Beruflich Dokumente
Kultur Dokumente
Galfan Coated Wires
Hochgeladen von
sreedhar0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
73 Ansichten9 SeitenGalfan coated wires and its high corrosion resistance properties reviewed in this paper
Copyright
© © All Rights Reserved
Verfügbare Formate
PDF oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenGalfan coated wires and its high corrosion resistance properties reviewed in this paper
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
73 Ansichten9 SeitenGalfan Coated Wires
Hochgeladen von
sreedharGalfan coated wires and its high corrosion resistance properties reviewed in this paper
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 9
GALFAN® GALVANIZING OFFERS IMPROVED CORROSION
RESISTANCE FOR WIRE
by
Marc Dewitte, N. V. Bekaert S.A.
Rolf Nunninghoff, Bergische University, Wuppertal
John L. Hostetler, ILZRO
for
Wire Association International
Mexico Technical Conference
October 9-12, 1994
Mexico City, Mexico
Introduction
The world-wide use of Galfan® galvanized wire for diverse end-use applications
has been steadily increasing since its introduction in Europe in 1984. Its superior
corrosion resistance has led to acceptance in uses such as wire ropes, fencing, springs,
gabions, nails, ACSR, automotive parts, vineyard wire and guy wire. Although Galfan
has the reputation of longer life, the explanation for is not as widely known.
Extensive research has led to a description of Galfan's unique corrosion
mechanism that explains its improved performance and allows better understanding of
the influence of the hot-dip process on its performance. The research has shown
increased magnitudes of superiority for three characteristics that were underestimated
before:
(1) Galfan's ability to maintain its best corrosion resistance at weld areas,
(2) Galfan’s remarkable galvanic properties, and
(3) the improvement of Galfan's passivity as it corrodes.
Welded Galfan® Wire Mesh
Investigations by TrefilARBED and N.V. Bekaert show significant advantages of
wire mesh made by welding wire pre-coated with Galfan as compared to welding
uncoated wire into a mesh followed by galvanizing. Table 1 shows comparative times
to red rust with salt spray tests (marine environment simulation) and with SO2 tests,
(industrial environment simulation) for mesh as used in two different applications.
Figure 1 shows that after 35 cycles in SOz, the Galfan mesh shows only white rust at the
welds and other areas, whereas the regularly galvanized mesh shows severe red
rusting.())
Testing by EFIM, an Italian Institute writing specifications for highway materials,
shows similar results that has led to greater use of Galfan wire for gabions and highway
fence.2)
Research at Bekaert has discovered the reason the corrosion resistance of Galfan
is not lost at weld areas. Typical optical micrographs through a weld section are shown
in Figures 2 and 3. It can be seen that part of the weld nugget and even part of the wire
near the nugget is not covered by a coating layer after welding.(1)
AES measurements made on numerous weld sections identify the bright phases
as essentially pure Fe while the surrounding material is 20%AI-75%Fe, and interface
between the wire and the rest of the Galfan coating. This interface is an intermetallic
with very low Zn, so its behavior will be similar to one of the Fe-Al compounds, FeAl,
Fe2Als or FeAls, all of which have melting points above 1150°C. Even with the small
amount of Zn in the intermetallic, its melting point will be well above the welding
temperature. This means that the distance from the uncoated wire and the
intermetallic Galfan interface is very small, allowing effective cathodic protection of the
steel.)
This is not the case when welding regular galvanized wires because their Fe-Zn_
intermetallic alloys present at the interface have melting points below 800°C that is
lower than the welding temperature. The welding heat causes them to be melted away.
The uncoated wire areas at the weld nugget are inadequately protected because the
distances from much of the nugget surface to the nearest coating surface are too great.
Not only is the cathodic potential greater because of the shorter distances over
which cathodic protection is required, but the electrical resistance of Galfan's
intermetallic (Al-Fe-Zn) is also lower than for regular galvanized (Fe-Zn). Therefore, its
cathodic efficiency is also greater.
Nails
Cathodic protection also improves the performance of nails made from Galfan
galvanized wire. Even though the points and heads are not coated, the Galfan nails can
outperform nails that are regular galvanized after shaping. Moreover, they offer the
advantage of possessing a more uniform coating thickness and are smoother and more
ductile. These benefits are especially important for automatic nailing machines.
‘When nails with different coatings of about 45 im thickness were exposed to 15,
cycles in a SO> test, the Galfan wire nail showed less corrosion on the heads and points
than aluminized, regular galvanized or sheradized nails.)
Ropes
Another application that can turn Galfan’s features into significant economic
benefits is ropes, especially marine and mining ropes because of the effects of greases
used for lubricating. Table 2 shows a performance comparison between regular and
Galfan galvanized fishing ropes in salt spray tests while Table 3 shows the comparison
including the effect of grease in a cyclical sea water test.(4)
Corrosion Mechanism
Galfan is one of several zinc-aluminum alloys introduced during the last two
decades. All have been attempts to provide more corrosion protection for the wire than
offered by regular galvanizing or aluminizing. Galfan is the eutectic ratio of the two
elements. That means it has the lowest melting: temperature and what is more
important, means that the two elements do not grossly separate during solidification.
Galfan solidifies into a lamellar microstructure consisting of alternating very thin (<1
um) Al-rich and Zn-rich plates within randomly oriented cells,®) as illustrated in Figure
4.
In this kind of microstructure, the more active zinc will corrode first, leaving the
aluminum to slowly oxidize and gradually form the corrosion products such as
ZngAl(OH)1¢ which are much harder, less porous and less easily washed away than
those from corrosion of regular galvanizing. An unusual feature is that the aluminum
3
enrichment continues with exposure so that the longer the exposure, the more
corrosion-resistant the coating becomes. Figure 5 shows a Scanning Auger Microprobe
(SAM) analysis of the outer surface of an uncorroded Galfan coating. Compare this with
Figure 6 showing a SAM indicating enriched aluminum levels on a corroded surface. (4)
Because of this unique microstructure and continuous aluminum enrichment of
the surface, Galfan’s surface becomes more passive with time, not less as will happen
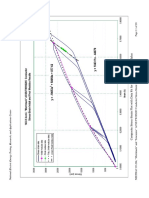
with regular galvanizing. Given this, it is not surprising to see that Galfan's weight loss
vs. time is not linear as other coatings are, but parabolic as seen in Figure 7.9) This is
confirmed by other investigations of long-term exposure tests. (5)
The thickness of the remaining coating at the time of first appearance of red rust
is still another difference due to the corrosion mechanisms. Regular galvanized wires
show red rust while there is still 15 to 20 um of residual coating left whereas Galfan
galvanized wires do not show red rust until the coating thickness is 5 um or less. See
Table 4.(4) The explanation for this mechanism characteristic is found in the galvanic
potential difference between the coating’s intermetallic layer and the free (eta) coating
layer. The difference in regular galvanizing is very small so that the corrodent can
penetrate directly toward the wire. The difference between Galfan's intermetallic and
eutectic layers is much higher so the less sacrificial intermetallic does not corrode until
the eutectic is completely consumed.
Producing Galfan Galvanized Wire
Steel sheet and tube are galvanized with Galfan using a single-dip process
similar to those used for regular hot-dip galvanizing. Although ILZRO has patented a
flux system for a single-dip process (SDEF), all Galfan galvanized wire has been
produced by a double-dip (2-D) process in which the wires are first hot-dipped in a
regular galvanizing bath, then immediately re-dipped in a Galfan bath. Wires
galvanized with Galfan using a 2-D process will have a different coating microstructure
than those done with the SDEF process.
The classical Fe-Zn alloys form the interface in the first dip. When the newly
galvanized wires are immersed in the Galfan bath however, aluminum from the bath
rapidly diffuses through the Fe-Zn alloys, transforming them into a more-or-less
homogeneous Al-Fe-Zn intermetallic compound as seen in Figure 8.
Whereas Fe-Zn alloy layers are brittle and quick to show red rust, the Galfan Al-
Fe-Zn intermetallic is very ductile, exceptionally corrosion-resistant, and is slow to
show red rust.
Future Demand
Galfan galvanized wire, both low-carbon and high-carbon, have been produced
in Belgium, France, Germany, Luxembourg and the United States since the mid-
eighties. Production by each licensee is increasing steadily. As more specifications are
modified to include Galfan, the demand should grow rapidly. Many new production
facilities in all parts of the world where galvanized wire is used will be licensed by
ILZRO to use the patented Galfan alloy .
The 2-D process will continue to be a popular method because it allows both
regular and Galfan galvanizing on the same line and because it has certain unique
coating microstructure characteristics. The SDEF process will also be used widely
because of its lower initial cost, simpler operating process, and lower production costs.
The technology for Galfan galvanizing small parts that are needed as accessories for
many of the wire products is developing rapidly so that Galfan systems can be offered to
the marketplace.
References
1. M. Dewitte and P. Lippens, N.V. Bekaert Co. Corrosion Resistance Study of Post-Welded
Galfan Wires,
2. Prof. R. Nunninghoff, Bergische Universitat, Wuppertal, Germany Report to the Galfan
Licensees Meeting, Tokyo, Japan, Oct., 1992 Part of this report includes work done by
Dr. Volker Hagebilling at Bergische Universitat.
3. Scott R. Bluni, Lehigh University, Report to the Galfan Licensees Meeting, Linz, Austria,
Oct., 1993. ‘i
4. M. Dewitte, S. DeBondt, etal, N.V. Bekaert Co., Galfan’s Efficient Galoanic Action
Provides Corrosion Protection.
5. Y. Hirose, Nisshin Steel Co., Ltd., Ten Year Atmospheric Corrosion Test Results of Galfan
Coated Steel.
TABI
TABLE 1
COMPARING REGULAR AND GALFAN GALVANIZED WELDED MESH
‘as SECURITY FENCING |
J vox 34mm dia wee |
Pecan | arenes RecN tee
(GALVANZED GALVANIZED.
COATING WEIGHT, gma 355
SALT SPRAY TEST (NaCh}:
Hours per gma
Gatan Ratio
KESTERNIGH TEST (S02}-
Number of cycles to 5% red rust
Cycles per g/m2
“Gallan Ratio.
Page 1
TAB2
TABLE 2
COMPARING REGULAR AND GALFAN GALUANIZED
FISHING ROPES IN SALT SPRAY TEST
COATING | SALT SPRAY TEST
WEIGHT | RESISTANCE IN HRS
G2 TO 5% RED RUST
ROPE CONSTRUCTION: CORE FABRIC CORD +647
REGULAR GALV. 140 216 1.54
130 1272 9.78
‘STRANDS: 7 # 1
167 456 2.73
GALFAN 150 1248 3.32 | 3.05:1
COATING
STRAND WIRE (before drawing)
410-436, 504 1.19
429-434 4272 2.94 | 2.471
Page 1
TABS.
TABLE 3
COMPARING REGULAR AND GALFAN GALUANIZING
IN CYCLICAL SEA WATER TEST
GALVANIZING
TRE
WITH NO GREASE
230 453 1.97
108 2558 35.17
WITH GREASE
230 208 0.90
108 296 2.74
TEST CONDITIONS
MAKE-UP OF SEAWATER. DAILY CYCLE
NaCl 28 gf 17 hrs immersed
MgSO4-7H20 _7/g/l 2 hrs dry
MgCi2 - 6H20 5 gf 3 hrs immersed
Cace 1.22 gf 2 hrs dry
NaHCO3 0.2 gf
Page 1
TAB 4
TABLE 4
RESIDUAL WIRE COATING THICKNESS
__AT'5% RED RUST IN SALT SPRAY TEST
INITIAL, INITIAL RESIDUAL COATING
GALVANIZING| INTERMETALLIC | TOTAL COATING THICKNESS
TYPE LAYER, THICKNESS,
MICRONS MICRONS: MICRONS:
LOW CARBON WIRE
8 to 10 41 to 62
GALFAN 4 to S 51 to 60 3
HIGH CARBON WIRE
64 to 78
1048
Page 1
Das könnte Ihnen auch gefallen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- Elevated Temperature Creep of AAC, AAAC, ACAR, AACSR, & ACSR ConductorsDokument8 SeitenElevated Temperature Creep of AAC, AAAC, ACAR, AACSR, & ACSR ConductorssreedharNoch keine Bewertungen
- ASM Reference TitleDokument1 SeiteASM Reference Titlesreedhar0% (1)
- Tension and LoadDokument1 SeiteTension and LoadsreedharNoch keine Bewertungen
- Ampacity Calculation - : Ac AcDokument1 SeiteAmpacity Calculation - : Ac AcsreedharNoch keine Bewertungen
- Invar36 MechanicalDokument1 SeiteInvar36 MechanicalsreedharNoch keine Bewertungen
- Stress StrainDokument1 SeiteStress StrainsreedharNoch keine Bewertungen
- NEMA Class 2Dokument1 SeiteNEMA Class 2sreedharNoch keine Bewertungen
- 795 ACCR Polynomial DerivatDokument18 Seiten795 ACCR Polynomial DerivatsreedharNoch keine Bewertungen
- Hole Win Wire SectionDokument1 SeiteHole Win Wire SectionsreedharNoch keine Bewertungen
- Order ProcedureDokument1 SeiteOrder ProceduresreedharNoch keine Bewertungen
- Creep Estimated IEEE 1283Dokument6 SeitenCreep Estimated IEEE 1283sreedharNoch keine Bewertungen
- Invar36 Char CurvesDokument1 SeiteInvar36 Char CurvessreedharNoch keine Bewertungen
- NEMA Class 1Dokument1 SeiteNEMA Class 1sreedharNoch keine Bewertungen
- Elevated Temperature CreepDokument2 SeitenElevated Temperature CreepsreedharNoch keine Bewertungen
- Materials Groups in TablesDokument1 SeiteMaterials Groups in TablessreedharNoch keine Bewertungen
- PrefaceDokument1 SeitePrefacesreedharNoch keine Bewertungen
- University - VIT APDokument1 SeiteUniversity - VIT APsreedharNoch keine Bewertungen
- Contributors and Revision ComDokument1 SeiteContributors and Revision ComsreedharNoch keine Bewertungen
- Gateway To Engg-VITDokument1 SeiteGateway To Engg-VITsreedharNoch keine Bewertungen
- Gateway To Engg-VIT - Progrms2Dokument1 SeiteGateway To Engg-VIT - Progrms2sreedharNoch keine Bewertungen
- MCL ACSR-MetricDokument1 SeiteMCL ACSR-MetricsreedharNoch keine Bewertungen
- MCL Acsr AstmDokument1 SeiteMCL Acsr AstmsreedharNoch keine Bewertungen
- C Users Sreedhar Downloads DASABrochureUG2021 18sep2021Dokument2 SeitenC Users Sreedhar Downloads DASABrochureUG2021 18sep2021sreedharNoch keine Bewertungen
- Math Handout (Trigonometry) Trig Formulas Web PageDokument11 SeitenMath Handout (Trigonometry) Trig Formulas Web PageAyushNoch keine Bewertungen
- Complexes Part2Dokument1 SeiteComplexes Part2sreedharNoch keine Bewertungen
- Low Resistance Testing PDFDokument19 SeitenLow Resistance Testing PDFAaqib MujtabaNoch keine Bewertungen
- ComplexesDokument1 SeiteComplexessreedharNoch keine Bewertungen
- Titanium Boron Aluminium Grain Refiners: Alloy Designation Color Code Ti B Si Fe V Others Each Total FormDokument1 SeiteTitanium Boron Aluminium Grain Refiners: Alloy Designation Color Code Ti B Si Fe V Others Each Total FormsreedharNoch keine Bewertungen
- Grain Refiner: The World'S Most EfficientDokument1 SeiteGrain Refiner: The World'S Most EfficientsreedharNoch keine Bewertungen
- Titanium aluminium and carbon master alloysDokument1 SeiteTitanium aluminium and carbon master alloyssreedharNoch keine Bewertungen
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)