (Neumeis-
arousing information (Seeley et al., 2007). Hyper-reactivity in ter et al., 2006) and glucocorticoid receptors, which are abun-
the nodes of this network, or enhanced connectivity between dant in amygdala, have been linked to enhanced anxiety and
these nodes, could contribute to hypervigilance, exaggerated fear (Schulkin et al., 2005), but direct evidence for a mechanistic
threat detection, exaggerated physiological reactivity, and other pathway through these systems to enhanced threat detection in
core symptoms of PTSD. PTSD is still missing.
Enhanced threat sensitivity expressed as hypervigilance is Diminished Executive Function and Emotion Regulation
indeed a fundamental feature of PTSD. PTSD patients are An additional mechanism that could contribute to PTSD patho-
biased to attend to threat (Buckley et al., 2000), at both supra- physiology involves regulatory processes that modulate re-
liminal (Bryant and Harvey, 1995) and subliminal (Harvey et al., sponses to emotionally evocative stimuli like threat. Successful
18 Neuron 92, October 5, 2016
Neuron
Perspective
Figure 3. Brain Regions Involved in
Executive Function and Emotional
Regulation
Abnormal function in these regions has been
implicated in the Diminished Executive Function
and Emotional Regulation model of PTSD (see
text).
There is considerable face validity to
the idea that deficits in cognitive control
and associated top-down inhibitory mod-
ulation of autonomic and emotional acti-
vation areas might play a role in PTSD.
Deficits in EF and/or ER may contribute
to functional impairment following trauma
and underlie some manifestations of
PTSD, including biased attention to
navigation in daily life, including avoidance of danger, requires trauma cues, heightened emotionality, deficits in memory pro-
holding information in mind, resisting distractors, switching be- cesses, irritability, and impulsivity (Powers et al., 2015). There
tween tasks, and planningtasks that are considered compo- is in fact growing evidence of dysfunction within EF/ER cir-
nents of Executive Function (EF). EF is a family of capacities by cuits in PTSD patients. For example, EF-related networks
which we manage cognitive processes and includes mecha- show impaired within-network connectivity in dorsal attention
nisms of working memory, attention, inhibition, and task shifting. frontoparietal regions in PTSD (A. Etkin, personal communica-
Deficits in multiple EF domains are seen in psychiatric disorders tion) and others have reported altered connectivity within and
and associated with poor social and occupational functioning between EF networks in PTSD (Sripada et al., 2012; Chen and
(Chen and Etkin, 2013). The same mechanisms, and their neural Etkin, 2013; Du et al., 2015; Dunkley et al., 2015; Kaiser et al.,
substrates, are also involved in regulation of emotions (ER), 2015; Zhang et al., 2015). However, few PTSD studies have
in that these cognitive resources are used to modulate emotion- examined volitional ER. Only two imaging studies examined
ally driven behavior. There are multiple aspects of PTSD that cognitive reappraisal in PTSD, with evidence suggesting
could stem from deficits in EF/ER, including memory deficits, generally reduced lateral and medial prefrontal activation dur-
exaggerated emotional responses to salient cues, irritability, ing reappraisal (Rabinak et al., 2014; New et al., 2009). Reap-
and impulsivity. praisal-related changes were also examined before and after
Neural activity in dorsal cortical networks, like fronto-parietal PTSD treatment, with evidence that treatment with selective
attentional networks, has been linked to EF (Niendam et al., serotonin inhibitors can ameliorate initial deficits in ER prefron-
2012). EF participates in shifting neural engagement from a tal brain function, and pretreatment ER-region deficits nega-
default mode network (DMN), involved in internally oriented tively predict treatment-related gains (MacNamara et al.,
self-directed mentation, to salience and task networks. Imbal- 2016).
ance and discoordination between DMN and task networks The cellular/molecular mechanisms involved in deficient
may contribute to lapses in attention and decrements in perfor- ER/EF are unknown, largely due to the fact that valid animal
mance (Anticevic et al., 2012), as well as to psychiatric disorders models of volitional (and involuntary) emotional regulation
(Sheline et al., 2009; Chen and Etkin, 2013; Bartova et al., 2015). are yet to be developed. Noradrenergic hyper-reactivity, postu-
ER involves some of the same cognitive control systems, such as lated to be characteristic of PTSD, has been linked to dimin-
memory and attention (Ochsner et al., 2002, 2012), supporting ished frontal lobe-mediated executive capacities (e.g., working
the notion of a common set of prefrontal regions that are impli- memory; Arnsten, 2009) and could contribute to diminished
cated in the regulation of affective and non-affective responses EF. Changes in ANS have been associated with reduced
(Figure 3). For example, the lPFC and mPFC regions activated performance in a number of EF tasks (e.g., working memory,
by emotional regulation (Ochsner et al., 2002) overlap with those attention) (Thayer et al., 2009). Whether the peripheral changes
activated in working memory and response selection tasks drive these deficits in CNS function or are downstream
(Miller and Cohen, 2001; Smith and Jonides, 1999; Buhle et al., effects that feedback is not known. In either case, they could
2014; Kohn et al., 2014). The most commonly studied type of be contributing to EF abnormalities in PTSD. However, while
emotion regulation is reappraisal, which involves conscious, ER deficits are intuitively appealing and somewhat promising
volitional efforts to modulate emotional responses by imposing based on pilot data, direct evidence for them as contributing
a less emotional cognitive frame on them. In concert with the factors to PTSD pathophysiology, using established ER para-
notion of common EF/ER mechanisms, reappraisal activates digms, remains sparse.
overlapping regions including dlPFC, vlPFC, dmPFC, dACC, Explanatory Gaps and Remaining Questions
lateral orbitofrontal cortex (OFC), and the posterior parietal The Fear Learning/Generalization, Threat Detection, and
lobe (Buhle et al., 2014; Phan et al., 2005). Emotional Regulation models can account for many of the signs
Neuron 92, October 5, 2016 19
Neuron
Perspective
and symptoms of PTSD. Abnormal Fear Learning and General- tion that is used to modulate emotional responses (Liberzon and
ization readily account for key features like the dominance of Sripada, 2008; Maren et al., 2013). This model is firmly rooted in
fear over safety memories, generalization of fear responses to prior work but it expands upon previous models, by reframing
any trauma-related stimuli, exaggerated psychophysiologic them through the lens of context processing, in an effort to better
and adrenergic reactivity, and hypervigilance. Insofar as avoid- integrate existing understanding and evidence with the missing
ance of trauma reminders represents a defensive strategy to pieces that remain unexplained.
prevent recurrence of these strong fear responses, avoidance Functionally, the concept of context has been used to refer to
symptoms could be explained as bi-products of altered fear general cognitive, semantic, or emotional backgrounds that
learning. The Abnormal Fear Learning model, however, does allow one to derive situation-informed meaning from the world
not offer satisfying explanations for other key PTSD features (Maren et al., 2013). Contexts are composed of many stimulus
such as spontaneous (non-cue-triggered) intrusions, nightmares elements that are assembled into configural, or contextual,
and other sleep disturbances, emotional numbing or pervasive representations, perceived as a gestalt and acquired rapidly
negative affect, or the behavioral recklessness often seen in (Maren et al., 2013). Salient cues may require radically different
PTSD, leading to re-traumatization. Emotional numbing and re- responses in different situationsthe critical function of context
exposure to traumatic environments are in fact particularly processing is to match cued responses to the needs of a given
difficult to reconcile with a view of PTSD as a disorder of exag- situation or context (Maren et al., 2013). For example, spotting a
gerated fear and diminished safety learning. mountain lion in ones back yard is life threatening, but in a zoo it
Exaggerated Threat Detection and Diminished EF/ER models, is exciting (even though the safety features can be well hidden).
on the other hand, offer plausible explanation for the presence of Contextual information allows us to freeze, flee, or enjoy the view
threat-focused attentional bias, working memory deficits and of the lion, depending on the situation. Considering the central
hyperarousal in PTSD, which could lead to hypervigilance, and role of context in the flexible representation and retrieval of infor-
exaggerated emotional and physiologic responses. Exagger- mation and in resolving ambiguity, contextual processing defi-
ated emotional responses and failure to regulate emotions could cits would lead to cue-focused behavioral reactivity, which is
contribute to pervasive anger and impulsivity. They could also less flexible and more likely to be situationally inappropriate,
generate avoidance behavior and contribute to the emergence potentially contributing to a broad range of PTSD symptoms.
of intrusive memories in response to traumatic reminder cues. The neural circuits involved in context processing have
These models, however, have difficulty explaining the pervasive- been extensively studied, largely through studies of associative
ness of trauma memories and, like the FL model, cannot explain learning (Bouton, 1993, 1994), building foundation for CP models
spontaneous recollections and intrusions, sleep abnormalities, of psychopathology by revealing the fundamental mechanisms
emotional numbing or re-traumatization. whereby contexts are encoded and how they modulate memory
The main PTSD symptoms left unexplained by these three retrieval (Corcoran and Maren, 2001). These studies have shown
models are spontaneous (non-cued) recollections, nightmares that the hippocampus plays a critical role in contextual learning
and other sleep abnormalities, emotional numbing, and reckless and memory (Holland and Bouton, 1999), which is consistent
behavior leading to re-traumatization. Neurobiologically, they with its role in episodic memory and spatial representation.
can account for exaggerated psychophysiologic reactivity and Unique contextual representations are established in the hippo-
a hyperadrenergic state, triggered by trauma or trauma-like campus by population coding of spatial properties of the envi-
cues. However, they cannot readily integrate other neurobiologic ronment, integrating them with non-spatial information (e.g.,
abnormalties, such as structural hippocampal deficits, HPA axis time, prior experience, internal states) into a gestalt that be-
dysregulation, genetic risk factors like FKBP5 or ADRB2, and comes context (Hayman et al., 2003). Converging findings from
altered sleep physiology. These models also require a number animal and human studies (Alvarez et al., 2008; Marschner
of independent pathophysiologic processes in several areas of et al., 2008) indicate that the role of the hippocampus is critical,
the CNS to explain as much as they can explain, while leaving although not exclusive, in context encoding, and that hippocam-
important symptoms and findings unexplained. Furthermore, pal and prefrontal cortical regions interact in context retrieval
as described above, existing empirical evidence in support of (Figure 4). The hippocampus establishes new memory represen-
the proposed mechanisms is limited in some places and often tations, minimizing overlap with previous memories, but it also
contradictory. PTSD could actually be a collection of somewhat helps to retrieve old memory based on partial information. These
distinct neurobiological entities. If so, the FL, TD, and EF models processes are known as pattern separation and pattern comple-
might correspond to specific disorder subtypes. However, given tion, respectively (see Yassa and Stark, 2011 for review). The
the empirical gaps noted and important phenomenological and ability to differentiate the zoos diorama from a real mountain
neurobiological features still unexplained, ongoing pursuit of a landscape, even though the former was designed to invoke the
more general and parsimonious explanation of PTSDs patho- latter, depends upon this process of pattern separation, helping
physiology is clearly still warranted. to differentiate safety from threat. In contrast, pattern completion
processes allow one to retrieve memory based on partial or
A New Model: Deficient Context Processing incomplete information, for example, allowing one to take quick,
In search of a more comprehensive and parsimonious explana- protective action with just a glimpse of a lion-like silhouette in
tion of the full range of PTSD symptoms and the disorders the backyard. These processes have clear relevance to PTSD,
neurobiology, we have hypothesized a core pathophysiological where a bias toward pattern completion in the face of a partial
deficit within neural circuits that process the contextual informa- threat cue, and difficulty reading the contextual nuances
20 Neuron 92, October 5, 2016
Neuron
Perspective
Figure 4. Brain Circuits Involved in
Contextual Processing Functions
Abnormal function within these circuits is impli-
cated in the Deficient Contextual Processing
model of PTSD (see text). Information flow in this
circuit includes Locus Coeruleus (LC) adrenergic
neurons (shown in schematic but not visible in
depicted brain slices).
create vulnerability to over-react to po-
tential indicators of threat, enhancing vig-
ilance, providing face validity for potential
relevance to PTSD.
Indeed, evidence is accumulating that
dysfunction within this interconnected
context processing circuitrywhich in-
volves hippocampus, prefrontal cortex,
thalamus, and amygdalamay play a
central role in the pathophysiology of
PTSD. With direct relevance to fear and
safety learning, human studies have
confirmed the engagement of mPFC re-
needed for pattern separation, would make it difficult to discrim- gions (including subgenual ACC, vmPFC, and OFC) (Gottfried
inate threat from safety and would foster threat generalization and Dolan, 2004; Phelps et al., 2004; Lang et al., 2009), dACC,
(Kheirbek and Hen, 2014; Kheirbek et al., 2012), contributing to rostral mPFC (LaBar et al., 1998; Veit et al., 2002; Gottfried
hypervigilance and hyperarousal. Impairment in hippocampal and Dolan, 2004; Knight et al., 2004; Phelps et al., 2004; Milad
subregions involved in pattern separation (i.e., the dentate gyrus) et al., 2007; Lang et al., 2009), and hippocampus (Knight et al.,
could result in recurrent retrieval of trauma memories in res- 2004) in extinction of fear memory, a process that is inherently
ponse to partial cues, leading to generalized fear responses context dependent. At the same time, diminished mPFC signal
that are incongruous with the current context. had been repeatedly demonstrated in PTSD and diminished
The mPFC is another critical component of context processing vmPFC signal is linked to PTSD deficits in extinction recall/reten-
circuitry. It is highly interconnected with hippocampus, and bidi- tion (Shin and Liberzon, 2010; Etkin and Wager, 2007). In con-
rectional interactions between hippocampus and mPFC have cert, abnormalities in processing of contextual information can
been implicated in contextual memory and in PTSD (Jin and Ma- lead to cue-elicited fear responses, in association with hypo-
ren, 2015). Ensembles of mPFC neurons track environmental activation in vmPFC and hyper-activation of dACC (Rouge-
contexts (Hyman et al., 2012) and integrate contextual recogni- mont-Bucking et al., 2011). Diminished vmPFC activation is
tion and the context-US association during fear-associated also seen during a Go/NoGo inhibitory task in PTSD subjects
learning (Zelikowsky et al., 2014). Lesions in mPFC disrupt and linked to enhanced fear-potentiated startle responses dur-
remote context memory (Quinn et al., 2008), suggesting that ing safety signal learning (Jovanovic et al., 2013). Together,
mPFC helps maintain context representations over time, since this emerging evidence suggested to us that abnormalities
the role of the hippocampus in this function appears to be time seen in PTSD in retention of extinction or in fear renewal, or fail-
limited (see Sutherland and Lehmann, 2011). While ventral hip- ure to learn or recall safety cues, could all stem from a general
pocampus is thought to encode non-spatial contextual informa- deficit in contextual processing, rooted in a hippocampal-pre-
tion such as odors, bodily states, and emotions (Pennartz et al., frontal-thalamic circuitry dysfunction. As an initial test of this
2011), mPFC creates associations between events, contexts, lo- hypothesis, we extended a standard conditioning/extinction
cations, and emotional responses (Euston et al., 2012). Preston paradigm into a renewal phase, making the counter-intuitive pre-
and Eichenbaum (2013) suggest that while hippocampus forms diction that PTSD patients would fail to detect danger signals,
novel memories, the prefrontal cortex .accumulates features despite their continuous hypervigilance for threat, if those signals
of related memories that compose the context of a set of con- were contextual in nature. We hypothesized that extinction recall
nected experiences (p. 766). In this role, mPFC both guides deficits and fear renewal deficits would both be present due to
appropriate memory retrieval by using the context to resolve disrupted hippocampal functioning, since both extinction recall
conflicting information and participates in memory formation and fear renewal are context- and hippocampal-dependent pro-
by resolving conflicts between pre-existing schemas and new cesses. If PTSD patients indeed exhibit such a general deficit,
events. This process of organizing multiple features into a single, then when presented again with the original conditioning context
coherent representation, integrating temporal and spatial infor- in a renewal test, they would fail to display an appropriate re-
mation, involves theta-oscillations that functionally bind hippo- turn of the conditioned fear response, similar to their reported
campus and PFC (Herweg et al., 2016). If this system is not inability to recall extinction when exposed to the extinction
working well, (being a bit unmoored in time and space), it could context. This is exactly what happened (Figure 5, bottom panel).
Neuron 92, October 5, 2016 21
Neuron
Perspective
Figure 5. Contextual Processing Abnormalities in the Single Prolonged Stress Animal Model of PTSD and in PTSD Patients
(A) Schematic depiction of the SPS paradigm, which involves multiple stressors delivered sequentially in 1 day and then a 7 day rest period, followed by fear
conditioning, extinction, and renewal testing.
(B) Data using this paradigm show abnormal fear renewal in SPS animals (seen on the right, in line graph and summary bar graph).
(C) Schematic depiction (including screen shots) of the paradigm used to examine fear conditioning, extinction, extinction recall and fear renewal in fMRI ex-
periments with humans.
(D and E) Data using this paradigm with PTSD patients and combat control (CC) subjects show abnormal extinction recall (D) and abnormal fear renewal (E) in
PTSD (skin conductance data shown on right in both panels). Differences in fMRI activations in PTSD compared to controls, in response to previously conditioned
and then extinguished stimuli (CS+E), are shown on the left in both panels.
PTSD patients failed to properly recall extinction and failed to warning cue in a stop signal anticipation task, supporting the
renew conditioned fear, and they also displayed reduced activa- idea that the context processing deficit in PTSD is general and
tion in the hippocampus and vmPFC (Garfinkel et al., 2014). not isolated to fear processing (van Rooij et al., 2015).
Subsequent studies from other laboratories have corrobo- In addition to modulating fear-associated learning, contextual
rated contextual processing deficits in PTSD. In a similar SCR processing plays a key role in shaping memory processes,
fear conditioning paradigm, PTSD patients failed to properly emotional responses and regulation, and in shaping flexible
renew conditioned fear when re-exposed to the conditioning behavioral choices. As such, a context processing deficit based
context following full extinction that occurred 24 hr after the in dysfunction of a hippocampal-prefrontal-thalamic network
conditioning phase (E. Shvil et al., 2014, 34th annual meeting could contribute to multiple aspect of post-traumatic psychopa-
of the Anxiety & Depression Association of America, confer- thology, beyond the documented specific deficits in extinction
ence). Another laboratory further corroborated these results retention and fear renewal, and their associated symptom-
by showing that PTSD patients failed to utilize a contextual atology. For example, mPFC-Hpc circuitry creates associations
22 Neuron 92, October 5, 2016
Neuron
Perspective
between contexts, events, and corresponding emotional re- PTSD-like phenotype (Knox et al., 2012). In concert, the
sponses (Euston et al., 2012; Preston and Eichenbaum, 2013), emerging genetic and genomic findings in PTSD implicate
so dysfunction in this system could link erroneous emotional molecular pathways that might alter hippocampal-prefrontal-
contexts to a given situation, triggering emotional responses thalamic circuitryperhaps impacting hippocampal volume or
that are inappropriate. For example, erroneous retrieval of an context-relevant hippocampal function like neurogenesis and
intensely negative emotional context of loss and pain during nor- LTP. For example, polymorphisms in BDNF (Gatt et al., 2009;
mally joyful/happy events will prevent experiencing the joyful Aas et al., 2014), ADBR2 and FKBP5 (Fani et al., 2013), or methyl-
event as happy, which can be experienced as emotional numb- ation of SLC6A4 (Dannlowski et al., 2014) or FKBP5 (Fani et al.,
ing. Similarly, excessive anger can emerge, following relatively 2013), all have hippocampal effects, and each has evidence for
innocuous triggers, if the context is perceived as dangerous or association with PTSD. These findings resonate with reports of
threatening. If context is identified as unsafe, attention is hippocampal size differences seen in PTSD, which may consti-
focused on potential threat cues, leading to hypervigilance; but tute a risk factor for PTSD development (Gilbertson et al.,
if contextual information that should alert one to danger is 2002; Bremner et al., 2003; Kitayama et al., 2005). Indeed,
missed, this might lead to recklessness and retraumatization. PTSD patients exhibit impaired performance on hippocampal-
Being unmoored from current contexts that keeps trauma dependent tasks including visual memory recall tests and verbal
memories from emerging in response to partial cues can lead memory tasks (Lindauer et al., 2006; Hayes et al., 2011), and
to the emergence of intrusive memories. These hypothesized these also may predate PTSD onset. Together, these converging
links between CP and a broad range of PTSD symptomology findings suggest a potential causative link between genetic and
are speculative, and extensive work is needed to test these developmental factors that shape hippocampal-mPFC circuitry
ideas, but initial support linking connectivity within the CP circuit and the development of signature HPA abnormalities and
to specific PTSD symptoms has been emerging. For example, lead to contextual processing deficits that can create much of
we have shown that diminished vmPFC/hippocampus connec- PTSDs phenomenology.
tivity at rest was associated with the severity of each of the three The CP model also nicely resonates with recent electrophysi-
PTSD symptom clusters (i.e., intrusive, avoidant and hyper- ologic and sleep findings. Hippocampal-prefrontal communica-
arousal) and that enhanced connectivity of these regions with tion involves additional nodes in the midline thalamus, such as
SN regions was associated specifically with enhanced hyper- the reuniens and rhomboid nuclei, which participate in con-
arousal symptoms (Sripada et al., 2012). A more recent solidation of enduring memories at a systems level (Pereira de
resting-state connectivity study replicated these findings, re- Vasconcelos and Cassel, 2015). This process requires sleep-
porting that re-experiencing symptoms in PTSD are linked to specific field-potential oscillations occurring during slow-wave
disconnection of hippocampus from right PFC, interpreted rapid eye movement sleep (REM), which, in turn, rely upon spe-
by the authors as a failure of contextualization and associated cific function of locus coeruleus (LC) neurons (Vanderheyden
overgeneralization of trauma memories (Spielberg et al., 2015). et al., 2014). Thus, effective encoding, consolidation, updating,
In addition to providing a broad and parsimonious explanation and retrieval of contextual memory within hippocampal-prefron-
of PTSD symptoms, the CP model also provides a robust frame- tal-thalamic circuitry likely require appropriate modulation by LC
work for integrating much of what is known, neurobiologically, firing. This raises the possibility that adrenergic dysregulation,
about this disorder. The cellular and molecular processes that LC firing, and sleep problems might be contributing to disruption
underlie context-processing have been revealed in animal of the appropriate encoding and retrieval of contextual informa-
studies (Maren et al., 2013). These data, coupled with data tion, by affecting hippocampal-prefrontal communication. This is
from animal models of PTSD (Knox et al., 2012) and human obviously only one potential etiologic pathway to mPFC-Hpc
genetic and genomic studies, support the relevance of context dysfunction (see below) and associated CP abnormalities, but
processing and its neurobiology to PTSD pathophysiology. For these linkages pull adrenergic/autonomic function and sleep
example, mediation of contextual fear depends upon activation disruption into our context processing model, suggesting how
of glucocorticoid receptors in prefrontal cortex (Reis et al., sleep problems can play a key role in the emergence or perpet-
2016). Glucocorticoid receptor (GR) hypersensitivity (with uation of CP deficits in a way that is consistent with sleep neuro-
enhanced HPA negative feedback) has been repeatedly demon- biology in PTSD. Normal rewiring of memory networks in the
strated in PTSD. Diminished mPFC function has also been hippocampus (including contextual memories) during sleep
consistently reported in PTSD (Shin and Liberzon, 2010) and requires bi-directional plasticity (both synaptic strengthening
confirmed by meta-analyses (Etkin and Wager, 2007). In our [LTP] and weakening [DP] [Kemp and Manahan-Vaughan,
PTSD animal model (SPS), which successfully replicates the 2004]) specifically during REM and transition to REM (TR). During
enhanced HPA negative feedback seen in patients (Yamamoto REM and TR, to allow bi-directional plasticity (Poe et al., 2010),
et al., 2009), abnormalities in fear renewal are also seen (Figure 5, the noradrenergic (NE) neurons in the LC must turn off, which
top panel), and upregulation of glucocorticoid receptors in is essential for synaptic depotentiation (Thomas et al., 1996;
mPFC has been shown to be critical for expression of the Booth and Poe, 2006). If a hyperadrenergic state is present dur-
PTSD-like phenotype (George et al., 2015). Similarly, glucocorti- ing REM and TR, the necessary synaptic depotentiation would
coid signaling in Hpc plays a critical role in pattern separation be impaired, disrupting the rewiring of memory networks in the
and can induce PTSD-like memory abnormalities in a mouse hippocampus (Vanderheyden et al., 2014), and potentially
model (Kaouane et al., 2012); and in our SPS rat model, upregu- contributing to emergence of contextual processing abnormal-
lation of GR in Hpc also appears critical to emergence of the ities. The origins of the hyperadrenergic state could be partly
Neuron 92, October 5, 2016 23
Neuron
Perspective
genetic, given the ADRB2 findings in PTSD (Liberzon et al., (genetic, neuroendocrine, immunological, and psychophysio-
2014), which may thus contribute to development of deficits in logic) and to mechanisms of symptom development and disease
hippocampal-prefrontal-thalamic circuit function. Indeed, there maintenanceis an ongoing effort that has gathered substantial
is evidence of linkage between disrupted REM and memory in momentum in the past decade. This signifies both a paradigm
PTSD (Lipinska et al., 2014) and evidence that REM abnormal- shift and remarkable progress in the trauma field, and clinical
ities may play a mechanistic role in PTSD development (Marshall neuroscience in general, as similar brain-based comprehensive
et al., 2014). It is also possible that pre-existing CP deficits, models of psychopathology have yet to be developed in the
rooted in Hpc-mPFC circuit disruption after trauma exposure fields of schizophrenia, depression, or bipolar disorder research,
(recent or past), undermine adaptive, post-traumatic processing despite longer and better funded efforts. Our progress promises
and contribute to the nocturnal hyperadrenergic state, disrupting both early translational and longer-term conceptual and more
sleep-dependent synaptic remodeling, and perpetuating and fundamental scientific benefits.
exacerbating the CP deficits. There are thus multiple potential Among the early translational/clinical benefits are potential
etiologic pathways to Hpc-mPFC dysfunction and CP deficits, advancements in development of more specific, mechanism-
but the CP model does offer a parsimonious way to integrate based treatments. If, as we suggest, PTSD is actually a collection
long-standing evidence that PTSD involves sleep, ANS and of somewhat distinct neurobiological entities, with the FL, TD,
HPA axis abnormalities, noradrenergic/HPA axis interaction ef- and EF models perhaps representing somewhat different endo-
fects on memory processes (Kukolja et al., 2011), and under- phenotypes, then identification of the dominant phenotype in a
standing of specific roles played by hippocampal-prefrontal- given patient might lead to a specific type of treatment, moving
thalamic circuits in shaping episodic and contextual memory. us toward a personalized medicine approach. For a patient
So what are the immediate implications and the next steps for with an Altered FL phenotype, enhancing extinction training
development of the CP model, beyond improved conceptual by using a pharmacological enhancer (e.g., D-cycloserine) in
coherence and explanatory power? A set of refutable hypothesis conjunction with prolonged exposure might be the most prom-
will need to be tested to enhance confidence in the model and ising avenue. For an individual with a CP phenotype, use of
demonstrate its utility in shaping future studies. First step will glucocorticoid/adrenergic agents in combination with contextual
be to further document that the PTSD deficits seen in fear-asso- processing retraining might be more promising. Individually
ciated learning paradigms are also present in a broader range of tailored treatment packages could be further augmented by
tasks that involve contextual processing and modulation. These development of behavioral treatments specifically designed to
include replication of fear renewal findings, and examination of strengthen contextualizing skills via enhanced function in Hpc-
fear reinstatement, contextual conditioning, and contextual mPFC circuitry.
modulation of responses to reward cues. Second, the hypothe- Improved pathophysiological models can also perhaps lead to
sized key role of Hpc-mPFC dysfunction should be examined by better use of existing treatment strategies. For example, evi-
testing other Hpc-mPFC dependent functions in PTSD. These dence for noradrenergic abnormalities in PTSD, along with evi-
should include tasks that probe pattern separation and pattern dence for their effects on memory, has led to efforts to treat or
completion capacities, visual-spatial navigation tasks, and tasks prevent PTSD using the beta receptor antagonist propranolol.
that require utilization of internal context (e.g., hunger, arousal). Propranolol was administered immediately or soon after trauma
Finally the integrative, organizing concept of the CP deficit role exposure, to try to prevent consolidation of traumatic memories.
predicts that other aspects of PTSD symptomatology, like While initial studies appeared promising (Pitman et al., 2002),
emotional numbing, anger outbursts hypervigilance, etc., should subsequent reports did not support this approach (Sijbrandij
be associated with CP deficits in both human studies and animal et al., 2015). If, as the CP deficit model suggests, hyperadrener-
models. gic activity is most relevant to PTSD development during sleep,
Animal work will also be critical in determining the implications and REM in particular, the failure of some propranolol trials
and value of this model, and it will require studies that directly could be due to the wrong timing of propranolol administration.
examine contextual processing and Hpc-mPFC circuits. To Adrenergic interventions might have to be directed toward
date, animal studies have largely utilized paradigms rooted in nocturnal hyperadrenergic states. Similar logic might apply to
fear conditioning/extinction with a focus on amygdala function. attempts to treat/prevent PTSD using glucocorticoid administra-
These paradigms have face validity and have contributed a great tion. Glucocorticoids dose dependently impact learning pro-
deal, but it may be time to develop models rooted in other theory- cesses with an inverted U-shaped dose-response curve (Salehi
based constructs like CP. These models may offer great value in et al., 2010). Knowledge of a trauma exposed individuals
translational and back-translational efforts. Indeed, some steps glucocorticoid status could be essential to insuring beneficial
in this direction have already been taken by various groups effects, since over-treatment in some cases could enhance
studying contextual processing (Jin and Maren, 2015), PTSD trauma memory consolidation or contribute to fear learning
models (Knox et al., 2012), and glucocorticoid modulation of generalization. On the other hand, with appropriate under-
memory processes (Kaouane et al., 2012). standing of the brain mechanisms involved, glucocorticoid
administration could perhaps be used to enhance context
Conclusions and Implications processing capacities, allowing traumatic memory to be better
The identification of neural circuits involved in PTSD pathophys- contextualized, in a flexible way, facilitating an appropriate
iology and the development of comprehensive modelsto balance between mechanisms of pattern separation and
link dysfunction in these circuits to existing established findings pattern completion in the hippocampus. Indeed evidence that
24 Neuron 92, October 5, 2016
Neuron
Perspective
glucocorticoid administration may enhance efficacy of pro- disposition and hippocampal plasticity. Proc. Natl. Acad. Sci. USA 106,
1412014125.
longed exposure therapy for PTSD has been recently reported,
but efficacy depends on the individuals GC receptor sensitivity Agorastos, A., Boel, J.A., Heppner, P.S., Hager, T., Moeller-Bertram, T., Haji,
(Yehuda et al., 2015). U., Motazedi, A., Yanagi, M.A., Baker, D.G., and Stiedl, O. (2013). Diminished
vagal activity and blunted diurnal variation of heart rate dynamics in posttrau-
The long-term implications of this progress are more funda- matic stress disorder. Stress 16, 300310.
mental and more extensive. Developing comprehensive brain-
Aizenberg, M., and Geffen, M.N. (2013). Bidirectional effects of aversive
based models that describe abnormalities in memory deposition
learning on perceptual acuity are mediated by the sensory cortex. Nat. Neuro-
and retrieval as the result of specific neurobiological processes sci. 16, 994996.
and molecular risk factors significantly advances our under-
Alvarez, R.P., Biggs, A., Chen, G., Pine, D.S., and Grillon, C. (2008). Contextual
standing of basic cold and hot memory mechanisms. It fear conditioning in humans: cortical-hippocampal and amygdala contribu-
also deepens understanding of stress response systems and tions. J. Neurosci. 28, 62116219.
brain mechanisms underlying generation and modulation of
Amstadter, A.B., Nugent, N.R., Yang, B.Z., Miller, A., Siburian, R., Moorjani, P.,
emotions. This work has implications beyond PTSD translation- Haddad, S., Basu, A., Fagerness, J., Saxe, G., et al. (2011). Corticotrophin-
ally as well. The key brain pathways and physiological processes releasing hormone type 1 receptor gene (CRHR1) variants predict posttrau-
matic stress disorder onset and course in pediatric injury patients. Dis.
involved in PTSD pathophysiology likely have relevance to our Markers 30, 8999.
understanding of other disorders, as well as stress sensitivity
and resilience more generally. It is very unlikely that any psychi- Anticevic, A., Cole, M.W., Murray, J.D., Corlett, P.R., Wang, X.J., and Krystal,
J.H. (2012). The role of default network deactivation in cognition and disease.
atric disorder can be fully explained by dysfunction of a single Trends Cogn. Sci. 16, 584592.
neurobiological circuit; and given the interconnected nature of
these circuits, the disruption in functions of any particular circuit Arnsten, A.F.T. (2009). Stress signalling pathways that impair prefrontal cortex
structure and function. Nat. Rev. Neurosci. 10, 410422.
is likely to be seen in disorders whose pathophysiology inter-
sects with Hpc-mPFC circuits. Substance Dependence is one Aupperle, R.L., Allard, C.B., Grimes, E.M., Simmons, A.N., Flagan, T., Beh-
rooznia, M., Cissell, S.H., Twamley, E.W., Thorp, S.R., Norman, S.B., et al.
example, where contextual factors have already been invoked (2012). Dorsolateral prefrontal cortex activation during emotional anticipation
to explain relapse vulnerability (Crombag and Shaham, 2002). and neuropsychological performance in posttraumatic stress disorder. Arch.
Exaggerated reactivity to drug cues, rather than trauma cues, Gen. Psychiatry 69, 360371.
may stem from CP deficits, in this case leading to abnormal Baker, D.G., West, S.A., Nicholson, W.E., Ekhator, N.N., Kasckow, J.W., Hill,
approach behavior rather than the avoidance seen in PTSD. K.K., Bruce, A.B., Orth, D.N., and Geracioti, T.D., Jr. (1999). Serial CSF corti-
cotropin-releasing hormone levels and adrenocortical activity in combat veter-
Determining the origins and roles of a particular Hpc-mPFC ans with posttraumatic stress disorder. Am. J. Psychiatry 156, 585588.
dysfunction in the etiological pathway of a specific disorder will
require additional research, but examining CP broadly as a key Baldi, E., Lorenzini, C.A., and Bucherelli, C. (2004). Footshock intensity and
generalization in contextual and auditory-cued fear conditioning in the rat.
brain function of relevance to a broad range of psychopathol- Neurobiol. Learn. Mem. 81, 162166.
ogies may facilitate progress.
As this work moves forward, we will be better able to define Bartova, L., Meyer, B.M., Diers, K., Rabl, U., Scharinger, C., Popovic, A., Pail,
G., Kalcher, K., Boubela, R.N., Huemer, J., et al. (2015). Reduced default mode
our disorders based on specific disruptions within particular network suppression during a working memory task in remitted major depres-
brain circuits leading to impaired function, and this more precise, sion. J. Psychiatr. Res. 64, 918.
neurobiologically based understanding will allow increasingly Bergado-Acosta, J.R., Sangha, S., Narayanan, R.T., Obata, K., Pape, H.C.,
efficient, impactful, and individually tailored treatments. This and Stork, O. (2008). Critical role of the 65-kDa isoform of glutamic acid decar-
progress will also slowly put to rest age-old debates about na- boxylase in consolidation and generalization of Pavlovian fear memory. Learn.
Mem. 15, 163171.
ture versus nurture and psychological versus biological in our
study and understanding of psychiatric disorders, and thereby Bilchick, K.C., and Berger, R.D. (2006). Heart rate variability. J. Cardiovasc.
Electrophysiol. 17, 691694.
help to combat stigmatization and further open the door to better
treatments for all who need them. Binder, E.B., Bradley, R.G., Liu, W., Epstein, M.P., Deveau, T.C., Mercer, K.B.,
Tang, Y., Gillespie, C.F., Heim, C.M., Nemeroff, C.B., et al. (2008). Association
of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic
ACKNOWLEDGMENTS stress disorder symptoms in adults. JAMA 299, 12911305.
The writing of this manuscript was supported by grants to one or both authors Blair, H.T., Schafe, G.E., Bauer, E.P., Rodrigues, S.M., and LeDoux, J.E.
(2001). Synaptic plasticity in the lateral amygdala: a cellular hypothesis of
from the Department of Defense (W81XWH-13-1-0377) and the National Insti-
fear conditioning. Learn. Mem. 8, 229242.
tute of Mental Health (R24 MH075999 and RO1 MH093486). We also thank
Jony Sheynin, PhD for invaluable help in preparation of this manuscript for Blechert, J., Michael, T., Vriends, N., Margraf, J., and Wilhelm, F.H. (2007).
publication, and Arieh Shalev, MD for help with conceptual development. Fear conditioning in posttraumatic stress disorder: evidence for delayed
extinction of autonomic, experiential, and behavioural responses. Behav.
Res. Ther. 45, 20192033.
REFERENCES
Booth, V., and Poe, G.R. (2006). Input source and strength influences overall
Aas, M., Haukvik, U.K., Djurovic, S., Tesli, M., Athanasiu, L., Bjella, T., Hans- firing phase of model hippocampal CA1 pyramidal cells during theta: relevance
son, L., Cattaneo, A., Agartz, I., Andreassen, O.A., and Melle, I. (2014). Inter- to REM sleep reactivation and memory consolidation. Hippocampus 16,
play between childhood trauma and BDNF val66met variants on blood 161173.
BDNF mRNA levels and on hippocampus subfields volumes in schizophrenia
spectrum and bipolar disorders. J. Psychiatr. Res. 59, 1421. Bordi, F., and LeDoux, J. (1992). Sensory tuning beyond the sensory system:
an initial analysis of auditory response properties of neurons in the lateral
Admon, R., Lubin, G., Stern, O., Rosenberg, K., Sela, L., Ben-Ami, H., and amygdaloid nucleus and overlying areas of the striatum. J. Neurosci. 12,
Hendler, T. (2009). Human vulnerability to stress depends on amygdalas pre- 24932503.
Neuron 92, October 5, 2016 25
Neuron
Perspective
Bordi, F., and LeDoux, J.E. (1994). Response properties of single units in areas Critchley, H.D. (2004). The human cortex responds to an interoceptive chal-
of rat auditory thalamus that project to the amygdala. I. Acoustic discharge lenge. Proc. Natl. Acad. Sci. USA 101, 63336334.
patterns and frequency receptive fields. Exp. Brain Res. 98, 261274.
Crombag, H.S., and Shaham, Y. (2002). Renewal of drug seeking by contextual
Boscarino, J.A., Erlich, P.M., Hoffman, S.N., Rukstalis, M., and Stewart, W.F. cues after prolonged extinction in rats. Behav. Neurosci. 116, 169173.
(2011). Association of FKBP5, COMT and CHRNA5 polymorphisms with PTSD
among outpatients at risk for PTSD. Psychiatry Res. 188, 173174. Dannlowski, U., Kugel, H., Redlich, R., Halik, A., Schneider, I., Opel, N., Grote-
gerd, D., Schwarte, K., Schettler, C., Ambree, O., et al. (2014). Serotonin trans-
Bouton, M.E. (1993). Context, time, and memory retrieval in the interference porter gene methylation is associated with hippocampal gray matter volume.
paradigms of Pavlovian learning. Psychol. Bull. 114, 8099. Hum. Brain Mapp. 35, 53565367.
Bouton, M.E. (1994). Context, ambiguity, and classical-conditioning. Curr. Dir. Davis, M. (1992). The role of the amygdala in fear and anxiety. Annu. Rev. Neu-
Psychol. Sci. 3, 4953. rosci. 15, 353375.
Bremner, J.D., Vythilingam, M., Vermetten, E., Southwick, S.M., McGlashan, Davis, M., Rainnie, D., and Cassell, M. (1994). Neurotransmission in the rat
T., Nazeer, A., Khan, S., Vaccarino, L.V., Soufer, R., Garg, P.K., et al. (2003). amygdala related to fear and anxiety. Trends Neurosci. 17, 208214.
MRI and PET study of deficits in hippocampal structure and function in women
with childhood sexual abuse and posttraumatic stress disorder. Am. J. Psychi- Du, M.Y., Liao, W., Lui, S., Huang, X.Q., Li, F., Kuang, W.H., Li, J., Chen, H.F.,
atry 160, 924932. Kendrick, K.M., and Gong, Q.Y. (2015). Altered functional connectivity in the
brain default-mode network of earthquake survivors persists after 2 years
Bremner, J.D., Elzinga, B., Schmahl, C., and Vermetten, E. (2008). Structural despite recovery from anxiety symptoms. Soc. Cogn. Affect. Neurosci. 10,
and functional plasticity of the human brain in posttraumatic stress disorder. 14971505.
Prog. Brain Res. 167, 171186.
Dunkley, B.T., Doesburg, S.M., Jetly, R., Sedge, P.A., Pang, E.W., and Taylor,
Bryant, R.A., and Harvey, A.G. (1995). Processing threatening information in M.J. (2015). Characterising intra- and inter-intrinsic network synchrony in com-
posttraumatic stress disorder. J. Abnorm. Psychol. 104, 537541. bat-related post-traumatic stress disorder. Psychiatry Res. 234, 172181.
Bryant, R.A., Kemp, A.H., Felmingham, K.L., Liddell, B., Olivieri, G., Peduto, A., Dunsmoor, J.E., Prince, S.E., Murty, V.P., Kragel, P.A., and LaBar, K.S. (2011).
Gordon, E., and Williams, L.M. (2008). Enhanced amygdala and medial pre- Neurobehavioral mechanisms of human fear generalization. Neuroimage 55,
frontal activation during nonconscious processing of fear in posttraumatic 18781888.
stress disorder: an fMRI study. Hum. Brain Mapp. 29, 517523.
Duvarci, S., and Pare, D. (2014). Amygdala microcircuits controlling learned
Buckley, T.C., Blanchard, E.B., and Neill, W.T. (2000). Information processing fear. Neuron 82, 966980.
and PTSD: a review of the empirical literature. Clin. Psychol. Rev. 20, 1041
1065. Eichenbaum, H. (1996). Learning from LTP: a comment on recent attempts to
identify cellular and molecular mechanisms of memory. Learn. Mem. 3, 6173.
Buhle, J.T., Silvers, J.A., Wager, T.D., Lopez, R., Onyemekwu, C., Kober, H.,
Weber, J., and Ochsner, K.N. (2014). Cognitive reappraisal of emotion: a Elzinga, B.M., and Bremner, J.D. (2002). Are the neural substrates of memory
meta-analysis of human neuroimaging studies. Cereb. Cortex 24, 29812990. the final common pathway in posttraumatic stress disorder (PTSD)? J. Affect.
Disord. 70, 117.
Campeau, S., and Davis, M. (1995). Involvement of the central nucleus and
basolateral complex of the amygdala in fear conditioning measured with Etkin, A., and Wager, T.D. (2007). Functional neuroimaging of anxiety: a meta-
fear-potentiated startle in rats trained concurrently with auditory and visual analysis of emotional processing in PTSD, social anxiety disorder, and specific
conditioned stimuli. J. Neurosci. 15, 23012311. phobia. Am. J. Psychiatry 164, 14761488.
Chavez, C.M., McGaugh, J.L., and Weinberger, N.M. (2009). The basolateral Etkin, A., Buchel, C., and Gross, J.J. (2015). The neural bases of emotion regu-
amygdala modulates specific sensory memory representations in the cerebral lation. Nat. Rev. Neurosci. 16, 693700.
cortex. Neurobiol. Learn. Mem. 91, 382392.
Euston, D.R., Gruber, A.J., and McNaughton, B.L. (2012). The role of medial
Chen, A.C., and Etkin, A. (2013). Hippocampal network connectivity and acti- prefrontal cortex in memory and decision making. Neuron 76, 10571070.
vation differentiates post-traumatic stress disorder from generalized anxiety
disorder. Neuropsychopharmacology 38, 18891898. Fani, N., Gutman, D., Tone, E.B., Almli, L., Mercer, K.B., Davis, J., Glover, E.,
Jovanovic, T., Bradley, B., Dinov, I.D., et al. (2013). FKBP5 and attention bias
Ciocchi, S., Herry, C., Grenier, F., Wolff, S.B., Letzkus, J.J., Vlachos, I., Ehrlich, for threat: associations with hippocampal function and shape. JAMA Psychia-
I., Sprengel, R., Deisseroth, K., Stadler, M.B., et al. (2010). Encoding of condi- try 70, 392400.
tioned fear in central amygdala inhibitory circuits. Nature 468, 277282.
Fanselow, M.S., and LeDoux, J.E. (1999). Why we think plasticity underlying
Cohen, H., Benjamin, J., Geva, A.B., Matar, M.A., Kaplan, Z., and Kotler, M. Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23,
(2000). Autonomic dysregulation in panic disorder and in post-traumatic stress 229232.
disorder: application of power spectrum analysis of heart rate variability at rest
and in response to recollection of trauma or panic attacks. Psychiatry Res. 96, Garfinkel, S.N., Abelson, J.L., King, A.P., Sripada, R.K., Wang, X., Gaines,
113. L.M., and Liberzon, I. (2014). Impaired contextual modulation of memories in
PTSD: an fMRI and psychophysiological study of extinction retention and
Corcoran, K.A., and Maren, S. (2001). Hippocampal inactivation disrupts fear renewal. J. Neurosci. 34, 1343513443.
contextual retrieval of fear memory after extinction. J. Neurosci. 21, 1720
1726. Gatt, J.M., Nemeroff, C.B., Dobson-Stone, C., Paul, R.H., Bryant, R.A., Scho-
field, P.R., Gordon, E., Kemp, A.H., and Williams, L.M. (2009). Interactions be-
Corcoran, K.A., and Quirk, G.J. (2007). Recalling safety: cooperative functions tween BDNF Val66Met polymorphism and early life stress predict brain and
of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS arousal pathways to syndromal depression and anxiety. Mol. Psychiatry 14,
Spectr. 12, 200206. 681695.
Courtin, J., Chaudun, F., Rozeske, R.R., Karalis, N., Gonzalez-Campo, C., Genud-Gabai, R., Klavir, O., and Paz, R. (2013). Safety signals in the primate
Wurtz, H., Abdi, A., Baufreton, J., Bienvenu, T.C., and Herry, C. (2014). Pre- amygdala. J. Neurosci. 33, 1798617994.
frontal parvalbumin interneurons shape neuronal activity to drive fear expres-
sion. Nature 505, 9296. George, S.A., Rodriguez-Santiago, M., Riley, J., Rodriguez, E., and Liberzon, I.
(2015). The effect of chronic phenytoin administration on single prolonged
Craig, A.D. (2003). A new view of pain as a homeostatic emotion. Trends Neu- stress induced extinction retention deficits and glucocorticoid upregulation
rosci. 26, 303307. in the rat medial prefrontal cortex. Psychopharmacology (Berl.) 232, 4756.
Craig, A.D. (2009). How do you feelnow? The anterior insula and human Ghosh, S., and Chattarji, S. (2015). Neuronal encoding of the switch from spe-
awareness. Nat. Rev. Neurosci. 10, 5970. cific to generalized fear. Nat. Neurosci. 18, 112120.
26 Neuron 92, October 5, 2016
Neuron
Perspective
Gilbertson, M.W., Shenton, M.E., Ciszewski, A., Kasai, K., Lasko, N.B., Orr, Kaouane, N., Porte, Y., Vallee, M., Brayda-Bruno, L., Mons, N., Calandreau, L.,
S.P., and Pitman, R.K. (2002). Smaller hippocampal volume predicts patho- Marighetto, A., Piazza, P.V., and Desmedt, A. (2012). Glucocorticoids can
logic vulnerability to psychological trauma. Nat. Neurosci. 5, 12421247. induce PTSD-like memory impairments in mice. Science 335, 15101513.
Goosens, K.A., and Maren, S. (2002). Long-term potentiation as a substrate for Kemp, A., and Manahan-Vaughan, D. (2004). Hippocampal long-term depres-
memory: evidence from studies of amygdaloid plasticity and Pavlovian fear sion and long-term potentiation encode different aspects of novelty acquisi-
conditioning. Hippocampus 12, 592599. tion. Proc. Natl. Acad. Sci. USA 101, 81928197.
Gottfried, J.A., and Dolan, R.J. (2004). Human orbitofrontal cortex mediates Kheirbek, M.A., and Hen, R. (2014). Add neurons, subtract anxiety. Sci. Am.
extinction learning while accessing conditioned representations of value. 311, 6267.
Nat. Neurosci. 7, 11441152.
Kheirbek, M.A., Klemenhagen, K.C., Sahay, A., and Hen, R. (2012). Neurogen-
Grillon, C. (2002). Startle reactivity and anxiety disorders: aversive condition- esis and generalization: a new approach to stratify and treat anxiety disorders.
ing, context, and neurobiology. Biol. Psychiatry 52, 958975. Nat. Neurosci. 15, 16131620.
Grillon, C., Morgan, C.A., 3rd, Davis, M., and Southwick, S.M. (1998). Effects of Kitayama, N., Vaccarino, V., Kutner, M., Weiss, P., and Bremner, J.D. (2005).
experimental context and explicit threat cues on acoustic startle in Vietnam Magnetic resonance imaging (MRI) measurement of hippocampal volume in
veterans with posttraumatic stress disorder. Biol. Psychiatry 44, 10271036. posttraumatic stress disorder: a meta-analysis. J. Affect. Disord. 88, 7986.
Harvey, A.G., Bryant, R.A., and Rapee, R.M. (1996). Preconscious processing Klavir, O., Genud-Gabai, R., and Paz, R. (2013). Functional connectivity be-
of threat in posttraumatic stress disorder. Cognit. Ther. Res. 20, 613623. tween amygdala and cingulate cortex for adaptive aversive learning. Neuron
80, 12901300.
Hayes, J.P., LaBar, K.S., McCarthy, G., Selgrade, E., Nasser, J., Dolcos, F.,
and Morey, R.A.; VISN 6 Mid-Atlantic MIRECC workgroup (2011). Reduced Knight, D.C., Cheng, D.T., Smith, C.N., Stein, E.A., and Helmstetter, F.J.
hippocampal and amygdala activity predicts memory distortions for trauma re- (2004). Neural substrates mediating human delay and trace fear conditioning.
minders in combat-related PTSD. J. Psychiatr. Res. 45, 660669. J. Neurosci. 24, 218228.
Hayman, R.M., Chakraborty, S., Anderson, M.I., and Jeffery, K.J. (2003). Knox, D., Nault, T., Henderson, C., and Liberzon, I. (2012). Glucocorticoid re-
Context-specific acquisition of location discrimination by hippocampal place ceptors and extinction retention deficits in the single prolonged stress model.
cells. Eur. J. Neurosci. 18, 28252834. Neuroscience 223, 163173.
Herweg, N.A., Apitz, T., Leicht, G., Mulert, C., Fuentemilla, L., and Bunzeck, N. Kohn, N., Eickhoff, S.B., Scheller, M., Laird, A.R., Fox, P.T., and Habel, U.
(2016). Theta-Alpha Oscillations Bind the Hippocampus, Prefrontal Cortex, (2014). Neural network of cognitive emotion regulationan ALE meta-analysis
and Striatum during Recollection: Evidence from Simultaneous EEG-fMRI. and MACM analysis. Neuroimage 87, 345355.
J. Neurosci. 36, 35793587.
Krystal, J.H., and Neumeister, A. (2009). Noradrenergic and serotonergic
Holland, P.C., and Bouton, M.E. (1999). Hippocampus and context in classical
mechanisms in the neurobiology of posttraumatic stress disorder and resil-
conditioning. Curr. Opin. Neurobiol. 9, 195202.
ience. Brain Res. 1293, 1323.
Hyman, J.M., Ma, L., Balaguer-Ballester, E., Durstewitz, D., and Seamans, J.K.
Kukolja, J., Klingmuller, D., Maier, W., Fink, G.R., and Hurlemann, R. (2011).
(2012). Contextual encoding by ensembles of medial prefrontal cortex neu-
Noradrenergic-glucocorticoid modulation of emotional memory encoding in
rons. Proc. Natl. Acad. Sci. USA 109, 50865091.
the human hippocampus. Psychol. Med. 41, 21672176.
Jasnow, A.M., Ehrlich, D.E., Choi, D.C., Dabrowska, J., Bowers, M.E., McCul-
lough, K.M., Rainnie, D.G., and Ressler, K.J. (2013). Thy1-expressing neurons LaBar, K.S., Gatenby, J.C., Gore, J.C., LeDoux, J.E., and Phelps, E.A. (1998).
in the basolateral amygdala may mediate fear inhibition. J. Neurosci. 33, Human amygdala activation during conditioned fear acquisition and extinc-
1039610404. tion: a mixed-trial fMRI study. Neuron 20, 937945.
Javanbakht, A., King, A.P., Evans, G.W., Swain, J.E., Angstadt, M., Phan, K.L., Lang, S., Kroll, A., Lipinski, S.J., Wessa, M., Ridder, S., Christmann, C., Schad,
and Liberzon, I. (2015). Childhood Poverty Predicts Adult Amygdala and Fron- L.R., and Flor, H. (2009). Context conditioning and extinction in humans: differ-
tal Activity and Connectivity in Response to Emotional Faces. Front. Behav. ential contribution of the hippocampus, amygdala and prefrontal cortex. Eur. J.
Neurosci. 9, 154. Neurosci. 29, 823832.
Jin, J., and Maren, S. (2015). Prefrontal-Hippocampal Interactions in Memory Lanius, R.A., Frewen, P.A., Girotti, M., Neufeld, R.W.J., Stevens, T.K., and
and Emotion. Front. Syst. Neurosci. 9, 170. Densmore, M. (2007). Neural correlates of trauma script-imagery in posttrau-
matic stress disorder with and without comorbid major depression: a func-
Jones, E. (2006). Historical approaches to post-combat disorders. Philos. tional MRI investigation. Psychiatry Res. 155, 4556.
Trans. R. Soc. Lond. B Biol. Sci. 361, 533542.
Laufer, O., and Paz, R. (2012). Monetary loss alters perceptual thresholds
Jovanovic, T., and Ressler, K.J. (2010). How the neurocircuitry and genetics of and compromises future decisions via amygdala and prefrontal networks.
fear inhibition may inform our understanding of PTSD. Am. J. Psychiatry 167, J. Neurosci. 32, 63046311.
648662.
LeDoux, J.E., Farb, C., and Ruggiero, D.A. (1990). Topographic organization of
Jovanovic, T., Norrholm, S.D., Fennell, J.E., Keyes, M., Fiallos, A.M., Myers, neurons in the acoustic thalamus that project to the amygdala. J. Neurosci. 10,
K.M., Davis, M., and Duncan, E.J. (2009). Posttraumatic stress disorder may 10431054.
be associated with impaired fear inhibition: relation to symptom severity. Psy-
chiatry Res. 167, 151160. Lee, E.A., and Theus, S.A. (2012). Lower heart rate variability associated with
military sexual trauma rape and posttraumatic stress disorder. Biol. Res. Nurs.
Jovanovic, T., Kazama, A., Bachevalier, J., and Davis, M. (2012). Impaired 14, 412418.
safety signal learning may be a biomarker of PTSD. Neuropharmacology 62,
695704. Letzkus, J.J., Wolff, S.B., Meyer, E.M., Tovote, P., Courtin, J., Herry, C., and
Luthi, A. (2011). A disinhibitory microcircuit for associative fear learning in
Jovanovic, T., Ely, T., Fani, N., Glover, E.M., Gutman, D., Tone, E.B., Norrholm, the auditory cortex. Nature 480, 331335.
S.D., Bradley, B., and Ressler, K.J. (2013). Reduced neural activation during an
inhibition task is associated with impaired fear inhibition in a traumatized Li, C.Q., Zhang, J.W., Dai, R.P., Wang, J., Luo, X.G., and Zhou, X.F. (2010).
civilian sample. Cortex 49, 18841891. Surgical incision induces anxiety-like behavior and amygdala sensitization: ef-
fects of morphine and gabapentin. Pain Res. Treat. 2010, 705874.
Kaiser, R.H., Andrews-Hanna, J.R., Wager, T.D., and Pizzagalli, D.A. (2015).
Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-anal- Liberzon, I., and Sripada, C.S. (2008). The functional neuroanatomy of PTSD: a
ysis of Resting-State Functional Connectivity. JAMA Psychiatry 72, 603611. critical review. Prog. Brain Res. 167, 151169.
Neuron 92, October 5, 2016 27
Neuron
Perspective
Liberzon, I., Abelson, J.L., Flagel, S.B., Raz, J., and Young, E.A. (1999a). Meewisse, M.L., Reitsma, J.B., de Vries, G.J., Gersons, B.P., and Olff, M.
Neuroendocrine and psychophysiologic responses in PTSD: a symptom prov- (2007). Cortisol and post-traumatic stress disorder in adults: systematic review
ocation study. Neuropsychopharmacology 21, 4050. and meta-analysis. Br. J. Psychiatry 191, 387392.
Liberzon, I., Taylor, S.F., Amdur, R., Jung, T.D., Chamberlain, K.R., Minosh- Melzig, C.A., Weike, A.I., Hamm, A.O., and Thayer, J.F. (2009). Individual dif-
ima, S., Koeppe, R.A., and Fig, L.M. (1999b). Brain activation in PTSD in ferences in fear-potentiated startle as a function of resting heart rate variability:
response to trauma-related stimuli. Biol. Psychiatry 45, 817826. implications for panic disorder. Int. J. Psychophysiol. 71, 109117.
Liberzon, I., King, A.P., Ressler, K.J., Almli, L.M., Zhang, P., Ma, S.T., Cohen, Milad, M.R., Wright, C.I., Orr, S.P., Pitman, R.K., Quirk, G.J., and Rauch, S.L.
G.H., Tamburrino, M.B., Calabrese, J.R., and Galea, S. (2014). Interaction of (2007). Recall of fear extinction in humans activates the ventromedial prefron-
the ADRB2 gene polymorphism with childhood trauma in predicting adult tal cortex and hippocampus in concert. Biol. Psychiatry 62, 446454.
symptoms of posttraumatic stress disorder. JAMA Psychiatry 71, 11741182.
Milad, M.R., Orr, S.P., Lasko, N.B., Chang, Y., Rauch, S.L., and Pitman, R.K.
Likhtik, E., and Paz, R. (2015). Amygdala-prefrontal interactions in (mal)adap- (2008). Presence and acquired origin of reduced recall for fear extinction in
tive learning. Trends Neurosci. 38, 158166. PTSD: results of a twin study. J. Psychiatr. Res. 42, 515520.
Milad, M.R., Pitman, R.K., Ellis, C.B., Gold, A.L., Shin, L.M., Lasko, N.B., Zei-
Likhtik, E., Stujenske, J.M., Topiwala, M.A., Harris, A.Z., and Gordon, J.A.
dan, M.A., Handwerger, K., Orr, S.P., and Rauch, S.L. (2009). Neurobiological
(2014). Prefrontal entrainment of amygdala activity signals safety in learned
basis of failure to recall extinction memory in posttraumatic stress disorder.
fear and innate anxiety. Nat. Neurosci. 17, 106113.
Biol. Psychiatry 66, 10751082.
Lindauer, R.J., Olff, M., van Meijel, E.P., Carlier, I.V., and Gersons, B.P. (2006).
Miller, E.K., and Cohen, J.D. (2001). An integrative theory of prefrontal cortex
Cortisol, learning, memory, and attention in relation to smaller hippocampal
function. Annu. Rev. Neurosci. 24, 167202.
volume in police officers with posttraumatic stress disorder. Biol. Psychiatry
59, 171177. Minassian, A., Geyer, M.A., Baker, D.G., Nievergelt, C.M., OConnor, D.T., and
Risbrough, V.B.; Marine Resiliency Study Team (2014). Heart rate variability
Lindauer, R.J.L., Booij, J., Habraken, J.B.A., van Meijel, E.P.M., Uylings, characteristics in a large group of active-duty marines and relationship to post-
H.B.M., Olff, M., Carlier, I.V.E., den Heeten, G.J., van Eck-Smit, B.L.F., and traumatic stress. Psychosom. Med. 76, 292301.
Gersons, B.P.R. (2008). Effects of psychotherapy on regional cerebral blood
flow during trauma imagery in patients with post-traumatic stress disorder: a Minassian, A., Maihofer, A.X., Baker, D.G., Nievergelt, C.M., Geyer, M.A., and
randomized clinical trial. Psychol. Med. 38, 543554. Risbrough, V.B.; Marine Resiliency Study Team (2015). Association of Prede-
ployment Heart Rate Variability With Risk of Postdeployment Posttraumatic
Lindley, S.E., Carlson, E.B., and Benoit, M. (2004). Basal and dexamethasone Stress Disorder in Active-Duty Marines. JAMA Psychiatry 72, 979986.
suppressed salivary cortisol concentrations in a community sample of patients
with posttraumatic stress disorder. Biol. Psychiatry 55, 940945. Morgan, C.A., 3rd, Grillon, C., Southwick, S.M., Davis, M., and Charney, D.S.
(1995). Fear-potentiated startle in posttraumatic stress disorder. Biol. Psychi-
Lipinska, M., Timol, R., Kaminer, D., and Thomas, K.G. (2014). Disrupted rapid atry 38, 378385.
eye movement sleep predicts poor declarative memory performance in post-
traumatic stress disorder. J. Sleep Res. 23, 309317. Morris, M.C., Compas, B.E., and Garber, J. (2012). Relations among posttrau-
matic stress disorder, comorbid major depression, and HPA function: a sys-
Lissek, S., and van Meurs, B. (2015). Learning models of PTSD: Theoretical ac- tematic review and meta-analysis. Clin. Psychol. Rev. 32, 301315.
counts and psychobiological evidence. Int. J. Psychophysiol. 98, 594605.
Neumeister, A., Drevets, W.C., Belfer, I., Luckenbaugh, D.A., Henry, S., Bonne,
Lissek, S., Powers, A.S., McClure, E.B., Phelps, E.A., Woldehawariat, G., O., Herscovitch, P., Goldman, D., and Charney, D.S. (2006). Effects of a alpha
Grillon, C., and Pine, D.S. (2005). Classical fear conditioning in the anxiety dis- 2C-adrenoreceptor gene polymorphism on neural responses to facial expres-
orders: a meta-analysis. Behav. Res. Ther. 43, 13911424. sions in depression. Neuropsychopharmacology 31, 17501756.
Lissek, S., Biggs, A.L., Rabin, S.J., Cornwell, B.R., Alvarez, R.P., Pine, D.S., New, A.S., Fan, J., Murrough, J.W., Liu, X., Liebman, R.E., Guise, K.G., Tang,
and Grillon, C. (2008). Generalization of conditioned fear-potentiated startle C.Y., and Charney, D.S. (2009). A functional magnetic resonance imaging
in humans: experimental validation and clinical relevance. Behav. Res. Ther. study of deliberate emotion regulation in resilience and posttraumatic stress
46, 678687. disorder. Biol. Psychiatry 66, 656664.
Lissek, S., Bradford, D.E., Alvarez, R.P., Burton, P., Espensen-Sturges, T., Niendam, T.A., Laird, A.R., Ray, K.L., Dean, Y.M., Glahn, D.C., and Carter, C.S.
Reynolds, R.C., and Grillon, C. (2014). Neural substrates of classically condi- (2012). Meta-analytic evidence for a superordinate cognitive control network
tioned fear-generalization in humans: a parametric fMRI study. Soc. Cogn. subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 12,
Affect. Neurosci. 9, 11341142. 241268.
Norrholm, S.D., Jovanovic, T., Olin, I.W., Sands, L.A., Karapanou, I., Bradley,
MacNamara, A., Rabinak, C.A., Kennedy, A.E., Fitzgerald, D.A., Liberzon, I.,
B., and Ressler, K.J. (2011). Fear extinction in traumatized civilians with post-
Stein, M.B., and Phan, K.L. (2016). Emotion Regulatory Brain Function and
traumatic stress disorder: relation to symptom severity. Biol. Psychiatry 69,
SSRI Treatment in PTSD: Neural Correlates and Predictors of Change. Neuro-
556563.
psychopharmacology 41, 611618.
Ochsner, K.N., Bunge, S.A., Gross, J.J., and Gabrieli, J.D. (2002). Rethinking
Malik, M., and Camm, A.J. (1995). Heart Rate Variability (Futura Publishing).
feelings: an FMRI study of the cognitive regulation of emotion. J. Cogn. Neuro-
sci. 14, 12151229.
Maren, S., Phan, K.L., and Liberzon, I. (2013). The contextual brain: implica-
tions for fear conditioning, extinction and psychopathology. Nat. Rev. Neuro- Ochsner, K.N., Silvers, J.A., and Buhle, J.T. (2012). Functional imaging studies
sci. 14, 417428. of emotion regulation: a synthetic review and evolving model of the cognitive
control of emotion. Ann. N Y Acad. Sci. 1251, E1E24.
Marschner, A., Kalisch, R., Vervliet, B., Vansteenwegen, D., and Buchel, C.
(2008). Dissociable roles for the hippocampus and the amygdala in human Ohman, A. (2005). The role of the amygdala in human fear: automatic detection
cued versus context fear conditioning. J. Neurosci. 28, 90309036. of threat. Psychoneuroendocrinology 30, 953958.
Marshall, A.J., Acheson, D.T., Risbrough, V.B., Straus, L.D., and Drummond, Orr, S.P., Metzger, L.J., Lasko, N.B., Macklin, M.L., Peri, T., and Pitman, R.K.
S.P. (2014). Fear conditioning, safety learning, and sleep in humans. (2000). De novo conditioning in trauma-exposed individuals with and without
J. Neurosci. 34, 1175411760. posttraumatic stress disorder. J. Abnorm. Psychol. 109, 290298.
Matynia, A., Kushner, S.A., and Silva, A.J. (2002). Genetic approaches to mo- Pennartz, C.M., Ito, R., Verschure, P.F., Battaglia, F.P., and Robbins, T.W.
lecular and cellular cognition: a focus on LTP and learning and memory. Annu. (2011). The hippocampal-striatal axis in learning, prediction and goal-directed
Rev. Genet. 36, 687720. behavior. Trends Neurosci. 34, 548559.
28 Neuron 92, October 5, 2016
Neuron
Perspective
Pereira de Vasconcelos, A., and Cassel, J.C. (2015). The nonspecific thal- Sandi, C., and Richter-Levin, G. (2009). From high anxiety trait to depression: a
amus: A place in a wedding bed for making memories last? Neurosci. Bio- neurocognitive hypothesis. Trends Neurosci. 32, 312320.
behav. Rev. 54, 175196.
Schechtman, E., Laufer, O., and Paz, R. (2010). Negative valence widens
Peri, T., Ben-Shakhar, G., Orr, S.P., and Shalev, A.Y. (2000). Psychophysio- generalization of learning. J. Neurosci. 30, 1046010464.
logic assessment of aversive conditioning in posttraumatic stress disorder.
Biol. Psychiatry 47, 512519. Schulkin, J., Morgan, M.A., and Rosen, J.B. (2005). A neuroendocrine mecha-
nism for sustaining fear. Trends Neurosci. 28, 629635.
Phan, K.L., Fitzgerald, D.A., Nathan, P.J., Moore, G.J., Uhde, T.W., and
Tancer, M.E. (2005). Neural substrates for voluntary suppression of negative Seeley, W.W., Menon, V., Schatzberg, A.F., Keller, J., Glover, G.H., Kenna, H.,
affect: a functional magnetic resonance imaging study. Biol. Psychiatry 57, Reiss, A.L., and Greicius, M.D. (2007). Dissociable intrinsic connectivity net-
210219. works for salience processing and executive control. J. Neurosci. 27, 2349
2356.
Phelps, E.A., and LeDoux, J.E. (2005). Contributions of the amygdala to
emotion processing: from animal models to human behavior. Neuron 48, Senn, V., Wolff, S.B., Herry, C., Grenier, F., Ehrlich, I., Grundemann, J., Fadok,
175187. J.P., Muller, C., Letzkus, J.J., and Luthi, A. (2014). Long-range connectivity de-
fines behavioral specificity of amygdala neurons. Neuron 81, 428437.
Phelps, E.A., Delgado, M.R., Nearing, K.I., and LeDoux, J.E. (2004). Extinction
learning in humans: role of the amygdala and vmPFC. Neuron 43, 897905. Sevenster, D., Hamm, A., Beckers, T., and Kindt, M. (2015). Heart rate pattern
and resting heart rate variability mediate individual differences in contextual
Pitman, R.K., and Orr, S.P. (1990). Twenty-four hour urinary cortisol and cate- anxiety and conditioned responses. Int. J. Psychophysiol. 98, 567576.
cholamine excretion in combat-related posttraumatic stress disorder. Biol.
Psychiatry 27, 245247. Shaban, H., Humeau, Y., Herry, C., Cassasus, G., Shigemoto, R., Ciocchi, S.,
Barbieri, S., van der Putten, H., Kaupmann, K., Bettler, B., and Luthi, A. (2006).
Pitman, R.K., Sanders, K.M., Zusman, R.M., Healy, A.R., Cheema, F., Lasko, Generalization of amygdala LTP and conditioned fear in the absence of pre-
N.B., Cahill, L., and Orr, S.P. (2002). Pilot study of secondary prevention of synaptic inhibition. Nat. Neurosci. 9, 10281035.
posttraumatic stress disorder with propranolol. Biol. Psychiatry 51, 189192.
Shah, A.J., Lampert, R., Goldberg, J., Veledar, E., Bremner, J.D., and Vaccar-
Pitman, R.K., Rasmusson, A.M., Koenen, K.C., Shin, L.M., Orr, S.P., Gilbert- ino, V. (2013). Posttraumatic stress disorder and impaired autonomic modula-
son, M.W., Milad, M.R., and Liberzon, I. (2012). Biological studies of post-trau- tion in male twins. Biol. Psychiatry 73, 11031110.
matic stress disorder. Nat. Rev. Neurosci. 13, 769787.
Shaikh al arab, A., Guedon-Moreau, L., Ducrocq, F., Molenda, S., Duhem, S.,
Poe, G.R., Walsh, C.M., and Bjorness, T.E. (2010). Cognitive neuroscience of Salleron, J., Chaudieu, I., Bert, D., Libersa, C., and Vaiva, G. (2012). Temporal
sleep. Prog. Brain Res. 185, 119. analysis of heart rate variability as a predictor of post traumatic stress disorder
in road traffic accidents survivors. J. Psychiatr. Res. 46, 790796.
Pole, N. (2007). The psychophysiology of posttraumatic stress disorder: a
meta-analysis. Psychol. Bull. 133, 725746.
Sheline, Y.I., Barch, D.M., Price, J.L., Rundle, M.M., Vaishnavi, S.N., Snyder,
A.Z., Mintun, M.A., Wang, S., Coalson, R.S., and Raichle, M.E. (2009). The
Powers, A., Etkin, A., Gyurak, A., Bradley, B., and Jovanovic, T. (2015). Asso-
default mode network and self-referential processes in depression. Proc.
ciations Between Childhood Abuse, Posttraumatic Stress Disorder, and Im-
Natl. Acad. Sci. USA 106, 19421947.
plicit Emotion Regulation Deficits: Evidence From a Low-Income, Inner-City
Population. Psychiatry 78, 251264.
Shepard, R.N. (1987). Toward a universal law of generalization for psycholog-
Preston, A.R., and Eichenbaum, H. (2013). Interplay of hippocampus and pre- ical science. Science 237, 13171323.
frontal cortex in memory. Curr. Biol. 23, R764R773.
Shin, L.M., and Liberzon, I. (2010). The neurocircuitry of fear, stress, and anx-
Quinn, J.J., Ma, Q.D., Tinsley, M.R., Koch, C., and Fanselow, M.S. (2008). In- iety disorders. Neuropsychopharmacology 35, 169191.
verse temporal contributions of the dorsal hippocampus and medial prefrontal
cortex to the expression of long-term fear memories. Learn. Mem. 15, Shin, L.M., Orr, S.P., Carson, M.A., Rauch, S.L., Macklin, M.L., Lasko, N.B.,
368372. Peters, P.M., Metzger, L.J., Dougherty, D.D., Cannistraro, P.A., et al. (2004).
Regional cerebral blood flow in the amygdala and medial prefrontal cortex dur-
Quirk, G.J., and Mueller, D. (2008). Neural mechanisms of extinction learning ing traumatic imagery in male and female Vietnam veterans with PTSD. Arch.
and retrieval. Neuropsychopharmacology 33, 5672. Gen. Psychiatry 61, 168176.
Rabinak, C.A., MacNamara, A., Kennedy, A.E., Angstadt, M., Stein, M.B., Lib- Sierra-Mercado, D., Padilla-Coreano, N., and Quirk, G.J. (2011). Dissociable
erzon, I., and Phan, K.L. (2014). Focal and aberrant prefrontal engagement roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolat-
during emotion regulation in veterans with posttraumatic stress disorder. eral amygdala in the expression and extinction of conditioned fear. Neuropsy-
Depress. Anxiety 31, 851861. chopharmacology 36, 529538.
Rasmusson, A.M., Hauger, R.L., Morgan, C.A., Bremner, J.D., Charney, D.S., Sijbrandij, M., Kleiboer, A., Bisson, J.I., Barbui, C., and Cuijpers, P. (2015).
and Southwick, S.M. (2000). Low baseline and yohimbine-stimulated plasma Pharmacological prevention of post-traumatic stress disorder and acute
neuropeptide Y (NPY) levels in combat-related PTSD. Biol. Psychiatry 47, stress disorder: a systematic review and meta-analysis. Lancet Psychiatry 2,
526539. 413421.
Reis, F.M., Almada, R.C., Fogaca, M.V., and Brandao, M.L. (2016). Rapid acti- Simmons, A., Matthews, S.C., Stein, M.B., and Paulus, M.P. (2004). Anticipa-
vation of glucocorticoid receptors in the prefrontal cortex mediates the expres- tion of emotionally aversive visual stimuli activates right insula. Neuroreport 15,
sion of contextual conditioned fear in rats. Cereb. Cortex 26, 26392649. 22612265.
Resnik, J., and Paz, R. (2015). Fear generalization in the primate amygdala. Smith, E.E., and Jonides, J. (1999). Storage and executive processes in the
Nat. Neurosci. 18, 188190. frontal lobes. Science 283, 16571661.
Resnik, J., Sobel, N., and Paz, R. (2011). Auditory aversive learning increases Southwick, S.M., Paige, S., Morgan, C.A., 3rd, Bremner, J.D., Krystal, J.H.,
discrimination thresholds. Nat. Neurosci. 14, 791796. and Charney, D.S. (1999). Neurotransmitter alterations in PTSD: catechol-
amines and serotonin. Semin. Clin. Neuropsychiatry 4, 242248.
Rougemont-Bucking, A., Linnman, C., Zeffiro, T.A., Zeidan, M.A., Lebron-Mi-
lad, K., Rodriguez-Romaguera, J., Rauch, S.L., Pitman, R.K., and Milad, M.R. Spielberg, J.M., McGlinchey, R.E., Milberg, W.P., and Salat, D.H. (2015). Brain
(2011). Altered processing of contextual information during fear extinction in network disturbance related to posttraumatic stress and traumatic brain injury
PTSD: an fMRI study. CNS Neurosci. Ther. 17, 227236. in veterans. Biol. Psychiatry 78, 210216.
Salehi, B., Cordero, M.I., and Sandi, C. (2010). Learning under stress: the in- Sripada, R.K., King, A.P., Welsh, R.C., Garfinkel, S.N., Wang, X., Sripada,
verted-U-shape function revisited. Learn. Mem. 17, 522530. C.S., and Liberzon, I. (2012). Neural dysregulation in posttraumatic stress
Neuron 92, October 5, 2016 29
Neuron
Perspective
disorder: evidence for disrupted equilibrium between salience and default Xu, W., and Sudhof, T.C. (2013). A neural circuit for memory specificity and
mode brain networks. Psychosom. Med. 74, 904911. generalization. Science 339, 12901295.
Sutherland, R.J., and Lehmann, H. (2011). Alternative conceptions of memory Yamamoto, S., Morinobu, S., Takei, S., Fuchikami, M., Matsuki, A., Yamawaki,
consolidation and the role of the hippocampus at the systems level in rodents. S., and Liberzon, I. (2009). Single prolonged stress: toward an animal model of
Curr. Opin. Neurobiol. 21, 446451. posttraumatic stress disorder. Depress. Anxiety 26, 11101117.
Thayer, J.F., Hansen, A.L., Saus-Rose, E., and Johnsen, B.H. (2009). Heart Yassa, M.A., and Stark, C.E. (2011). Pattern separation in the hippocampus.
rate variability, prefrontal neural function, and cognitive performance: the neu- Trends Neurosci. 34, 515525.
rovisceral integration perspective on self-regulation, adaptation, and health.
Ann. Behav. Med. 37, 141153. Yehuda, R. (2001). Biology of posttraumatic stress disorder. J. Clin. Psychiatry
62 (Suppl 17 ), 4146.
Thomas, M.J., Moody, T.D., Makhinson, M., and ODell, T.J. (1996). Activity-
dependent beta-adrenergic modulation of low frequency stimulation induced
Yehuda, R., Southwick, S.M., Nussbaum, G., Wahby, V., Giller, E.L., Jr., and
LTP in the hippocampal CA1 region. Neuron 17, 475482.
Mason, J.W. (1990). Low urinary cortisol excretion in patients with posttrau-
van der Kolk, B.A., Greenberg, M.S., Orr, S.P., and Pitman, R.K. (1989). Endog- matic stress disorder. J. Nerv. Ment. Dis. 178, 366369.
enous opioids, stress induced analgesia, and posttraumatic stress disorder.
Psychopharmacol. Bull. 25, 417421. Yehuda, R., Southwick, S.M., Krystal, J.H., Bremner, D., Charney, D.S., and
Mason, J.W. (1993). Enhanced suppression of cortisol following dexametha-
van Rooij, S.J., Geuze, E., Kennis, M., Rademaker, A.R., and Vink, M. (2015). sone administration in posttraumatic stress disorder. Am. J. Psychiatry 150,
Neural correlates of inhibition and contextual cue processing related to treat- 8386.
ment response in PTSD. Neuropsychopharmacology 40, 667675.
Yehuda, R., Boisoneau, D., Lowy, M.T., and Giller, E.L., Jr. (1995a). Dose-
van Rooij, S.J., Kennis, M., Vink, M., and Geuze, E. (2016). Predicting treat- response changes in plasma cortisol and lymphocyte glucocorticoid receptors
ment outcome in PTSD: a longitudinal functional MRI study on trauma-unre- following dexamethasone administration in combat veterans with and without
lated emotional processing. Neuropsychopharmacology 41, 11561165. posttraumatic stress disorder. Arch. Gen. Psychiatry 52, 583593.
Vanderheyden, W.M., Poe, G.R., and Liberzon, I. (2014). Trauma exposure and Yehuda, R., Kahana, B., Binder-Brynes, K., Southwick, S.M., Mason, J.W.,
sleep: using a rodent model to understand sleep function in PTSD. Exp. Brain and Giller, E.L. (1995b). Low urinary cortisol excretion in Holocaust survivors
Res. 232, 15751584. with posttraumatic stress disorder. Am. J. Psychiatry 152, 982986.
VanElzakker, M.B., Dahlgren, M.K., Davis, F.C., Dubois, S., and Shin, L.M. Yehuda, R., Teicher, M.H., Trestman, R.L., Levengood, R.A., and Siever, L.J.
(2014). From Pavlov to PTSD: the extinction of conditioned fear in rodents, hu- (1996). Cortisol regulation in posttraumatic stress disorder and major depres-
mans, and anxiety disorders. Neurobiol. Learn. Mem. 113, 318. sion: a chronobiological analysis. Biol. Psychiatry 40, 7988.
Veit, R., Flor, H., Erb, M., Hermann, C., Lotze, M., Grodd, W., and Birbaumer,
Yehuda, R., Brand, S.R., Golier, J.A., and Yang, R.K. (2006). Clinical correlates
N. (2002). Brain circuits involved in emotional learning in antisocial behavior
of DHEA associated with post-traumatic stress disorder. Acta Psychiatr.
and social phobia in humans. Neurosci. Lett. 328, 233236.
Scand. 114, 187193.
White, S., Acierno, R., Ruggiero, K.J., Koenen, K.C., Kilpatrick, D.G., Galea, S.,
Gelernter, J., Williamson, V., McMichael, O., Vladimirov, V.I., and Amstadter, Yehuda, R., Bierer, L.M., Pratchett, L.C., Lehrner, A., Koch, E.C., Van Manen,
A.B. (2013). Association of CRHR1 variants and posttraumatic stress symp- J.A., Flory, J.D., Makotkine, I., and Hildebrandt, T. (2015). Cortisol augmenta-
toms in hurricane exposed adults. J. Anxiety Disord. 27, 678683. tion of a psychological treatment for warfighters with posttraumatic stress dis-
order: Randomized trial showing improved treatment retention and outcome.
Wilker, S., Pfeiffer, A., Elbert, T., Ovuga, E., Karabatsiakis, A., Krumbholz, A., Psychoneuroendocrinology 51, 589597.
Thieme, D., Schelling, G., and Kolassa, I.T. (2016). Endocannabinoid concen-
trations in hair are associated with PTSD symptom severity. Psychoneuroen- Zelikowsky, M., Hersman, S., Chawla, M.K., Barnes, C.A., and Fanselow, M.S.
docrinology 67, 198206. (2014). Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex
track differential components of contextual fear. J. Neurosci. 34, 84628466.
Wingenfeld, K., Whooley, M.A., Neylan, T.C., Otte, C., and Cohen, B.E. (2015).
Effect of current and lifetime posttraumatic stress disorder on 24-h urinary cat- Zhang, Y., Liu, F., Chen, H., Li, M., Duan, X., Xie, B., and Chen, H. (2015). Intra-
echolamines and cortisol: results from the Mind Your Heart Study. Psycho- network and internetwork functional connectivity alterations in post-traumatic
neuroendocrinology 52, 8391. stress disorder. J. Affect. Disord. 187, 114121.
30 Neuron 92, October 5, 2016
Das könnte Ihnen auch gefallen
- Neuropsychiatric Guide to Modern Everyday PsychiatVon EverandNeuropsychiatric Guide to Modern Everyday PsychiatNoch keine Bewertungen
- Context Processing and The Neurobiology of Post Traumatic PDFDokument35 SeitenContext Processing and The Neurobiology of Post Traumatic PDFUSM San IgnacioNoch keine Bewertungen
- 2009 Nihms105893Dokument25 Seiten2009 Nihms105893IgnacioJoséCalderónPérezNoch keine Bewertungen
- Acupuntura AlgoDokument6 SeitenAcupuntura AlgoSergio SCNoch keine Bewertungen
- Estiulo Afectivo Correl Neurofisiol DavidsonDokument17 SeitenEstiulo Afectivo Correl Neurofisiol DavidsonAna Pia PiancatelliNoch keine Bewertungen
- Brain Circuit Dysfunction in Post - Traumatic Stress Disorder: From Mouse To Man - Robert J. FensterDokument17 SeitenBrain Circuit Dysfunction in Post - Traumatic Stress Disorder: From Mouse To Man - Robert J. FensterAlvaro Arboledas LoriteNoch keine Bewertungen
- Lanius & Frewen 2006 - Toward A PsychobiologyDokument15 SeitenLanius & Frewen 2006 - Toward A PsychobiologyCarlos Eduardo NorteNoch keine Bewertungen
- Nemeroff Heim PTSDDokument12 SeitenNemeroff Heim PTSDmariela100% (1)
- Neural Circuits Underlying The Pathophysiology of Mood DisordersDokument11 SeitenNeural Circuits Underlying The Pathophysiology of Mood Disordersrocambolescas perthNoch keine Bewertungen
- Biological Psychology: ArticleinfoDokument15 SeitenBiological Psychology: ArticleinfoManuela SilvaNoch keine Bewertungen
- 1 s2.0 S2352289519300438 MainDokument9 Seiten1 s2.0 S2352289519300438 MainOlga MichailidouNoch keine Bewertungen
- Aupperle 2012 - Executive Function and PTSDDokument9 SeitenAupperle 2012 - Executive Function and PTSDElena-Andreea MutNoch keine Bewertungen
- Hakdogggg SKKKKDokument2 SeitenHakdogggg SKKKKMay PerezNoch keine Bewertungen
- Phillips 2008Dokument25 SeitenPhillips 2008Donald Cabrera AstudilloNoch keine Bewertungen
- Cognition and PTSDPDFDokument14 SeitenCognition and PTSDPDFAndrés Felipe SarmientoNoch keine Bewertungen
- Paper 1Dokument16 SeitenPaper 1api-348971279Noch keine Bewertungen
- 2012 Nihms311812Dokument23 Seiten2012 Nihms311812IgnacioJoséCalderónPérezNoch keine Bewertungen
- Case Study - PTSD Final PaperDokument29 SeitenCase Study - PTSD Final PaperMay PerezNoch keine Bewertungen
- Emotion Modulation in PTSD: Clinical and Neurobiological Evidence For A Dissociative SubtypeDokument8 SeitenEmotion Modulation in PTSD: Clinical and Neurobiological Evidence For A Dissociative SubtypeKarla Katrina CajigalNoch keine Bewertungen
- Progress in Neuropsychopharmacology & Biological PsychiatryDokument12 SeitenProgress in Neuropsychopharmacology & Biological PsychiatryEmergencia y Trauma HICNoch keine Bewertungen
- State of The Art: The Biology of Fear-And Anxiety-Related BehaviorsDokument19 SeitenState of The Art: The Biology of Fear-And Anxiety-Related BehaviorsHarumWulansariNoch keine Bewertungen
- Psychiatric Manifestations of Neurologic DiseaseDokument14 SeitenPsychiatric Manifestations of Neurologic DiseaseMesay AssefaNoch keine Bewertungen
- NIH Public Access: Stress Risk Factors and Stress-Related Pathology: Neuroplasticity, Epigenetics and EndophenotypesDokument29 SeitenNIH Public Access: Stress Risk Factors and Stress-Related Pathology: Neuroplasticity, Epigenetics and EndophenotypesEntreNoch keine Bewertungen
- Levin 1999 Neuroimaging EMDRDokument14 SeitenLevin 1999 Neuroimaging EMDRPabloNoch keine Bewertungen
- Olive 2018 Superior Colliculus Resting State NDokument12 SeitenOlive 2018 Superior Colliculus Resting State Nbenoit berthailNoch keine Bewertungen
- The Circumplex Model of Affect: An Integrative Approach To Affective Neuroscience, Cognitive Development, and PsychopathologyDokument20 SeitenThe Circumplex Model of Affect: An Integrative Approach To Affective Neuroscience, Cognitive Development, and PsychopathologyFemke MeijersNoch keine Bewertungen
- Schwartz Volitional BrainDokument28 SeitenSchwartz Volitional BrainBadi VillarNoch keine Bewertungen
- Post-Traumatic: DirectionsDokument9 SeitenPost-Traumatic: DirectionsmarielaNoch keine Bewertungen
- Interface Between Hypothalamic-Pituitary-Adrenal Axis Andbrain-Derived Neurotrophic Factor in DepressionDokument13 SeitenInterface Between Hypothalamic-Pituitary-Adrenal Axis Andbrain-Derived Neurotrophic Factor in DepressioncarlosNoch keine Bewertungen
- Pittenger 2007Dokument22 SeitenPittenger 2007rocambolescas perthNoch keine Bewertungen
- Pascal 2019 Ooperation of The Vestibular and Cerebellar Networks in Anxiety Disorders and DepressionDokument56 SeitenPascal 2019 Ooperation of The Vestibular and Cerebellar Networks in Anxiety Disorders and DepressionJuan Hernández GarcíaNoch keine Bewertungen
- Fnbeh 08 00378Dokument8 SeitenFnbeh 08 00378Juan Alberto GonzálezNoch keine Bewertungen
- Defining Biotypes For Depression and Anxiety - Williams2016Dokument16 SeitenDefining Biotypes For Depression and Anxiety - Williams2016alina-loredana.popescu26Noch keine Bewertungen
- Lanius2017 - The Innate Alarm SystemDokument7 SeitenLanius2017 - The Innate Alarm Systembenoit berthailNoch keine Bewertungen
- Neurobiology of Resilience: ReviewDokument10 SeitenNeurobiology of Resilience: ReviewVissente TapiaNoch keine Bewertungen
- Resting State Mod Structurelle McsweeneyDokument24 SeitenResting State Mod Structurelle Mcsweeneyouazzani youssefNoch keine Bewertungen
- 1 s2.0 S0028393217302403 MainDokument8 Seiten1 s2.0 S0028393217302403 MaincursosNoch keine Bewertungen
- PV Interneuron Alterations in Stress RelDokument98 SeitenPV Interneuron Alterations in Stress RelSea SaltNoch keine Bewertungen
- Psychiatric Manifestations of Neurologic Disease Where Are We HeadedDokument15 SeitenPsychiatric Manifestations of Neurologic Disease Where Are We HeadedAlvaro StephensNoch keine Bewertungen
- The Circumplex Model of AffectDokument25 SeitenThe Circumplex Model of AffectMarcelo100% (1)
- Abramovitch2021 - The C Factor - Cognitive Dysfunction As A Transdiagnostic Dimension in PsychopathologyDokument27 SeitenAbramovitch2021 - The C Factor - Cognitive Dysfunction As A Transdiagnostic Dimension in PsychopathologyValter MachadoNoch keine Bewertungen
- Linking Molecules To Mood: New Insight Into The Biology of DepressionDokument16 SeitenLinking Molecules To Mood: New Insight Into The Biology of Depressiondudul pudlianNoch keine Bewertungen
- Pubs Shin 2010 Neural CorDokument8 SeitenPubs Shin 2010 Neural CorTJSNoch keine Bewertungen
- Neural Contributors To Trauma Resilience: A Review of Longitudinal Neuroimaging StudiesDokument17 SeitenNeural Contributors To Trauma Resilience: A Review of Longitudinal Neuroimaging StudiesHelena QuintNoch keine Bewertungen
- Andreescu (2012) Comorbid Anxiety and Depression. Bete Noire or Quick Fix - BJP - 179.fullDokument4 SeitenAndreescu (2012) Comorbid Anxiety and Depression. Bete Noire or Quick Fix - BJP - 179.fullKermit the frogNoch keine Bewertungen
- Well-Being and Affective Style: Neural Substrates and Biobehavioural CorrelatesDokument17 SeitenWell-Being and Affective Style: Neural Substrates and Biobehavioural CorrelatesGustavo Adolfo DomínguezNoch keine Bewertungen
- Vol. 6 No. 2 February 2019Dokument6 SeitenVol. 6 No. 2 February 2019Nilofa YasminNoch keine Bewertungen
- Review The Role of Inflammation and Microglial Activation in The Pathophysiology of Psychiatric DisordersDokument14 SeitenReview The Role of Inflammation and Microglial Activation in The Pathophysiology of Psychiatric DisordersDusan MladenovicNoch keine Bewertungen
- Richardson 2018Dokument7 SeitenRichardson 2018Maria Von ShaftNoch keine Bewertungen
- WFSBP Consensus Paper Biological Markers in DepressionDokument34 SeitenWFSBP Consensus Paper Biological Markers in Depressionscabrera_scribdNoch keine Bewertungen
- Allostasis and The Epigenetics of Brain and Body Health Over The Life Course The Brain On StressDokument2 SeitenAllostasis and The Epigenetics of Brain and Body Health Over The Life Course The Brain On StressSamadhi SalguedoNoch keine Bewertungen
- Fnins 17 1281401Dokument16 SeitenFnins 17 1281401adinjiaNoch keine Bewertungen
- 2010 Goh Vulnera - EngDokument5 Seiten2010 Goh Vulnera - EngFernandoCedroNoch keine Bewertungen
- DNB 22 2 J PDFDokument5 SeitenDNB 22 2 J PDFFlorinaNoch keine Bewertungen
- ANSLAB: Integrated Multichannel Peripheral Biosignal Processing in Psychophysiological ScienceDokument18 SeitenANSLAB: Integrated Multichannel Peripheral Biosignal Processing in Psychophysiological SciencemeliananitaNoch keine Bewertungen
- Neurobiología Del Control de Impulsos 2008Dokument11 SeitenNeurobiología Del Control de Impulsos 2008María Angélica MercadoNoch keine Bewertungen
- Material 1 Psicosis y TeaDokument12 SeitenMaterial 1 Psicosis y TeaALEJANDRO PALMA SOTONoch keine Bewertungen
- tmp33F4 TMPDokument7 Seitentmp33F4 TMPFrontiersNoch keine Bewertungen
- 1.psychiatric Neuroscience - Incorporating Pathophysiology Into Clinical Case Formulation - ClinicalKeyDokument43 Seiten1.psychiatric Neuroscience - Incorporating Pathophysiology Into Clinical Case Formulation - ClinicalKeyClaudia0% (1)
- Conversion DisorderDokument8 SeitenConversion DisorderMaria Von ShaftNoch keine Bewertungen
- Epilepsy A Global ApproachDokument237 SeitenEpilepsy A Global ApproachVictor CarrenoNoch keine Bewertungen
- Microglia 2017Dokument10 SeitenMicroglia 2017Victor CarrenoNoch keine Bewertungen
- Psychophysiology - 2022 - Smith - Approach Motivation and Loneliness Individual Differences and Parasympathetic ActivityDokument8 SeitenPsychophysiology - 2022 - Smith - Approach Motivation and Loneliness Individual Differences and Parasympathetic ActivityVictor CarrenoNoch keine Bewertungen
- Dopamine Modulates Adaptive Forgetting in Medial Prefrontal CortexDokument39 SeitenDopamine Modulates Adaptive Forgetting in Medial Prefrontal CortexVictor CarrenoNoch keine Bewertungen
- Cell Language Theory, The Connecting Mind and Matter PDFDokument610 SeitenCell Language Theory, The Connecting Mind and Matter PDFVictor CarrenoNoch keine Bewertungen
- 10 1001@jamapsychiatry 2016 4287Dokument10 Seiten10 1001@jamapsychiatry 2016 4287Victor CarrenoNoch keine Bewertungen
- Sv121 BlankeDokument12 SeitenSv121 BlankeVictor CarrenoNoch keine Bewertungen
- Insel 2005Dokument4 SeitenInsel 2005Victor CarrenoNoch keine Bewertungen
- 10 1001@jamapsychiatry 2016 3325Dokument9 Seiten10 1001@jamapsychiatry 2016 3325Victor CarrenoNoch keine Bewertungen
- Project For A Scientific Psychiatry Neuroscience Literacy: EditorialDokument1 SeiteProject For A Scientific Psychiatry Neuroscience Literacy: EditorialVictor CarrenoNoch keine Bewertungen
- 10 1001@jamapsychiatry 2016 4287Dokument10 Seiten10 1001@jamapsychiatry 2016 4287Victor CarrenoNoch keine Bewertungen
- Chung 2013Dokument7 SeitenChung 2013Victor CarrenoNoch keine Bewertungen
- R Evues 4Dokument28 SeitenR Evues 4Victor CarrenoNoch keine Bewertungen
- Esquizofrenia AnhedoniaDokument11 SeitenEsquizofrenia AnhedoniaVictor CarrenoNoch keine Bewertungen
- 10 1056@NEJMra1700312Dokument12 Seiten10 1056@NEJMra1700312Victor CarrenoNoch keine Bewertungen
- EsquizoobsesivoDokument4 SeitenEsquizoobsesivoVictor CarrenoNoch keine Bewertungen
- Voluntad Revista 3Dokument20 SeitenVoluntad Revista 3Victor CarrenoNoch keine Bewertungen
- Sinke2017 2Dokument15 SeitenSinke2017 2Victor CarrenoNoch keine Bewertungen
- Volluntad y Personalidad BorderlineDokument9 SeitenVolluntad y Personalidad BorderlineVictor CarrenoNoch keine Bewertungen
- EAWEDokument45 SeitenEAWEVictor CarrenoNoch keine Bewertungen
- Hadley 2012Dokument11 SeitenHadley 2012Victor CarrenoNoch keine Bewertungen
- Risas y TECIONDokument45 SeitenRisas y TECIONVictor CarrenoNoch keine Bewertungen
- Anhedonia, Depression, Anxiety, and Craving in Opiate Dependent Patients Stabilized On Oral Naltrexone or An Extended Release Naltrexone ImplantDokument8 SeitenAnhedonia, Depression, Anxiety, and Craving in Opiate Dependent Patients Stabilized On Oral Naltrexone or An Extended Release Naltrexone ImplantVictor CarrenoNoch keine Bewertungen
- Hutson Et Al-2015-Journal of NeurochemistryDokument11 SeitenHutson Et Al-2015-Journal of NeurochemistryVictor CarrenoNoch keine Bewertungen
- Direct Comparison of The Neural Substrates of Recognition Memory For Words and FacesDokument15 SeitenDirect Comparison of The Neural Substrates of Recognition Memory For Words and FacesVictor CarrenoNoch keine Bewertungen
- Systematic Evidence Synthesis of Treatments For ADHD in Children and Adolescents Indirect Treatment Comparisons of Lisdexamfetamine WithDokument14 SeitenSystematic Evidence Synthesis of Treatments For ADHD in Children and Adolescents Indirect Treatment Comparisons of Lisdexamfetamine WithVictor CarrenoNoch keine Bewertungen
- Suicidio y Via de La Kinurenina 2016 RCTDokument9 SeitenSuicidio y Via de La Kinurenina 2016 RCTVictor CarrenoNoch keine Bewertungen
- Suicidio e Inflamacion Revision 2016Dokument55 SeitenSuicidio e Inflamacion Revision 2016Victor CarrenoNoch keine Bewertungen
- Suicidio y Via de La Kinurenina 2016 RCTDokument9 SeitenSuicidio y Via de La Kinurenina 2016 RCTVictor CarrenoNoch keine Bewertungen
- International Journal of PharmaceuticsDokument6 SeitenInternational Journal of PharmaceuticsSjis11362Noch keine Bewertungen
- Immediate Care of The NewbornDokument4 SeitenImmediate Care of The NewbornMichelle GambolNoch keine Bewertungen
- Chapter 1 Essay NegligenceDokument3 SeitenChapter 1 Essay NegligenceVladimir Hechavarria100% (1)
- Case of Bataliny v. RussiaDokument29 SeitenCase of Bataliny v. RussiamrbtdfNoch keine Bewertungen
- DR Irza Wahid - Annemia Approach - 139Dokument60 SeitenDR Irza Wahid - Annemia Approach - 139single_ladyNoch keine Bewertungen
- Pemanfaatan Teknik Assisted Hatching Dalam Meningkatkan Implantasi EmbrioDokument10 SeitenPemanfaatan Teknik Assisted Hatching Dalam Meningkatkan Implantasi EmbrioMumutTeaNoch keine Bewertungen
- Intramedullary Nailing For Femur Fracture Web Version EnglishDokument6 SeitenIntramedullary Nailing For Femur Fracture Web Version EnglishCarima JatnoNoch keine Bewertungen
- Current Management of LabourDokument48 SeitenCurrent Management of Labourapi-3705046100% (4)
- Effectiveness of A Comprehensive Hand Hygiene Program ForDokument11 SeitenEffectiveness of A Comprehensive Hand Hygiene Program Form1k0eNoch keine Bewertungen
- Visual NeglectDokument2 SeitenVisual NeglectPierre A. RodulfoNoch keine Bewertungen
- Love Pain e Book PDFDokument14 SeitenLove Pain e Book PDFTina AnesNoch keine Bewertungen
- Buffalo LeprosyDokument4 SeitenBuffalo LeprosyALTAF HUSAINNoch keine Bewertungen
- Bentley Autopipe v8 CrackDokument3 SeitenBentley Autopipe v8 CrackAdi M. Mutawali100% (2)
- Girimananda Sutta: Discourse To Girimananda TheraDokument4 SeitenGirimananda Sutta: Discourse To Girimananda TheraMatt Aldridge100% (1)
- Raku Fire Dragon Way PDFDokument17 SeitenRaku Fire Dragon Way PDFmonipiron100% (4)
- Aseptic MeningitisDokument24 SeitenAseptic Meningitisidno1008100% (1)
- Pediatric Neuropsychology Case Studies PDFDokument358 SeitenPediatric Neuropsychology Case Studies PDFGabriela Hernandez100% (1)
- MEDDRA, PVPI, PVMF, PsMF-1Dokument28 SeitenMEDDRA, PVPI, PVMF, PsMF-1Nitin Shah100% (1)
- Pedia Small Notebook Edited PDFDokument17 SeitenPedia Small Notebook Edited PDFAshNoch keine Bewertungen
- Techniques For Parasite Egg Identification in Faecal SamplesDokument9 SeitenTechniques For Parasite Egg Identification in Faecal SamplesDr.Kedar Karki ,M.V.Sc.Preventive Vet.Medicine CLSU Philippines100% (2)
- NSCA Tools and Resources PDFDokument3 SeitenNSCA Tools and Resources PDFkunal mishraNoch keine Bewertungen
- Ais YesDokument15 SeitenAis YesLauriz Dillumas MachonNoch keine Bewertungen
- Principle of Laser Application in Medicine & LASER SAFETY5Dokument62 SeitenPrinciple of Laser Application in Medicine & LASER SAFETY5melisandrianaNoch keine Bewertungen
- HSE Campiagn - Safety Talk-01Dokument2 SeitenHSE Campiagn - Safety Talk-01NeeleshSoganiNoch keine Bewertungen
- Cardiovascular System QuizDokument4 SeitenCardiovascular System QuizIrams KitchenNoch keine Bewertungen
- A Regenerative Interventional Approach To The Management of Degenerative Low Back PainDokument16 SeitenA Regenerative Interventional Approach To The Management of Degenerative Low Back PainAthenaeum Scientific PublishersNoch keine Bewertungen
- 5 Meo DMTDokument18 Seiten5 Meo DMTJimmy Contreras Rey100% (1)
- Jordan University of Science and TechnologyDokument33 SeitenJordan University of Science and TechnologyNourAldin AbuSalehNoch keine Bewertungen
- Reasons For Extraction of Primary Teeth in Jordan-A Study.: August 2013Dokument5 SeitenReasons For Extraction of Primary Teeth in Jordan-A Study.: August 2013Mutia KumalasariNoch keine Bewertungen
- Leadership Compass WorkshopDokument15 SeitenLeadership Compass WorkshopMarisa HaireNoch keine Bewertungen
- The Obesity Code: Unlocking the Secrets of Weight LossVon EverandThe Obesity Code: Unlocking the Secrets of Weight LossBewertung: 4 von 5 Sternen4/5 (6)
- The Age of Magical Overthinking: Notes on Modern IrrationalityVon EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityBewertung: 4 von 5 Sternen4/5 (28)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsVon EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsNoch keine Bewertungen
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeVon EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeBewertung: 2 von 5 Sternen2/5 (1)
- ADHD is Awesome: A Guide to (Mostly) Thriving with ADHDVon EverandADHD is Awesome: A Guide to (Mostly) Thriving with ADHDBewertung: 5 von 5 Sternen5/5 (2)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionVon EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionBewertung: 4 von 5 Sternen4/5 (404)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsVon EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsBewertung: 5 von 5 Sternen5/5 (1)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisVon EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisBewertung: 4.5 von 5 Sternen4.5/5 (42)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedVon EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedBewertung: 5 von 5 Sternen5/5 (81)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsVon EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsBewertung: 3.5 von 5 Sternen3.5/5 (3)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisVon EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisBewertung: 4 von 5 Sternen4/5 (1)
- Why We Die: The New Science of Aging and the Quest for ImmortalityVon EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityBewertung: 4 von 5 Sternen4/5 (3)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaVon EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- The Comfort of Crows: A Backyard YearVon EverandThe Comfort of Crows: A Backyard YearBewertung: 4.5 von 5 Sternen4.5/5 (23)
- Sleep Stories for Adults: Overcome Insomnia and Find a Peaceful AwakeningVon EverandSleep Stories for Adults: Overcome Insomnia and Find a Peaceful AwakeningBewertung: 4 von 5 Sternen4/5 (3)
- Love Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)Von EverandLove Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)Noch keine Bewertungen
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisVon EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisBewertung: 3.5 von 5 Sternen3.5/5 (2)
- Gut: the new and revised Sunday Times bestsellerVon EverandGut: the new and revised Sunday Times bestsellerBewertung: 4 von 5 Sternen4/5 (392)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryVon EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryBewertung: 4 von 5 Sternen4/5 (44)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Von EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Bewertung: 4.5 von 5 Sternen4.5/5 (110)
- The Marshmallow Test: Mastering Self-ControlVon EverandThe Marshmallow Test: Mastering Self-ControlBewertung: 4.5 von 5 Sternen4.5/5 (58)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessVon EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessBewertung: 4.5 von 5 Sternen4.5/5 (328)
- Dark Psychology: Learn To Influence Anyone Using Mind Control, Manipulation And Deception With Secret Techniques Of Dark Persuasion, Undetected Mind Control, Mind Games, Hypnotism And BrainwashingVon EverandDark Psychology: Learn To Influence Anyone Using Mind Control, Manipulation And Deception With Secret Techniques Of Dark Persuasion, Undetected Mind Control, Mind Games, Hypnotism And BrainwashingBewertung: 4 von 5 Sternen4/5 (1138)
- Troubled: A Memoir of Foster Care, Family, and Social ClassVon EverandTroubled: A Memoir of Foster Care, Family, and Social ClassBewertung: 4.5 von 5 Sternen4.5/5 (27)