Beruflich Dokumente
Kultur Dokumente
Expt 4
Hochgeladen von
Rein MirandaCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Expt 4
Hochgeladen von
Rein MirandaCopyright:
Verfügbare Formate
Chan, Miranda, Tapel PhCh 135 PH2
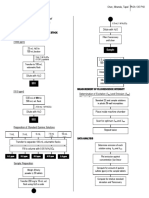
EXPERIMENT 4: Determination of
Quinine Sulfate in Tablets Using 0.5 mL 1 M H2SO4
Fluorescence Spectroscopy
Dilute with H2O
PREPARATION OF STANDARD QUININE STOCK
SOLUTION, 1000 ppm Filter if necessary
[1000 ppm] until clear
25 mL H2O in
Sample
100-mL beaker
5 mL 1 M H2SO4
0.1207 g quinine 2.5 µL sample solution in
Transfer to 100-mL 100-mL volumetric flask
volumetric flask
Dilute with 0.05 M H2SO4
Dilute with H2O
Measure fluorescence
SS1 intensities in triplicates
MEASUREMENT OF FLUORESCENCE INTENSITY
[10.0 ppm]
Determination of Excitation (lex) and Emission (lem) Wavelengths
5.00 mL of SS1 in
Standard (5) and sample solutions in
500-mL vol. flask
plastic 96-well microplates
25.0 mL 1 M H2SO4
Dilute with H2O Place inside machine chamber
SS2
Scan for optimum lex and lem
Preparation of Standard Quinine Solutions
Repeat twice
1 mL SS2 3 mL SS2 5 mL SS2 7.5 mL SS2 10 mL SS2
DATA ANALYSIS
Transfer to separate 100-mL volumetric flasks
Determine emission of each
Fill to volume with 0.05 M H2SO4 solution using lex and lem
0.1 ppm 0.3 ppm 0.5 ppm 0.75 ppm 1.0 ppm Plot emission against concentration
Use linear regression
Sample Preparation
Compute for amount of quinine
Weigh & finely
sulfate (ppm) in the sample tablets
powder ≥ 20 tablets
Compute for relative standard
Transfer 200 mg to 10-mL vol.
deviation and % recovery
flask using H2O as aide
Das könnte Ihnen auch gefallen
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Exercise Set BDokument1 SeiteExercise Set BRein MirandaNoch keine Bewertungen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Patient Counseling ActivityDokument1 SeitePatient Counseling ActivityRein MirandaNoch keine Bewertungen
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Reproductive StructuresDokument16 SeitenReproductive StructuresRein MirandaNoch keine Bewertungen
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Expt 4Dokument1 SeiteExpt 4Rein MirandaNoch keine Bewertungen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Expt 3 PDFDokument1 SeiteExpt 3 PDFRein MirandaNoch keine Bewertungen
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- Expt 3Dokument2 SeitenExpt 3Rein MirandaNoch keine Bewertungen
- Chapter 4 - Part 1 - Group 2Dokument48 SeitenChapter 4 - Part 1 - Group 2Ahmad SalahuddinNoch keine Bewertungen
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- Data Science - Machine LearningDokument3 SeitenData Science - Machine Learningfaheem ahmedNoch keine Bewertungen
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Aesthetics and PsychobiologyDokument8 SeitenAesthetics and PsychobiologyDavid RojasNoch keine Bewertungen
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Isolasi Dan Pemisahan Senyawa Alkaloid Dari Buah Mahkota DEWA (Phaleria Macrocarpa Boerl.) DENGAN METODE KROMATOGRAFI CAIRDokument7 SeitenIsolasi Dan Pemisahan Senyawa Alkaloid Dari Buah Mahkota DEWA (Phaleria Macrocarpa Boerl.) DENGAN METODE KROMATOGRAFI CAIRFitra DamanhuryNoch keine Bewertungen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- FS-726 Market ReserachDokument2 SeitenFS-726 Market ReserachBurhan HajeeNoch keine Bewertungen
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- SPSS Workshop: Utilizing and Implementing SPSS in Our OC-Math Statistics ClassesDokument11 SeitenSPSS Workshop: Utilizing and Implementing SPSS in Our OC-Math Statistics Classesgura1999Noch keine Bewertungen
- Falsafah / Paradigma PenyelidikanDokument28 SeitenFalsafah / Paradigma Penyelidikanying reenNoch keine Bewertungen
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Let's Calculate NA 2.0Dokument66 SeitenLet's Calculate NA 2.0Eyenee AbdullahNoch keine Bewertungen
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Creating A Data Analysis Plan What ToDokument7 SeitenCreating A Data Analysis Plan What TokaaanyuNoch keine Bewertungen
- Quantitative Methods Prelim Quiz 2 PerfectDokument4 SeitenQuantitative Methods Prelim Quiz 2 Perfectjulius obregonNoch keine Bewertungen
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- CHAPTER-III DoneDokument5 SeitenCHAPTER-III DoneThresia LozaritaNoch keine Bewertungen
- Practical Research 2 w1 w2Dokument47 SeitenPractical Research 2 w1 w2RA HI MANoch keine Bewertungen
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- How Is Standard Deviation Used To Determine Risk? (10 Points)Dokument2 SeitenHow Is Standard Deviation Used To Determine Risk? (10 Points)Rodin PaspasanNoch keine Bewertungen
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Artikel Rev Sisca (Repaired)Dokument19 SeitenArtikel Rev Sisca (Repaired)Zakky SugisNoch keine Bewertungen
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- Guidelines For Estimating and Reporting Measurement Uncertainty of Chemical Test ResultsDokument11 SeitenGuidelines For Estimating and Reporting Measurement Uncertainty of Chemical Test ResultsAlexandr ChuvakovNoch keine Bewertungen
- Research Methodology Through OnionDokument15 SeitenResearch Methodology Through OnionZarin Irfan100% (1)
- Integration of Well and Seismic Data Using Geostatistics-2Dokument101 SeitenIntegration of Well and Seismic Data Using Geostatistics-2qbirax100% (1)
- Chapter 3 Methodology Revised Partial Data GatheringDokument7 SeitenChapter 3 Methodology Revised Partial Data GatheringFhranzy VillegasNoch keine Bewertungen
- Use of Assessment in Outcomes AssessmentDokument11 SeitenUse of Assessment in Outcomes AssessmentUser LeftNoch keine Bewertungen
- Basic Marketing ResearchDokument159 SeitenBasic Marketing ResearchJason TongNoch keine Bewertungen
- A Fuzzy-Probabilistic Maintenance Optimization Cost ModelDokument1 SeiteA Fuzzy-Probabilistic Maintenance Optimization Cost ModelSemana de Engenharia '2010Noch keine Bewertungen
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (120)
- Improving Lean Healthcare EffectivenessDokument27 SeitenImproving Lean Healthcare EffectivenessHadi P.Noch keine Bewertungen
- Forecasting - NotesDokument65 SeitenForecasting - NotesMelvin KasiryeNoch keine Bewertungen
- Metrology & SQC - NewDokument4 SeitenMetrology & SQC - NewKashif RazaqNoch keine Bewertungen
- T-Test For Independent Samples: West Visayas State University College of Education Graduate SchoolDokument7 SeitenT-Test For Independent Samples: West Visayas State University College of Education Graduate SchoolChristian Jalandoni AlmanonNoch keine Bewertungen
- End of Year Project ExamplesDokument10 SeitenEnd of Year Project ExamplesMalik Fahad YounasNoch keine Bewertungen
- Nonlinear ARDL Model ManualDokument18 SeitenNonlinear ARDL Model ManualDumegã KokutseNoch keine Bewertungen
- Success of Mang InasalDokument44 SeitenSuccess of Mang Inasalsubmarine00170% (23)
- Sta 2200 Probability and Statistics IiDokument4 SeitenSta 2200 Probability and Statistics IimichaelNoch keine Bewertungen
- Unit 2 Reliability and Validity (External and Internal) : StructureDokument19 SeitenUnit 2 Reliability and Validity (External and Internal) : StructureVeenaVidhyaSri VijayakumarNoch keine Bewertungen
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)