Beruflich Dokumente
Kultur Dokumente
i1T5u20L.3: Scattering Angle (Deg.)
Hochgeladen von
vainateyagoldarOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
i1T5u20L.3: Scattering Angle (Deg.)
Hochgeladen von
vainateyagoldarCopyright:
Verfügbare Formate
2050 J. Electrochem. Soc., Vol. 144, No. 6, June 1997 The Electrochemical Society, Inc.
reacted on discharge, the starting material does not form
SCATTERING ANGLE (deg.) again. Sn peaks do not form after the first charge as
20 30 40 50 observed for SnO, Sn02, and SiSnO3. This could be due to
the fact that for Li2SnO3 3 mol of Li20 per mole of Li2 Sn03
form during the discharge (Eq. 5). This higher concentra-
tion of lithia could prevent large regions of tin metal from
forming and as a result we do not observe well defined tin
04
0
1 peaks in our XRD scan.
We believe that the lithia matrix that is formed during
the initial stages of lithium insertion in these materials
acts as a 'glue," which helps to hold the Li-Sn regions
together through the large volume changes in the alloy-
zw
Cl)
ing/dealloying process. This is based on the reversibility of
the processes as demonstrated electrochemically when
I-
z compared to metallic tin (Fig. 2). Table II shows the calcu-
lated volume per mole of Sn atoms in each of the respec-
tive Li-Sn phases. Notice the relatively small volume
increase factor to LiSn, 1.53, and the large volume in-
crease factor to the alloying limit of Li22Sn5, 3.59.
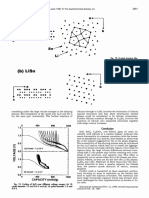
An investigation of the crystal structures of each of the
various phases shows that both Li2Sn, and LiSn are lay-
Fig. 10. In situ XRD results for SiSnO3; (a) end of charge —2.5 V, ered structures, whereas higher concentrations of lithium
(b) end of discharge —0.0 V, (c) calculated peak positions for Li5Sn2, lead to more complicated structures where layers or tun-
Li135n5, Li75n2, and U225n5 (the majority of peaks for all of these nels of Li atoms are not formed. Figure 12 is a diagram of
structures are around 22° and 381, and (d) first scan —2.5 V. the crystal structures of Li2Sn5 and LiSn. The crystal
Logarithmic intensities are plotted for scans in reference to the left-
structure of Li2Sn5 has some prominent features which are
hand vertical axis and linear intensifies are plotted for the calculat-
ed patterns (normalized to 100) in reference to the right-hand ver-
outlined by the solid lines in the right-hand diagram of
tical axis. Fig. 12a. The lithium resides within pentagonal structures
between the layers of Sn atoms. There are also square and
triangular sites which exist. If all the triangular and
rial does not reappear during the charge as can be deter- square sites were filled with Li, a stoichiometry of LiSn
mined by comparing Fig. iOa and d. would result. However there would be three inequivalent
Figure ii shows some of the in situ XED scans for types of Li atoms. A slight skewing to create an overall
Li2SnO3. Figure lid shows the first scan (2.5 V) and the coordination of 8 Sn per Li (square sites) and vice versa
corresponding calculated peaks for Li2SnO3 are shown by results in the crystal structure shown in Fig. i2b, or LiSn.
the broken lines in that figure. When the cell is fully dis- Over certain ranges the Li-Sn alloy structural transitions
charged, the broad features at 22° and 38°, which are are minimally disruptive and so should be quite reversible.
indicative of the lower Li-Sn alloy phases, appear We cycled cells of SnO over various voltage ranges in
(Fig. lib —0.0 V). The original peaks of Li2SnO3 do not order to confirm that the first two Li-Sn phases should
entirely disappear for this cell. The first discharge capaci- demonstrate good reversibility. Figure 13 shows the results
ty for this cell was approximately 1000 mAh/g instead of of two of these cycling experiments. In Fig. 13a we show a
approximately 1200 mAh/g for other cells (Fig. 2e). cell that has been cycled between 1.3 and 0.0 V Over this
Therefore we believe that not all of the starting material voltage range the process is not very reversible. We believe
has reacted in this in situ cell. By the end of charge that this is due to the cracking and crumbling of the mate-
(Fig. ha —2,5 V) the lower Li-Sn phases are no longer pre- rial by the large volume increases that are involved in real-
sent and, with the exception of the Li2SnO3 that has not izing the lower Li-Sn alloy phases. Figure 13b shows the
results for a SnO cell that has been discharged to 0.4 V and
then charged to 1.3 V for ten cycles. We can see that this
process is more reversible, and that the phase definition as
SCATTERING ANGLE (deg.) indicated by the plateaus seems to improve with cycling.
Since the first two Li-Sn phases, that is Li25n5 and LiSn,
20 30 40 50 are layered and related structures, then over this region
100 TT the reaction with Li is more reversible than if we were to
(a) End ot Charge
3 react over the full range of Li-Sn alloys.
Figure 14 is a schematic of the reaction mechanism that
P1
0r 10
100 —I—I i—Ill .(!l ii
{b) End of Discharge - 0.0 V
Ii occurs in the tin oxides and tin oxide composites that we
have studied. In the diagram we assume SnO as the start-
ing material, but this could he easily replaced by any of
3
10
>.
U) I I.i' I—S Jill iiii 4....—
the other starting materials by simply adjusting the stoi-
chiometry of the added lithium. We first introduce lithium
forming a Li2O "matrix-glue" which assists in the reversi-
zLu (C) Jll Li5Sn2, Li13Sn5, bility of the subsequent alloying/dealloying by resisting
1i7Sn2 Li229n5
I—
100 : :;j j L_.4_,; I
Table Il. Calculated volume per mol Sn (cm3/mol) and volume
i1T5u20L.3
3
increase factor (over Sn metal) for each of U-Sn alloy phases.
10
Phase Volume per mol Sn (cm°/mol) Volume increase factor
Fig. 11. In situ XRD results for Li25n03; (a) end of charge —2.5 V,
(b) end of discharge —0.0 V, (c) calculated peak positions for Li5Sn2, Sn 16.18 1.00
Li2Sn, 19.88 1.23
Li13Sn5, Li7Sn2, and LiSn5 (the majority of peaks for a11 of these 24.74 1.53
structures are around 22° and 381, and (d) first scan —2.5 V. LiSo
Li75n3 36.95 2.28
Logarithmic intensities are plotted for scans in reference to the left- 44.64 2.76
Li55n2 2.81
hand vertical axis and linear intensities are plotted for the calculat- Li3Sn, 45.45
ed patterns (normalized to 100) in reference to the right-hand ver- Li75n2 48.31 2.99
Li,25n5 58.14 3.59
tical axis.
Das könnte Ihnen auch gefallen
- 6.4li + Sno - 8.4li + Sno, - : A Reasonable Correlation Between The Calculated andDokument1 Seite6.4li + Sno - 8.4li + Sno, - : A Reasonable Correlation Between The Calculated andvainateyagoldarNoch keine Bewertungen
- Self-Heating - Induced Healing of Lithium Dendrites: BatteriesDokument5 SeitenSelf-Heating - Induced Healing of Lithium Dendrites: BatteriesSudeepta MondalNoch keine Bewertungen
- Cobalt Free CompositeDokument10 SeitenCobalt Free CompositeFadil KhayrNoch keine Bewertungen
- D and F-Block QuesDokument12 SeitenD and F-Block QuesCharmiNoch keine Bewertungen
- Rangasamy 2013Dokument6 SeitenRangasamy 2013Septia Kurniawati ArifahNoch keine Bewertungen
- D Block Imp QuestionsDokument4 SeitenD Block Imp QuestionsAnanya SrivastavaNoch keine Bewertungen
- Electrode Materials For Rechargeable SodiumDokument8 SeitenElectrode Materials For Rechargeable Sodiumvigalles_2Noch keine Bewertungen
- Electrorecrystallization of Metal AlloyDokument5 SeitenElectrorecrystallization of Metal AlloyWL JangNoch keine Bewertungen
- Electrochemical Impedance Spectroscopy Study of SnO and nano-SnODokument6 SeitenElectrochemical Impedance Spectroscopy Study of SnO and nano-SnOlukasz15432Noch keine Bewertungen
- Salter 2005Dokument7 SeitenSalter 2005Jabid LoteroNoch keine Bewertungen
- Photochemistry and Photophysics of Coordination Compounds of The Main Group MetalsDokument7 SeitenPhotochemistry and Photophysics of Coordination Compounds of The Main Group MetalsгогавагановNoch keine Bewertungen
- D and F Block ElementsDokument17 SeitenD and F Block ElementsAnushka MishraNoch keine Bewertungen
- CBSE Class 12 Chemistry Paper Sample Paper Solution Set 4Dokument14 SeitenCBSE Class 12 Chemistry Paper Sample Paper Solution Set 4Gamer ChannelNoch keine Bewertungen
- tmp1220 TMPDokument4 Seitentmp1220 TMPFrontiersNoch keine Bewertungen
- Scattering Angle (Deg.) Scattering Angle (Deg.)Dokument1 SeiteScattering Angle (Deg.) Scattering Angle (Deg.)vainateyagoldarNoch keine Bewertungen
- Dissolution Process - Sodium Chloride CisneDokument4 SeitenDissolution Process - Sodium Chloride CisneRICARDO AMORIMNoch keine Bewertungen
- The Mixing Mechanism During Lithiation of Si Negative Electrode in Li-Ion Batteries: An Ab Initio Molecular Dynamics StudyDokument7 SeitenThe Mixing Mechanism During Lithiation of Si Negative Electrode in Li-Ion Batteries: An Ab Initio Molecular Dynamics StudyParamita HaldarNoch keine Bewertungen
- S/Sciences/Physics/Optics/Lasertut Orial/Creating/Creating - HTMDokument13 SeitenS/Sciences/Physics/Optics/Lasertut Orial/Creating/Creating - HTMSuvankar ChakrabortyNoch keine Bewertungen
- Unit 3: Inorganic Chemistry: Recommended Prior KnowledgeDokument4 SeitenUnit 3: Inorganic Chemistry: Recommended Prior KnowledgeHubbak KhanNoch keine Bewertungen
- Martensitic Transformations in Nonferrous Shape Memory AlloysDokument17 SeitenMartensitic Transformations in Nonferrous Shape Memory AlloysAnang IhsanNoch keine Bewertungen
- D and F Block Questions With AnswersDokument11 SeitenD and F Block Questions With AnswersYash RajputNoch keine Bewertungen
- ResumoDokument17 SeitenResumoGabriel SachiNoch keine Bewertungen
- 1/2 Spins of The Ti3+ Ions (See, However,: Ch. 10 The Correlated Metallic StateDokument1 Seite1/2 Spins of The Ti3+ Ions (See, However,: Ch. 10 The Correlated Metallic StateSupriyaNoch keine Bewertungen
- A New Finding On The Role of LiNO3 in Lithium-Sulfur BatteryDokument7 SeitenA New Finding On The Role of LiNO3 in Lithium-Sulfur BatteryTong YichenNoch keine Bewertungen
- Anodic Behaviour of Tin in Potassium Hydroxide SolutionDokument7 SeitenAnodic Behaviour of Tin in Potassium Hydroxide SolutionSantiago EdingerNoch keine Bewertungen
- 1986 (Exp) BegemannDokument4 Seiten1986 (Exp) BegemannJose Aminadat Morato MarquezNoch keine Bewertungen
- CLS Aipmt 19 20 XII Che Study Package 1 Level 1 Chapter 1Dokument20 SeitenCLS Aipmt 19 20 XII Che Study Package 1 Level 1 Chapter 1ShubanghiNoch keine Bewertungen
- Chemistry The D and F Block Elements Q&A 5marksDokument14 SeitenChemistry The D and F Block Elements Q&A 5marksPramit RanjanNoch keine Bewertungen
- IntroductionDokument25 SeitenIntroductionRaj vermaNoch keine Bewertungen
- PhysRevB 41 7876Dokument3 SeitenPhysRevB 41 7876郑子豪Noch keine Bewertungen
- Blocking Lithium Dendrite Growth in Solid-State Batteries With An Ultrathin Amorphous Li-La-Zr-O Solid ElectrolyteDokument10 SeitenBlocking Lithium Dendrite Growth in Solid-State Batteries With An Ultrathin Amorphous Li-La-Zr-O Solid ElectrolyteclasyoonNoch keine Bewertungen
- Chapter 10 Electrochemistry Text Book ExerciseDokument31 SeitenChapter 10 Electrochemistry Text Book ExerciseshahidkakaNoch keine Bewertungen
- D& F BlockDokument29 SeitenD& F BlockKrish BhardwajNoch keine Bewertungen
- DF CompleteDokument11 SeitenDF Completeranaharshit994Noch keine Bewertungen
- Research Paper On D and F Block PDFDokument9 SeitenResearch Paper On D and F Block PDFVishwa RahulNoch keine Bewertungen
- Metal-Thiolate Bonds in Bioinorganic ChemistryDokument14 SeitenMetal-Thiolate Bonds in Bioinorganic ChemistrykawtherahmedNoch keine Bewertungen
- Cao 2015Dokument5 SeitenCao 2015Septia Kurniawati ArifahNoch keine Bewertungen
- A New Class of Solvent-in-Salt Electrolyte ForDokument9 SeitenA New Class of Solvent-in-Salt Electrolyte ForTong YichenNoch keine Bewertungen
- The D and F Block ElementsDokument16 SeitenThe D and F Block Elementssyedasifbasha1990Noch keine Bewertungen
- LiS BrewsterDokument8 SeitenLiS BrewsterTyler HermanNoch keine Bewertungen
- Scott - 1982 - The Vibrational Structure of Davydov Solitons PDFDokument9 SeitenScott - 1982 - The Vibrational Structure of Davydov Solitons PDFЮрий ЮрийNoch keine Bewertungen
- Ncert Sol D&FDokument16 SeitenNcert Sol D&FKAVERI JAINNoch keine Bewertungen
- CHAPTER 1: Semiconductor Materials & PhysicsDokument25 SeitenCHAPTER 1: Semiconductor Materials & PhysicsUma MaheswariNoch keine Bewertungen
- The "Golden Penny" DemonstrationDokument3 SeitenThe "Golden Penny" DemonstrationOren RosenfeldNoch keine Bewertungen
- Nature Synthesis of SrNbO3Dokument4 SeitenNature Synthesis of SrNbO3Rafael BritoNoch keine Bewertungen
- D and F Block ElementsDokument4 SeitenD and F Block Elementsishu010.comNoch keine Bewertungen
- Simon-1988-Angewandte Chemie InqqqqDokument26 SeitenSimon-1988-Angewandte Chemie InqqqqAria MoonNoch keine Bewertungen
- Oxidation States of Transition MetalsDokument5 SeitenOxidation States of Transition MetalskushanNoch keine Bewertungen
- 10 Chapter Electrochemistry Text Book ExerciseDokument31 Seiten10 Chapter Electrochemistry Text Book ExerciseSajid AzeemNoch keine Bewertungen
- Kinetics of Aqueous CorrosionDokument54 SeitenKinetics of Aqueous CorrosionjcscobucciNoch keine Bewertungen
- A Level Notes On Transition MetalsDokument18 SeitenA Level Notes On Transition Metalskmoiz427Noch keine Bewertungen
- 9.0 PeriodicityDokument22 Seiten9.0 PeriodicitygoverotaropafadzwaNoch keine Bewertungen
- Feature Article: Electronic Wave Functions in Semiconductor Clusters: Experiment and TheoryDokument6 SeitenFeature Article: Electronic Wave Functions in Semiconductor Clusters: Experiment and TheoryNitika GuptaNoch keine Bewertungen
- On The Moseley Diagram of The X-Ray Term Values: D/T/R (T RDokument8 SeitenOn The Moseley Diagram of The X-Ray Term Values: D/T/R (T RPepe LuisNoch keine Bewertungen
- BondingDokument12 SeitenBondingPAUL KOLERENoch keine Bewertungen
- 2.1 Fundamentals of Nucleation: 2. Literature ReviewDokument28 Seiten2.1 Fundamentals of Nucleation: 2. Literature ReviewEdon BediNoch keine Bewertungen
- 2018 Evolving Affinity Between Coulombic Reversibility and Hysteretic Phase Transformations in Nano-Structured Silicon-Based Lithium-Ion BatteriesDokument17 Seiten2018 Evolving Affinity Between Coulombic Reversibility and Hysteretic Phase Transformations in Nano-Structured Silicon-Based Lithium-Ion Batteriesruonan liNoch keine Bewertungen
- Amorphous Semiconductors: Structural, Optical, and Electronic PropertiesVon EverandAmorphous Semiconductors: Structural, Optical, and Electronic PropertiesNoch keine Bewertungen
- Endohedral Metallofullerenes: Fullerenes with Metal InsideVon EverandEndohedral Metallofullerenes: Fullerenes with Metal InsideNoch keine Bewertungen
- Electrodes for Li-ion Batteries: Materials, Mechanisms and PerformanceVon EverandElectrodes for Li-ion Batteries: Materials, Mechanisms and PerformanceNoch keine Bewertungen
- Algorithm 4 Procedure Next - Gen - GA Input The Current Population Output The Next Population While SNDokument1 SeiteAlgorithm 4 Procedure Next - Gen - GA Input The Current Population Output The Next Population While SNvainateyagoldarNoch keine Bewertungen
- 1, If 0, Otherwise : Iii. The Proposed FrameworkDokument1 Seite1, If 0, Otherwise : Iii. The Proposed FrameworkvainateyagoldarNoch keine Bewertungen
- C. Illustrated ExampleDokument5 SeitenC. Illustrated ExamplevainateyagoldarNoch keine Bewertungen
- K-Itemset. Let D (T: Ux uXTDokument1 SeiteK-Itemset. Let D (T: Ux uXTvainateyagoldarNoch keine Bewertungen
- Min - Util, Ce Is Not An HUI. The TU of T T TWU (Ce) TU (T: Tid T T T T T T T T T TDokument1 SeiteMin - Util, Ce Is Not An HUI. The TU of T T TWU (Ce) TU (T: Tid T T T T T T T T T TvainateyagoldarNoch keine Bewertungen
- Standard Steady State Genetic Algorithms Can Hillclimb Faster Than Mutation-Only Evolutionary AlgorithmsDokument14 SeitenStandard Steady State Genetic Algorithms Can Hillclimb Faster Than Mutation-Only Evolutionary AlgorithmsvainateyagoldarNoch keine Bewertungen
- Scattering Angle (Deg.) Scattering Angle (Deg.)Dokument1 SeiteScattering Angle (Deg.) Scattering Angle (Deg.)vainateyagoldarNoch keine Bewertungen
- Lo:an1jj: II II Lilt IIDokument1 SeiteLo:an1jj: II II Lilt IIvainateyagoldarNoch keine Bewertungen
- Proc. POWERCON 7, 1980, Pp. E3-1-E3-15.: OnclusionDokument1 SeiteProc. POWERCON 7, 1980, Pp. E3-1-E3-15.: OnclusionvainateyagoldarNoch keine Bewertungen
- Fig. 5. Double-Sided Switching Pattern in A Cycle Period: Casadei Et Al.: Matrix Converter Modulation Strategies 375Dokument1 SeiteFig. 5. Double-Sided Switching Pattern in A Cycle Period: Casadei Et Al.: Matrix Converter Modulation Strategies 375vainateyagoldarNoch keine Bewertungen
- S - 5 S - SS - : (A) Li2Sn5Dokument1 SeiteS - 5 S - SS - : (A) Li2Sn5vainateyagoldarNoch keine Bewertungen
- Matrix Converter Modulation Strategies: A New General Approach Based On Space-Vector Representation of The Switch StateDokument1 SeiteMatrix Converter Modulation Strategies: A New General Approach Based On Space-Vector Representation of The Switch StatevainateyagoldarNoch keine Bewertungen
- Fig. 1. Basic Scheme of Matrix Converters.: Casadei Et Al.: Matrix Converter Modulation Strategies 371Dokument1 SeiteFig. 1. Basic Scheme of Matrix Converters.: Casadei Et Al.: Matrix Converter Modulation Strategies 371vainateyagoldarNoch keine Bewertungen
- Binder2 5Dokument1 SeiteBinder2 5vainateyagoldarNoch keine Bewertungen
- Fig. 2. Double-Sided Switching Pattern in A Cycle PeriodDokument1 SeiteFig. 2. Double-Sided Switching Pattern in A Cycle PeriodvainateyagoldarNoch keine Bewertungen
- Casadei Et Al.: Matrix Converter Modulation Strategies 373: A. SVM TechniqueDokument1 SeiteCasadei Et Al.: Matrix Converter Modulation Strategies 373: A. SVM TechniquevainateyagoldarNoch keine Bewertungen
- Matrix Converter Modulation Strategies: A New General Approach Based On Space-Vector Representation of The Switch StateDokument1 SeiteMatrix Converter Modulation Strategies: A New General Approach Based On Space-Vector Representation of The Switch StatevainateyagoldarNoch keine Bewertungen
- Binder1 40Dokument1 SeiteBinder1 40vainateyagoldarNoch keine Bewertungen
- Flanges and Pipe Fitting ManufacturerDokument12 SeitenFlanges and Pipe Fitting Manufacturer9823458877Noch keine Bewertungen
- Environmental Statement Form V of TSJ Works Tata Steel Limited For The Year 2020 2021Dokument12 SeitenEnvironmental Statement Form V of TSJ Works Tata Steel Limited For The Year 2020 2021nikhil pawarNoch keine Bewertungen
- Ecological Bricks' Made With Clays and Steel Dust PollutantsDokument13 SeitenEcological Bricks' Made With Clays and Steel Dust PollutantsMaria Isabel Meza MorejonNoch keine Bewertungen
- SGS Speeds Feeds GPDokument2 SeitenSGS Speeds Feeds GPnammarisNoch keine Bewertungen
- Chemistry of Noble GasesDokument26 SeitenChemistry of Noble GasesjaqNoch keine Bewertungen
- Selangor Skema Kimia Kertas 2 (Set 1)Dokument17 SeitenSelangor Skema Kimia Kertas 2 (Set 1)SITI RAIHANI BINTI KAMSO MoeNoch keine Bewertungen
- Paper 4Dokument10 SeitenPaper 4UmaibalanNoch keine Bewertungen
- SL Topic 2: Atomic Structure: © DR Geoffrey Neuss, InthinkingDokument8 SeitenSL Topic 2: Atomic Structure: © DR Geoffrey Neuss, InthinkingCarlos Moreno BorralloNoch keine Bewertungen
- Science 9 Wlas QTR 2 Week 3 ValidatedDokument10 SeitenScience 9 Wlas QTR 2 Week 3 ValidatedMYLENE B. ZABALLERONoch keine Bewertungen
- Filler Metal Technical Bulletin ERCuNi - FM67Dokument2 SeitenFiller Metal Technical Bulletin ERCuNi - FM67jromero_rpgNoch keine Bewertungen
- Chemical Resistance Guide For Metal - Excelente PDFDokument8 SeitenChemical Resistance Guide For Metal - Excelente PDFAmiLkar Lastre100% (1)
- Anglais ScientifiqueDokument4 SeitenAnglais Scientifiqueali BourenaneNoch keine Bewertungen
- Chapter 11Dokument53 SeitenChapter 11dertas6641100% (1)
- Alcohol, Ether & Phenol: Chapter Practice ProblemsDokument6 SeitenAlcohol, Ether & Phenol: Chapter Practice ProblemsAtharva GanjuNoch keine Bewertungen
- Hydrometallurgical Treatment of Used Printed Circuit Boards After Thermal TreatmentDokument6 SeitenHydrometallurgical Treatment of Used Printed Circuit Boards After Thermal TreatmentIoannis KapageridisNoch keine Bewertungen
- Naming Chemical Compounds WorksheetDokument4 SeitenNaming Chemical Compounds WorksheetSam Jo100% (1)
- Band Theory of The Electronic Properties of SolidsDokument6 SeitenBand Theory of The Electronic Properties of SolidsSrinivas SaiNoch keine Bewertungen
- Chem 12123123Dokument4 SeitenChem 12123123ZombieSlayer1730Noch keine Bewertungen
- Bpharm 1 Sem Pharmaceutical Inorganic Chemistry BP 104 T 2018 19Dokument1 SeiteBpharm 1 Sem Pharmaceutical Inorganic Chemistry BP 104 T 2018 19Himanshu Sharma100% (2)
- 맥머리유기화학8판Dokument1.177 Seiten맥머리유기화학8판이경식Noch keine Bewertungen
- Chapter 2 MineralsDokument48 SeitenChapter 2 MineralsDiasphroNoch keine Bewertungen
- IGCSE Chemistry - Unit 12 - The Periodic TableDokument6 SeitenIGCSE Chemistry - Unit 12 - The Periodic TableRaffaella LaxaldeNoch keine Bewertungen
- 9701 s04 QP 2 PDFDokument8 Seiten9701 s04 QP 2 PDFSanthi RamanNoch keine Bewertungen
- B&M Series 1000 SwitchesDokument12 SeitenB&M Series 1000 SwitchesscribdkhatnNoch keine Bewertungen
- Important Questions For CBSE Class 8 Science Chapter 6Dokument10 SeitenImportant Questions For CBSE Class 8 Science Chapter 6Ishant Kumar PandaNoch keine Bewertungen
- PDF SN O2 - 00-041-1445 FTODokument2 SeitenPDF SN O2 - 00-041-1445 FTOolgaNoch keine Bewertungen
- Case Study chm133Dokument2 SeitenCase Study chm133hasanahNoch keine Bewertungen
- File NameDokument25 SeitenFile NameGagan VarshneyNoch keine Bewertungen
- IS 228 Part 23Dokument6 SeitenIS 228 Part 23Gurdeep SinghNoch keine Bewertungen
- CPIDokument6 SeitenCPIPrincess SamianoNoch keine Bewertungen