Beruflich Dokumente
Kultur Dokumente
Half Book Tests 2nd Year Ch7-15
Hochgeladen von
Haroon EjazOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Half Book Tests 2nd Year Ch7-15
Hochgeladen von
Haroon EjazCopyright:
Verfügbare Formate
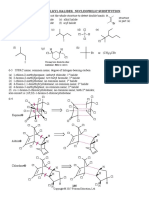
PUNJAB GROUP OF COLLEGES
ALI PUR CHATHA
Class: 2nd year Half Book Test Marks: 17 Time: 20 min
Subject: Chemistry Syllabus: Chapter 7-15
(Objective Type)
Q1: Encircle the correct option. (1 x 17 = 17)
1. Linear shape is associated with which set of hybrid orbitals.
(a). sp (b). sp2 (c). sp3 (d). dsp2
2. Which gas is used for artificial ripening of fruits?
(a). Ethane (b). Ethyne (c). Methane (d). Propane.
3. Halogenation of alkanes in the presence of sunlight undergoes:
(a). SN1 mechanism ( b). SN2 mechanism
(c). Free radical mechanism (d). Acid –base catalysed mechanism
4. Benzene cannot undergo
(a). Substitution reactions (b). Addition reactions (c). Oxidation reactions (d). Elimination
reactions.
5. Ortho, para derivatives are obtained by halogenation of:
(a). Nitrobenzene (b). Toluene (c). Benzaldehyde (d). Benzene
6. SN2 reactions are given by:
(a). Primary alkyl halides (b). Secondary alkyl halides (c). Secondary alcohols (d). Primary alcohols.
7. For which mechanisms the first step involved is the same:
(a). E1 and E2 (b). E1 and SN1 (c). E1 and SN2 (d). E2 and SN2
8. Which woody raw material is used for the manufacture of paper pulp?
(a). Cotton (b). Bagasse (c). Poplar (d). Rice straw.
9. Which compound is universal solvent:
(a). H2O (b). CH3OH (c). C2H5OH (d). CH3 – O – CH3
10. Cannizzaro’s reaction is not given by:
(a). Formaldehyde (b). Acetaldehyde (c). Benzaldehyde (d). Trimethyl acetaldehyde
11. Methanol can be prepared from hydrogenation of:
(a). CH3CN (b). CH3Br (c). HCHO (d). CH3CHO
12. Which compound is polyprotic acid
(a). CH3COOH (b). C6H4(OH)COOH (c). (COOH)2 (d). C6H5OH
13. Amino acids are prepared by
(a). Williamson’s synthesis (b). Strecker’s synthesis (c). Wurtz’s synthesis (d). Perkin’s reaction.
14. One of the following does not react with NaHSO3
(a). Methanal (b). Propanone (c). Butanone (d). 3 – pentanone
15. Ketones are prepared by oxidation of:
(a). Primary alcohols (b). Secondary alcohols (c). Tertiary alcohols (d). None of these.
16. Which of the following is a condensation polymer?
(a). Benzene (b). Bakelite (c). PVC (d). Polystyrene
17. Which is a synthetic polymer?
(a). animal fat (b).cellulose (c). starch (d). polyester
PUNJAB GROUP OF COLLEGES
ALI PUR CHATHA
Class: 2nd year Half Book Test Marks: 68 Time: 2 hr 10 min
Subject: Chemistry Syllabus: Chapter 7-15
(Subjective Type)
(SECTION I)
Q2: Write answers of any eight (8) questions (2 x 8 = 16)
1. What is vital force theory? How was it rejected?
2. Differentiate between alicyclic and aromatic compounds.
3. Define tautomerism with example.
4. What is catalytic cracking? Give an example.
5. 2 – Butyne does not show geometric isomerism but 2 – butene does. Give reason.
6. Why alkenes are called olefins?
7. What is Baeyer’s Test?
8. Convert ethene to 1 – butanol.
9. Explain why alkanes are less reactive than alkenes.
10. Describe catalytic oxidation of methane.
11. Convert: (a). CH4 into CH3NO2
(SECTION II)
Q3: Write answers of any eight (8) questions (2 x 8 = 16)
1. What is the difference between rectified spirit and absolute alcohol?
2. Why phenoxide is more stable than the ethoxide ion?
3. Define fermentation. Give one example.
4. Alcohols react with conc.H2SO4 and give different products at different conditions, give reactions
5. How does ethyl alcohol reacts with the following reagents?(a)Conc.H 2SO4(b)PCl5
6. How acetone is prepared from propyne.
7. How can you prepare (i)Formalddehyde (ii)Ethylene glycol from ethene.
8. What is condensation reaction? Give an example.
9. Discuss oxidation of aliphatic aldehdye with Fehling‘s solution.
10. What is haloform reaction?
11. Write method to prepare formaldehyde and acetaldehyde.
(SECTION III)
Q4: Write answers of any six (6) questions (2 x 6 = 12)
1. What are polyamide resins? Explain it with an example.
2. Define saponification number and iodine number.
3. What is the hardening of oil? Give an example.
4. What are Steroids? Give their general structure.
5. Define lipids and give their types.
6. What is clinker?
7. What are the four main non – woody raw materials used in the production pulp and paper?
8. Give reactions taking place in first 24 hours in setting of cement.
9. What are macronutrients?
Q: Write answers of any three (3) question. (3 x 8 = 24)
Q5: (a) What is meant by Reforming of Petroleum? Explain knocking and octane number in that regard. 4
(b) Give mechanism of Alkylation and Acylation of benzene. 4
Q6: Complete the following reaction in balanced form: 8
(i) Ethene + Alkaline KMnO4
(ii) Ethene + Sulphur monochloride
(iii) Ethyne + Ammonical silver nitrate
(iv) Methane + HNO3
Q7: (a) Explain the structure of benzene on the basis of atomic orbital treatment. 4
(b) Define hybridization. Discuss the structure of ethyne on its basis. 4
Q8: (a) How Grignard reagent reacts with the followings? 3
║ (b). H2C – CH2 (c). C2H5 – OH
(a). CH3 – C – CH3 O
(b) What is Aldol Condensation? Give two examples and mechanism of the reaction. 5
Q9: (a) What is haloform reaction? Give two examples. 4
(b) What are the factors affecting the enzyme activity? 4
Das könnte Ihnen auch gefallen
- Half Book Tests 2nd Year Ch1-6 & 16Dokument4 SeitenHalf Book Tests 2nd Year Ch1-6 & 16Haroon Ejaz80% (5)
- Chemistry Part II (1st Half)Dokument4 SeitenChemistry Part II (1st Half)Muhammad Qasim100% (1)
- Chemistry Pre-Board 2023Dokument3 SeitenChemistry Pre-Board 2023Muhammad AhsanNoch keine Bewertungen
- 1st HALF BOOK TESTS 2ND YEAR - BackupDokument3 Seiten1st HALF BOOK TESTS 2ND YEAR - Backupimran khalid100% (3)
- MCQ Chapter 8 Coordination CompoundDokument7 SeitenMCQ Chapter 8 Coordination CompoundSavien Brandan100% (3)
- Chemistry Equilibrium MCQs Chapter NotesDokument6 SeitenChemistry Equilibrium MCQs Chapter NotesAmina Khan100% (3)
- 9th Class Chemistry Mcqs English MediumDokument18 Seiten9th Class Chemistry Mcqs English MediumCh Umar Khatana100% (1)
- Facebook for Biology NotesDokument30 SeitenFacebook for Biology NotesRao Asif RajpootNoch keine Bewertungen
- Coordination CompoundDokument19 SeitenCoordination CompoundJatindra PatelNoch keine Bewertungen
- 11th Chemistry Book Back Questions New BookDokument38 Seiten11th Chemistry Book Back Questions New BookNaveen KumarNoch keine Bewertungen
- Class 12 MCQs on Alcohols, Phenols and EthersDokument9 SeitenClass 12 MCQs on Alcohols, Phenols and EthersGyanendra Vikram Maurya100% (1)
- 4 Chapter Liquids and Solids McqsDokument6 Seiten4 Chapter Liquids and Solids McqsAáwáíź Jútt0% (1)
- 2nd Year Special Exams 2021 by Bismillah Academy 0300-7980055Dokument40 Seiten2nd Year Special Exams 2021 by Bismillah Academy 0300-7980055Najeeb Ullah100% (2)
- MCQs pdf-1 PDFDokument5 SeitenMCQs pdf-1 PDFEmman Ann100% (3)
- COORDINATION CHEMISTRY TITLEDokument11 SeitenCOORDINATION CHEMISTRY TITLESubhasish Sau100% (2)
- All MCQS First YearDokument85 SeitenAll MCQS First YearNazimEhsanMalik0% (3)
- Multiple Choice Questions D AN BLOCKDokument11 SeitenMultiple Choice Questions D AN BLOCKMahrishiShukla100% (1)
- RAZA ACADEMY 10th English Test PaperDokument11 SeitenRAZA ACADEMY 10th English Test Papersamar khan75% (4)
- 10 Chapter Electrochemistry MCQS PDFDokument11 Seiten10 Chapter Electrochemistry MCQS PDFAbdulkhaliq Khan64% (14)
- 1st Year Half Book TestDokument43 Seiten1st Year Half Book Testumair talash100% (1)
- Chemistry FSC 2nd Year Solved MCQs NotesDokument72 SeitenChemistry FSC 2nd Year Solved MCQs Notesbushra3ansari83% (194)
- 1st Year Chemistry All MCQS Short Questions For Federal Board Punjab BoardDokument8 Seiten1st Year Chemistry All MCQS Short Questions For Federal Board Punjab BoardDaniyal yousaf100% (1)
- BIOMOLECULES MHT CET SynopsisDokument4 SeitenBIOMOLECULES MHT CET SynopsisAbhishek Mandlik100% (3)
- Co Ordination Compounds (Question Bank)Dokument11 SeitenCo Ordination Compounds (Question Bank)Karan83% (6)
- Redox MCQsDokument7 SeitenRedox MCQsHarsh Walavalkar100% (1)
- SPSC Assistant (BPS-16) QuestionsDokument14 SeitenSPSC Assistant (BPS-16) QuestionsScope Pk100% (2)
- Chemistry MCQs HandoutsDokument26 SeitenChemistry MCQs HandoutsOsama Hasan91% (11)
- Benzene and Aromatic Compounds ExamDokument18 SeitenBenzene and Aromatic Compounds ExamNidhi Sisodia100% (3)
- CLASS XI IUPAC RULES & PRACTICE SHEET GUIDEDokument24 SeitenCLASS XI IUPAC RULES & PRACTICE SHEET GUIDETara Singh100% (2)
- Haloalkanes and Haloarenes Class 12 Chemistry MCQs PDFDokument33 SeitenHaloalkanes and Haloarenes Class 12 Chemistry MCQs PDFSanjana Sanjay100% (1)
- Class 10 Science MCQ on Periodic Classification of ElementsDokument30 SeitenClass 10 Science MCQ on Periodic Classification of ElementsAymen WaelNoch keine Bewertungen
- MCQ - Coordination CompoundsDokument20 SeitenMCQ - Coordination Compoundstharoonsays100% (1)
- Mcqs From Past Papers Physics All in One 2nd Year - Taleemtutor PDFDokument35 SeitenMcqs From Past Papers Physics All in One 2nd Year - Taleemtutor PDFMuhammad Mohsin Raza83% (12)
- 02 Unit# 2Dokument8 Seiten02 Unit# 2Muhammad Bilal ChemIstNoch keine Bewertungen
- VBT Metal-Ligand BondingDokument67 SeitenVBT Metal-Ligand BondingKehkashan GhauriNoch keine Bewertungen
- Mcqs Class 1st Year Chemistry Chapter WiseDokument64 SeitenMcqs Class 1st Year Chemistry Chapter Wisezeerak shafiqNoch keine Bewertungen
- Industrial Chemistry MCQDokument69 SeitenIndustrial Chemistry MCQSatvik BeheraNoch keine Bewertungen
- Lecturer Chemistry Mcqs PSC Past PaperDokument26 SeitenLecturer Chemistry Mcqs PSC Past Paperlog man63% (8)
- Chapter 3rd GASES MCQsDokument7 SeitenChapter 3rd GASES MCQsbushra3ansari25% (4)
- Soved MCQ 2nd Phy - 1 by Asif Rasheed (General Notes On First Year and Second Year Physics by Asif RasheedDokument6 SeitenSoved MCQ 2nd Phy - 1 by Asif Rasheed (General Notes On First Year and Second Year Physics by Asif RasheedAsif Rasheed Rajput0% (1)
- Part - I: Subjective Questions: Section (A) : VSEPR TheoryDokument17 SeitenPart - I: Subjective Questions: Section (A) : VSEPR TheoryRavindar PurohitNoch keine Bewertungen
- Model Paper of Chemistry 9th Class For Peshawar Board PDFDokument2 SeitenModel Paper of Chemistry 9th Class For Peshawar Board PDFAfzaal Jan100% (1)
- Chemistry MCQ Module I-III ReviewDokument22 SeitenChemistry MCQ Module I-III ReviewNo NameNoch keine Bewertungen
- 9th Class CHEMISTRY MCQsDokument26 Seiten9th Class CHEMISTRY MCQsArham SultanNoch keine Bewertungen
- Worksheet 20 - Coordination Compounds Nomenclature WorksheetDokument2 SeitenWorksheet 20 - Coordination Compounds Nomenclature WorksheetKarmendra100% (1)
- Poetry Analysis: Elements, Forms, Devices and Sir Henry Wotton's "The Character of a Happy LifeDokument27 SeitenPoetry Analysis: Elements, Forms, Devices and Sir Henry Wotton's "The Character of a Happy LifeJawad86% (7)
- X I I Past Papers (From 1993 - 2012)Dokument96 SeitenX I I Past Papers (From 1993 - 2012)asif_zehravi804888% (16)
- Benzene Derivatives: Key Concepts and ReactionsDokument14 SeitenBenzene Derivatives: Key Concepts and ReactionsRaj ModiNoch keine Bewertungen
- Alkyl Halides and Amines Mcqs KeyDokument3 SeitenAlkyl Halides and Amines Mcqs KeySameer HussainNoch keine Bewertungen
- Question Bank OrganometallicsDokument6 SeitenQuestion Bank OrganometallicsHimanshu Gusain100% (3)
- Overall CHP 7 and 12Dokument4 SeitenOverall CHP 7 and 12faheemNoch keine Bewertungen
- Punjab Group of Colleges: Quarter Test # 3 (CH # 9, 11, 13, 14) F.SC Part II ChemistryDokument2 SeitenPunjab Group of Colleges: Quarter Test # 3 (CH # 9, 11, 13, 14) F.SC Part II ChemistryNimra Maqbool HashmiNoch keine Bewertungen
- Section II Q No. 2. Attempt Any Eight Parts Out of TwelveDokument4 SeitenSection II Q No. 2. Attempt Any Eight Parts Out of TwelveUsama IjazNoch keine Bewertungen
- 2nd Year ChemistryDokument2 Seiten2nd Year ChemistryTariq RayNoch keine Bewertungen
- Monthly Test Xii Chemistry October 2023-24Dokument4 SeitenMonthly Test Xii Chemistry October 2023-24soumityachaudharyNoch keine Bewertungen
- 12 1st HalfDokument2 Seiten12 1st HalfSheraz ShahNoch keine Bewertungen
- 116180HSSC IichemistryDokument2 Seiten116180HSSC IichemistryMughal usmanNoch keine Bewertungen
- QP - Chem-07-FEB 2024Dokument5 SeitenQP - Chem-07-FEB 2024Tanuj MohiteNoch keine Bewertungen
- Test 12Dokument2 SeitenTest 12Sheraz ShahNoch keine Bewertungen
- Second Year: English Book-II Part-IDokument22 SeitenSecond Year: English Book-II Part-IMuhammad Tayyab IqbalNoch keine Bewertungen
- English Chemistry Biology Physics Urdu Pakistan Studies: (Intimation) Formno: 909983Dokument1 SeiteEnglish Chemistry Biology Physics Urdu Pakistan Studies: (Intimation) Formno: 909983Haroon EjazNoch keine Bewertungen
- Correction of Address PDFDokument1 SeiteCorrection of Address PDFHaroon EjazNoch keine Bewertungen
- Continue Admission Form PDFDokument1 SeiteContinue Admission Form PDFHaroon EjazNoch keine Bewertungen
- English Chemistry Biology Physics Urdu Pakistan Studies: (Intimation) Formno: 909983Dokument1 SeiteEnglish Chemistry Biology Physics Urdu Pakistan Studies: (Intimation) Formno: 909983Haroon EjazNoch keine Bewertungen
- Shaheen Aslam: D/O Muhammad AslamDokument1 SeiteShaheen Aslam: D/O Muhammad AslamHaroon EjazNoch keine Bewertungen
- Application FormDokument2 SeitenApplication FormHaroon EjazNoch keine Bewertungen
- Shapes of FDokument1 SeiteShapes of FHaroon EjazNoch keine Bewertungen
- B.Sc. Botany Elective-I: Detail of CoursesDokument4 SeitenB.Sc. Botany Elective-I: Detail of CoursesHaroon EjazNoch keine Bewertungen
- Vilsmeier ReactionDokument9 SeitenVilsmeier ReactionMartha GraciaNoch keine Bewertungen
- Ncert SolutionsDokument41 SeitenNcert SolutionsAni PatelNoch keine Bewertungen
- Chapter 6-Alkyl Halides Nucleophilic Substitution: You May Have Drawn The Other Enantiomer. Either Is CorrectDokument23 SeitenChapter 6-Alkyl Halides Nucleophilic Substitution: You May Have Drawn The Other Enantiomer. Either Is Correct張湧浩Noch keine Bewertungen
- Alkyl and Aryl Halide ClassificationDokument21 SeitenAlkyl and Aryl Halide ClassificationLitmus GodNoch keine Bewertungen
- Solomon-2ed Organic ChemistryDokument20 SeitenSolomon-2ed Organic ChemistryBala Subramanian67% (3)
- Test Bank For Organic Chemistry A Short Course 13th by Hart DownloadDokument12 SeitenTest Bank For Organic Chemistry A Short Course 13th by Hart Downloaddannyriddle05051994ieq100% (23)
- Alkyl Halide PDFDokument29 SeitenAlkyl Halide PDFSantosh Potdar100% (2)
- Organic Mock Exam QuestionsDokument119 SeitenOrganic Mock Exam QuestionsAriel Raye Rica100% (1)
- Exp10 PDFDokument3 SeitenExp10 PDFعمر العنزيNoch keine Bewertungen
- Chapter 6 Acid –Base Chemistry: Theories and StrengthsDokument63 SeitenChapter 6 Acid –Base Chemistry: Theories and StrengthsAlyssa BaltazarNoch keine Bewertungen
- Exam 3 Organic Reactions and MechanismsDokument6 SeitenExam 3 Organic Reactions and MechanismsVarokah VarNoch keine Bewertungen
- ALKYL, ARYL HALIDES REACTIONSDokument15 SeitenALKYL, ARYL HALIDES REACTIONSSahilNoch keine Bewertungen
- BenzeneDokument39 SeitenBenzenesar34ws100% (1)
- 56 5 3 ChemistryDokument24 Seiten56 5 3 ChemistrygettotonnyNoch keine Bewertungen
- Part - I: Objective Questions: Section (A) : Unimolecular Nucleophilic Substitution Reactions of Alkyl Halides (S 1)Dokument24 SeitenPart - I: Objective Questions: Section (A) : Unimolecular Nucleophilic Substitution Reactions of Alkyl Halides (S 1)Deeptam BharNoch keine Bewertungen
- NGP ReactionDokument6 SeitenNGP ReactionjeeadvanceNoch keine Bewertungen
- 5358chemistry Class XII Question Bank (First Part) (2022-23)Dokument27 Seiten5358chemistry Class XII Question Bank (First Part) (2022-23)Jiya PandeyNoch keine Bewertungen
- 1000 Mcqs ChemistryDokument113 Seiten1000 Mcqs ChemistryMariam IshtiaqNoch keine Bewertungen
- Chapter 6 - 542Dokument26 SeitenChapter 6 - 542Nada KhanNoch keine Bewertungen
- Green Park Educational Institutions, Namakkal: Long Term - Chemistry (Worksheet)Dokument4 SeitenGreen Park Educational Institutions, Namakkal: Long Term - Chemistry (Worksheet)Monalisa PremkumarNoch keine Bewertungen
- Dehalogenation Process OverviewDokument26 SeitenDehalogenation Process Overviewvnikhar123100% (1)
- Organic Chemistry Unit 2Dokument13 SeitenOrganic Chemistry Unit 2ABDULLAH SHAHZADNoch keine Bewertungen
- 235practice Exam 2 AnswerDokument9 Seiten235practice Exam 2 Answernbobs7Noch keine Bewertungen
- Ch 7 Alcohols, Phenols, Thiols Properties ReactionsDokument8 SeitenCh 7 Alcohols, Phenols, Thiols Properties ReactionsImran ParvezNoch keine Bewertungen
- Chapter 21 - Test Bank Chem 200Dokument64 SeitenChapter 21 - Test Bank Chem 200Jan Chester Chan67% (3)
- Organic Halide Classification TestsDokument3 SeitenOrganic Halide Classification TestsROSEMARIE ONGNoch keine Bewertungen
- DPP - 06 - Substitution ReactionDokument4 SeitenDPP - 06 - Substitution ReactionWhite Pubg BrothersNoch keine Bewertungen
- First Year Reactions For Chem1200Dokument4 SeitenFirst Year Reactions For Chem1200ElliotNoch keine Bewertungen
- Organic Chemistry Chapter Wise Previous Year Question PDFDokument13 SeitenOrganic Chemistry Chapter Wise Previous Year Question PDFVishal SNoch keine Bewertungen
- UNITIII SN1andSN2reactionsDokument24 SeitenUNITIII SN1andSN2reactionsRams ChanderNoch keine Bewertungen