Beruflich Dokumente
Kultur Dokumente
I. Experiment Title: Aldehyde, Ketone and Carboxylic Acid
Hochgeladen von
Laily SafitriOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
I. Experiment Title: Aldehyde, Ketone and Carboxylic Acid
Hochgeladen von
Laily SafitriCopyright:
Verfügbare Formate
I.
EXPERIMENT TITLE : Aldehyde, ketone and Carboxylic Acid
Identification
II. EXPERIMENT DATE : Tuesday, February 27th 2018 at 10.20 a.m.
– 16.20 p.m.
III. EXPERIMENT PURPOSE :
1. To identify organic compound that contain aldehyde group.
2. To identify organic compound that contain ketone group.
3. To identify organic compound that contain carboxylic group.
4. To differentiate between aldehyde, ketone, and carboxylic acid in the
organic compound.
IV. BASIC THEORY
A. Definition of Aldehyde, Ketone and Carboxylic Acid
Everyone has at least some first hand sensory knowledge of
aldehydes and ketones. Some aldehydes are responsible for very pleasant
tastes and odors, such as vanillin from vanilla beans, benzaldehyde from
almonds, and cinnamaldehyde from cinnamon. On the other hand,
acetaldehyde causes unpleasant hangover feeling that can result from
consuming alcoholic beverages, and formaldehyde (methanal) is highly
toxic and has a very pungent odor, as do many other aldehydes.
Structurally, the difference between the chemical causes of
hangovers and the taste of vanilla ice cream is simply a methyl group
versus a substituted phenyl group. But, what a difference this switch
makes ice cream would not be pleased in their dessert were replaced
replaced by acetaldehyde.
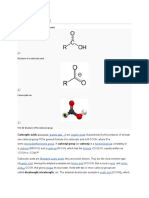
An aldehyde is an organic compound. It contains a formyl group.
A formyl group is a part of a molecule with the structure R-CHO. It is
made of a carbon double bonded to oxygen. The oxygen is also bonded
to hydrogen and an R group. A side chain is the rest of the molecule. The
group without the side chain is called the aldehyde group or formyl group.
Aldehydes are different from ketones because the formyl group is at the
end of the molecule in an aldehyde. Ketones have the formyl group in the
middle of the molecule. Aldehydes are common in organic chemistry.
Many fragrances (smell producing compounds) are aldehydes. Aldehydes
have a carbon double bonded to an oxygen. Because of this, they are
slightly polar. This causes aldehydes to have a number of properties. One
of the most important of these is solubility in water.
Aldehydes can be subjected to a number of reactions. In fact, the
Virtual Organic Chemistry Book lists over 10 common reactions of
aldehydes. A key similarity in the reactions of aldehydes is that the
carbonyl is the site of reactivity. The carbonyl carbon of aldehyde is
slightly positive because it is double bonded to an electronegative oxygen.
Due to the positive nature of the carbonyl carbon, aldehydes are
considered electrophiles. Electrophiles, or electron 'loving' molecules,
attract the electrons from molecules called nucleophiles. A nucleophile is a
molecule capable of donating a pair of electrons to form a new covalent
bond. You can think of electrophiles and nucleophiles like magnets. The
positive magnet (electrophile) attracts the negative magnet (nucleophile).
When aldehydes react with nucleophiles, the nucleophile forms a
new bond with the carbonyl carbon. This action is called a nucleophilic
attack. Molecules that act as nucleophiles typically have oxygens, such as
H2O, or nitrogen's, as in NH3, with lone pairs of electron free to 'attack'
the carbonyl carbon. In organic chemistry, the nucleophilic attack of
aldehydes is classified as an addition reaction. Often in reactions of
aldehydes, nucleophilic attack is followed by loss of water. This process is
called condensation.
Ketone, any of a class of organic compounds characterized by the
presence of a carbonyl group in which the carbonatom is covalently
bonded to an oxygenatom. The remaining two bonds are to other carbon
atoms or hydrocarbon radicals.
Ketone compounds have important physiological properties. They
are found in several sugars and in compounds for medicinal use, including
natural andsynthetic steroid hormones. Molecules of the anti-inflammatory
agent cortisonecontain three ketone groups.
Only a small number of ketones are manufactured on a large scale
in industry. They can be synthesized by a wide variety of methods, and
because of their ease of preparation, relative stability, and high reactivity,
they are nearly ideal chemical intermediates. Many complex organic
compounds are synthesized using ketones as building blocks. They are
most widely used as solvents, especially in industries
manufacturing explosives, lacquers, paints, and textiles. Ketones are also
used in tanning, as preservatives, and in hydraulic fluids.
The most important ketone is acetone (CH3COCH3), a liquid with a
sweetish odour. Acetone is one of the few organic compounds that is
infinitely soluble inwater (i.e., soluble in all proportions); it also dissolves
many organic compounds. For this reason—and because of its low boiling
point (56 °C [132.8 °F]), which makes it easy to remove by evaporation
when no longer wanted—it is one of the most important industrial
solvents, being used in such products as paints, varnishes, resins, coatings,
and nail-polish removers.
Carboxylic acid, any of a class of organic compounds in which
a carbon (C) atom is bonded to an oxygen (O) atom by a double bond and
to a hydroxyl group (−OH) by a single bond. A fourth bond links the
carbon atom to a hydrogen (H) atom or to some other univalent combining
group. The carboxyl (COOH) group is so-named because of the carbonyl
group (C=O) and hydroxyl group.
The chief chemical characteristic of the carboxylic acids is their
acidity. They are generally more acidic than other
organiccompounds containing hydroxyl groups but are generally weaker
than the familiar mineral acids (e.g., hydrochloric acid, HCl,sulfuric acid,
H2SO4, etc.).
Carboxylic acids occur widely in nature. Thefatty acids are
components of glycerides, which in turn are components of fat. Hydroxyl
acids, such as lactic acid (found in sour-milk products) and citric
acid (found in citrus fruits), and many keto acids are important metabolic
products that exist in most living cells. Proteins are made up ofamino
acids, which also contain carboxyl groups.
Compounds in which the −OH of the carboxyl group is replaced by
certain other groups are called carboxylic acid derivatives, the most
important of which are acyl halides, acid anhydrides, esters, and amides.
Carboxylic acid derivatives have varied applications. For example,
in addition to its use as a disinfectant, formic acid, the simplest carboxylic
acid, is employed intextile treatment and as an acid reducing agent. Acetic
acid is extensively used in the production of cellulose plastics and
esters.Aspirin, the ester of salicylic acid, is prepared from acetic acid.
Palmitic acid and stearic acid are important in the manufacture of soaps,
cosmetics, pharmaceuticals, candles, and protective coatings. Stearic acid
also is used in rubbermanufacture. Acrylic acid is employed as an ester in
the production of polymers (long-chain molecules) known as acrylates.
Methacrylic acid serves as an ester and is polymerized to form Lucite.
Oleic acid is used in the manufacture of soaps and detergents and of
textiles.
B. Reaction of Aldehyde. Ketone and Carboxylic Acid
Tollens’ test, also known as silver-mirror test, is a qualitative
laboratory test used to distinguish between an aldehyde and a ketone. It
exploits the fact that aldehydes are readily oxidized (see oxidation),
whereas ketones are not. Tollens’ test uses a reagent known as Tollens’
reagent, which is a colorless, basic, aqueous solution containing silver ions
coordinated to ammonia [Ag(NH3)2+][Ag(NH3)2+]. It is prepared using a
two-step procedure.
Step 1: Aqueous silver nitrate is mixed with aqueous sodium hydroxide.
AgNO3+NaOH2AgOH→AgOH+NHO3→Ag2O+H2OAgNO3+NaOH→
AgOH+NHO32AgOH→Ag2O+H2O
Step 2: Aqueous ammonia is added drop-wise until the precipitated silver
oxide completely dissolves.
Ag2O+4NH3+H2O→2Ag(NH3)+2+2OH−Ag2O+4NH3+H2O→2Ag(NH
3)2++2OH−
Tollens’ reagent oxidizes an aldehyde into the corresponding carboxylic
acid.
The reaction is accompanied by the reduction of silver ions in Tollens’
reagent into metallic silver, which, if the test is carried out in a clean glass
test tube, forms a mirror on the test tube.
Fehling's reagent, a blue colored basic solution of
bistartratocuprate(II) complex, is added to three different aqueous sugar
solutions immersed in beakers of warm water. A brick-red precipitate
forms in the solutions containing glucose and fructose. There is no
reaction in the test tube containing sucrose solution.
Fehling's solution is used as a chemical test used to differentiate
between water-soluble aldehyde and ketone functional groups, and as a
test for monosaccharides. The test was developed by German
chemist Hermann von Fehling in 1849.[1]
Fehling's solution is always prepared fresh in the laboratory. It is
made initially as two separate solutions, known as Fehling's A and
Fehling's B. Fehling's A is a blue aqueous solution of copper(II)
sulfate pentahydrate crystals, while Fehling's B is a clear solution of
aqueous potassium sodium tartrate (also known as Rochelle salt) and a
strong alkali (commonly sodium hydroxide).
Equal volumes of the two mixtures are mixed together to get the
final Fehling's solution, which is a deep blue colour. In this final mixture,
aqueous tartrate ions from the dissolved Rochelle salt chelate to Cu2+ (aq)
ions from the dissolved copper sulfate crystals, as bidentate ligands giving
the bistartratocuprate(II)complex as shown in the accompanying
illustration.
Fehling's can be used to determine whether a carbonyl-
containing compound is an aldehyde or a ketone. The bistartratocuprate(II)
complex in Fehling's solution is anoxidizingagent and the active reagent in
the test. The compound to be tested is added to the Fehling's solution and
the mixture is heated. Aldehydes are oxidized, giving a positive result, but
ketones do not react, unless they are alpha-hydroxy-ketones. The
bistartratocuprate(II) complex oxidizes the aldehyde to a carboxylateanion,
and in the process the copper(II) ions of the complex are reduced to
copper(I) ions. Red copper(I) oxide then precipitates out of the reaction
mixture, which indicates a positive result i.e. that redox has taken place
(this is the same positive result as with Benedict's solution. A negative
result is the absence of the red precipitate; it is important to note that
Fehling's will not work with aromaticaldehydes; in this case Tollens'
reagent should be used. Aldehydes are oxidised to corresponding
carboxylate anion. Aromatic aldehydes do not respond to this test.
Sodium bisulfite (NaHSO3) adds to aldehydes and some ketones to
give what is usually known as a bisulfite addition compound. The reaction
occurs by nucleophilic attack of a lone pair on the carbonyl group. This
leaves a positively charged sulfur atom but a simple proton transfer leads
to the product.
The products are useful for two reasons. They are usually crystalline and
so can be used to purify liquid aldehydes by recrystallization. This is of
value only because this reaction, like is reversible. The bisulfite
compounds are made by mixing the aldehyde or ketone with saturated
aqueous sodium bisulfite in an ice bath, shaking, and crystallizing. After
purifi cation the bisulfite addition compound can be hydrolysed back to the
aldehyde in dilute aqueous acid or base.
2,4-Dinitrophenylhydrazine (2,4-DNP or 2,4-DNPH) reacts readily
with aldehydes and ketones viaa condensation reaction (the lone pair of
electrons on the terminal amino group in 2,4-DNPH makes it a strong
nucleophile and the condensation starts by the nucleophilic 2,4-DNPH
attacking the electrophilic carbonyl carbon) to produce the corresponding
hydrazone. The hydrazine is usually a brightly colored yellow, orange or
red compound, so the reaction is often used to test for the presences of an
aldehyde or ketone.
2,4-DNPH does not react with amides, esters or carboxylic acids. As
shown below for the case of an ester, an extra resonance structure can be
drawn for these 3 types of compounds as compared to a ketone. This extra
resonance structure delocalizes some of the positive charge away from the
carbonyl carbon onto the adjacent hetero-atom (oxygen in the case of the
ester or carboxylic acid, nitrogen in the case of an amide). This makes the
carbonyl carbon less electrophilic in these compounds, and consequently
attack by the nucleophilic 2,4-DNPH is less favored.
Ketones having an α - hydrogen atom react readily with halogens
and β- haloketones are obtained as the product. The reaction is promoted
both by acids and bases.
In presence of the base, multiple halogenation occurs to give the
trihalo product.
The trihalo group is a good leaving group and the trihalo ketone
reacts with OH– which finally gives a carboxylate ion and a haloform.
This reaction is called the haloform reaction after the name of the product.
If iodine is used as the halogen, then we getiodoform( CHI3 ) as the
product. The iodoform is a bright yellow solid having a characterstic
melting point. This reaction, thus, forms the basis of the iodoform test.
Thus, methyl ketones give a positive iodoform test.
Aldehydes and ketones having at least one α-hydrogen undergo a
reaction in the presence of dilute alkali as catalyst to form β-hydroxy
aldehydes (aldol) or β-hydroxy ketones (ketol), respectively. This is
known as Aldol reaction.
The name aldol is derived from the names of the two functional groups,
aldehyde and alcohol, present in the products. The aldol and ketol
readily lose water to give α,β-unsaturated carbonyl compounds which
are aldol condensation products and the reaction is called Aldol
condensation. Though ketones give ketols (compounds containing a
keto and alcohol groups), the general name aldol condensation still
applies to the reactions of ketones due to their similarity with
aldehydes.
Carbon compounds containing a carboxyl functional group, –
COOH are called carboxylic acids. The carboxyl group, consists of a
carbonyl group attached to a hydroxyl group, hence its name carboxyl.
Carboxylic acids may be aliphatic (RCOOH) or aromatic (ArCOOH)
depending on the group, alkyl or aryl, attached to carboxylic carbon.
Large number of carboxylic acids are found in nature. Some higher
members of aliphatic carboxylic acids (C12 – C18) known as fatty
acids, occur in natural fats as esters of glycerol. Carboxylic acids serve
as starting material for several other important organic compounds such
as anhydrides, esters, acid chlorides, amides, etc.
Das könnte Ihnen auch gefallen
- 7E Lesson Plan in Types of Chemical Reaction FINALDokument4 Seiten7E Lesson Plan in Types of Chemical Reaction FINALangelica calica83% (6)
- 2012 Chemistry (Stage 3) Marking KeyDokument24 Seiten2012 Chemistry (Stage 3) Marking KeyMichael BobNoch keine Bewertungen
- Worksheet 5.3Dokument3 SeitenWorksheet 5.3Kelso ZwariyaNoch keine Bewertungen
- AldehydeDokument29 SeitenAldehydeJan michael ChivaNoch keine Bewertungen
- Name: Andi Ria Indahsari Asik ID: 1913442004: AldehydeDokument6 SeitenName: Andi Ria Indahsari Asik ID: 1913442004: AldehydeAndi RiaNoch keine Bewertungen
- Aldehydes and Ketones by Group 6 - 20230920 - 072912 - 0000Dokument18 SeitenAldehydes and Ketones by Group 6 - 20230920 - 072912 - 0000ۦۦ CristineNoch keine Bewertungen
- Lab 4 Aldehyde and KetonsDokument13 SeitenLab 4 Aldehyde and KetonsalihusseinNoch keine Bewertungen
- Laporan IGF AlimDokument11 SeitenLaporan IGF Alimppg.risdaniar99130Noch keine Bewertungen
- Module 5 Aldehydes, Ketones, and Carboxylic Acids PDFDokument19 SeitenModule 5 Aldehydes, Ketones, and Carboxylic Acids PDFRica Pearl ZorillaNoch keine Bewertungen
- Aldehydes and KetoneDokument18 SeitenAldehydes and KetoneAli AlisonNoch keine Bewertungen
- Exp 10Dokument9 SeitenExp 10ChantalDanaNoch keine Bewertungen
- Explain Chemical Reactions of Alcohol CompoundsDokument2 SeitenExplain Chemical Reactions of Alcohol CompoundsIrfan DanialNoch keine Bewertungen
- Share My Portfolio in Chem 102Dokument61 SeitenShare My Portfolio in Chem 102Remar PabalayNoch keine Bewertungen
- Siml Geometric Presentation by SlidesgoDokument62 SeitenSiml Geometric Presentation by SlidesgobltrnNoch keine Bewertungen
- Alcohol Alcohol, Any of A Class of Organic CompoundsDokument4 SeitenAlcohol Alcohol, Any of A Class of Organic CompoundsJason Orolfo Salvadora HLNoch keine Bewertungen
- Structure of Aldehydes: Aldehyde, Any of A Class ofDokument13 SeitenStructure of Aldehydes: Aldehyde, Any of A Class ofBellaPeruchaNoch keine Bewertungen
- I. Tittle: Aldehyde and Ketone II. Date of Experiment: 13th Mart 2013 Iii. PurposeDokument27 SeitenI. Tittle: Aldehyde and Ketone II. Date of Experiment: 13th Mart 2013 Iii. PurposeNurel HidayahNoch keine Bewertungen
- Tyndale-Biscoe and Mallinson Society: Senior DepartmentDokument11 SeitenTyndale-Biscoe and Mallinson Society: Senior DepartmentAlima IqbalNoch keine Bewertungen
- Hydroxyl Group: EthanolDokument8 SeitenHydroxyl Group: EthanolJen AdvientoNoch keine Bewertungen
- 5 Hydrocarbon Derivatives 2Dokument28 Seiten5 Hydrocarbon Derivatives 2Marivic TayabanNoch keine Bewertungen
- Structure of A Carboxylic AcidDokument7 SeitenStructure of A Carboxylic AcidKajal SinghNoch keine Bewertungen
- Final FinalDokument36 SeitenFinal FinalaprilrosesanchezNoch keine Bewertungen
- ESTERSDokument36 SeitenESTERSAbdihakemNoch keine Bewertungen
- Title of ExperimentDokument5 SeitenTitle of ExperimentAmeliaNoch keine Bewertungen
- Carboxylic Acid and Derivatives PDFDokument12 SeitenCarboxylic Acid and Derivatives PDFÏt's RîçkgãrçīäNoch keine Bewertungen
- Alcohols LabDokument7 SeitenAlcohols Lab7sky7harveyNoch keine Bewertungen
- Experiment 9 Formal Report On Classification Test of Hydroxyl-Containing and Carbonyl-Containing Organic CompoundsDokument16 SeitenExperiment 9 Formal Report On Classification Test of Hydroxyl-Containing and Carbonyl-Containing Organic CompoundsLuisGabito100% (1)
- Chem Notes No6Dokument31 SeitenChem Notes No6AnyhaNoch keine Bewertungen
- Reactions of AlcoholsDokument3 SeitenReactions of AlcoholsKimNoch keine Bewertungen
- Names: Bryle Kristiann CamaroteDokument18 SeitenNames: Bryle Kristiann CamaroteKateNoch keine Bewertungen
- Commonly Encountered Alcohols Methyl Alcohol (Methanol)Dokument4 SeitenCommonly Encountered Alcohols Methyl Alcohol (Methanol)Peanut DucNoch keine Bewertungen
- Aldehydes and KetonesDokument25 SeitenAldehydes and Ketonesabigail_acostaNoch keine Bewertungen
- Quarter 2 Lesson 7 Organic Chemistry and Functional Groups 1Dokument71 SeitenQuarter 2 Lesson 7 Organic Chemistry and Functional Groups 1Lhyn DE Leon DumayaNoch keine Bewertungen
- CHM 121 Lecture NoteDokument13 SeitenCHM 121 Lecture NoteOyedotun TundeNoch keine Bewertungen
- Final ScriptDokument6 SeitenFinal ScriptKresley GamayNoch keine Bewertungen
- تقرير العضويه العملي الكحول PDFDokument6 Seitenتقرير العضويه العملي الكحول PDFزينب هانيNoch keine Bewertungen
- Lab 3 AlcoholDokument11 SeitenLab 3 AlcoholalihusseinNoch keine Bewertungen
- Carberylic Acid.Dokument22 SeitenCarberylic Acid.afrNoch keine Bewertungen
- Chemistry Reference Materials 8 and 9Dokument47 SeitenChemistry Reference Materials 8 and 9Colb MLGNoch keine Bewertungen
- Carboxylic AcidDokument6 SeitenCarboxylic AcidVishu AgrawalNoch keine Bewertungen
- Carboxylic Acid: From Wikipedia, The Free EncyclopediaDokument11 SeitenCarboxylic Acid: From Wikipedia, The Free EncyclopediaDipti BagdeNoch keine Bewertungen
- Synthetic of CyclohexanoneDokument15 SeitenSynthetic of CyclohexanoneRadiatul Awalia AmirNoch keine Bewertungen
- Organic ChemistryDokument13 SeitenOrganic ChemistryKC De leonNoch keine Bewertungen
- 2-28!3!14 Oxidation ReductionDokument11 Seiten2-28!3!14 Oxidation ReductionNadine Harajli HamzehNoch keine Bewertungen
- Genesis B Organic ChemDokument4 SeitenGenesis B Organic ChemGenesis PalangiNoch keine Bewertungen
- Formal Report Exp 9Dokument13 SeitenFormal Report Exp 9Rianne SolivenNoch keine Bewertungen
- ACFrOgCOwz PAkA13N5r VLYfL3oWwwtpBeynrtTTiAe7DxlxTe14VfengPMQr4yoxJo06fry085T9L SFpJHU1GU25RVvZBKewvuYmlT7941NOjlUV2jM1 e21KB XVLoyxAFlunlO IPMct8hyDokument10 SeitenACFrOgCOwz PAkA13N5r VLYfL3oWwwtpBeynrtTTiAe7DxlxTe14VfengPMQr4yoxJo06fry085T9L SFpJHU1GU25RVvZBKewvuYmlT7941NOjlUV2jM1 e21KB XVLoyxAFlunlO IPMct8hykateNoch keine Bewertungen
- Aldehyde and KetonesDokument14 SeitenAldehyde and KetonesBismah SaeedNoch keine Bewertungen
- 3 Ethoxy 4 HydroxybenzaldehydeDokument2 Seiten3 Ethoxy 4 HydroxybenzaldehydemeimeiliuNoch keine Bewertungen
- Aldehydes, Ketones, Carboxylic Acids, and EstersDokument11 SeitenAldehydes, Ketones, Carboxylic Acids, and EstersNATURE COMPUTERNoch keine Bewertungen
- Carbon & Its Compounds - pdf-28Dokument5 SeitenCarbon & Its Compounds - pdf-28WARRIORS100% (1)
- Module 9. Alcohols: Nomenclature, Preparation and ReactionsDokument3 SeitenModule 9. Alcohols: Nomenclature, Preparation and ReactionsPeña, RodolfoNoch keine Bewertungen
- Aldehyde KetoneDokument25 SeitenAldehyde KetoneIpshita PathakNoch keine Bewertungen
- Carboxylic AcidsDokument15 SeitenCarboxylic Acidsmadhu bonamNoch keine Bewertungen
- 6 SikloheksenaDokument13 Seiten6 SikloheksenaHasrilia BeskaraNoch keine Bewertungen
- CNC XDokument8 SeitenCNC Xvandanachaudhary075Noch keine Bewertungen
- CarbohydratesDokument12 SeitenCarbohydratesDynamic & CannyNoch keine Bewertungen
- Encyclopedia of Chemistry & Explosives MaterialsDokument143 SeitenEncyclopedia of Chemistry & Explosives Materialskaustubhvjoshi3617100% (4)
- F334 - What's in A Medicine?Dokument11 SeitenF334 - What's in A Medicine?Becky Tenney100% (1)
- Transition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesVon EverandTransition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesNoch keine Bewertungen
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersVon EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNoch keine Bewertungen
- Aliphatic Compounds: Trihydric Alcohols, Their Oxidation Products and Derivatives, Penta- and Higher Polyhydric Alcohols, Their Oxidation Products and Derivatives; Saccharides, Tetrahydric Alcohols, Their Oxidation Products and DerivativesVon EverandAliphatic Compounds: Trihydric Alcohols, Their Oxidation Products and Derivatives, Penta- and Higher Polyhydric Alcohols, Their Oxidation Products and Derivatives; Saccharides, Tetrahydric Alcohols, Their Oxidation Products and DerivativesNoch keine Bewertungen
- Lanes Work + Ex Res MedanDokument7 SeitenLanes Work + Ex Res MedanLaily SafitriNoch keine Bewertungen
- Answer Questions N Nh4Dokument3 SeitenAnswer Questions N Nh4Laily SafitriNoch keine Bewertungen
- Spiritual Attitude Assessment Observation Sheet: Basic CompetenceDokument8 SeitenSpiritual Attitude Assessment Observation Sheet: Basic CompetenceLaily SafitriNoch keine Bewertungen
- No. Expr. Procedure Result Observation Hypothesis/Reactions Conclusion Before AfterDokument2 SeitenNo. Expr. Procedure Result Observation Hypothesis/Reactions Conclusion Before AfterLaily SafitriNoch keine Bewertungen
- Medallists: NOC Medal NameDokument1 SeiteMedallists: NOC Medal NameLaily SafitriNoch keine Bewertungen
- Syllaby: Subject: Chemistry "Hydrocarbon Compound" Senior High School: SMA Core Competences: KI.1: K.I.2Dokument3 SeitenSyllaby: Subject: Chemistry "Hydrocarbon Compound" Senior High School: SMA Core Competences: KI.1: K.I.2Laily SafitriNoch keine Bewertungen
- 10th Experiment - Copy-1Dokument4 Seiten10th Experiment - Copy-1Laily SafitriNoch keine Bewertungen
- Let'S See Who We'Re On "Anime Show": A Special Invitation For All Student of 6 ShsDokument1 SeiteLet'S See Who We'Re On "Anime Show": A Special Invitation For All Student of 6 ShsLaily SafitriNoch keine Bewertungen
- Final TestDokument5 SeitenFinal TestArriane Joy VillariscoNoch keine Bewertungen
- EDTADokument2 SeitenEDTARay AribatoNoch keine Bewertungen
- Ebook Chemistry For Today General Organic and Biochemistry PDF Full Chapter PDFDokument67 SeitenEbook Chemistry For Today General Organic and Biochemistry PDF Full Chapter PDFrobert.davidson233100% (20)
- Literature ReviewDokument19 SeitenLiterature ReviewRoxana100% (1)
- The Folin-Ciocalteu Assay RevisitedDokument10 SeitenThe Folin-Ciocalteu Assay RevisitedoniatrdNoch keine Bewertungen
- Joint Entrance Test (Jet) - 2019: General Instructions For Admission ToDokument27 SeitenJoint Entrance Test (Jet) - 2019: General Instructions For Admission ToKd Gurjar KhuriNoch keine Bewertungen
- 02 Full Length Paper-2 (Student Copy)Dokument15 Seiten02 Full Length Paper-2 (Student Copy)jawaidaliNoch keine Bewertungen
- Lower Secondary Science 7 Scheme of WorkDokument21 SeitenLower Secondary Science 7 Scheme of WorksumnamundethNoch keine Bewertungen
- Chemical Kinetics (M) PDFDokument41 SeitenChemical Kinetics (M) PDFNalla Umapathi Reddy75% (4)
- Thermodynamic Modeling and Analysis of Biomass Gasification For Hydrogen Production in Supercritical WaterDokument12 SeitenThermodynamic Modeling and Analysis of Biomass Gasification For Hydrogen Production in Supercritical Waterprashant_salima6377Noch keine Bewertungen
- Holy Angel University Basic Education Department Senior High School Perfomance Task General Chemistry 2 - Third QuarterDokument6 SeitenHoly Angel University Basic Education Department Senior High School Perfomance Task General Chemistry 2 - Third QuarterRuskee PatawaranNoch keine Bewertungen
- Separations 09 00307Dokument27 SeitenSeparations 09 00307Mi doremiNoch keine Bewertungen
- Heat of NeutralizationDokument7 SeitenHeat of NeutralizationReymar Suello UngabNoch keine Bewertungen
- Lett 9b03210Dokument5 SeitenLett 9b03210Yarkali KrishnaNoch keine Bewertungen
- 010 - UOP Manual de Plataforma Manual PRT1 PDFDokument263 Seiten010 - UOP Manual de Plataforma Manual PRT1 PDFROMAN ALEXANDER FERNANDEZ ROCHA100% (2)
- KimiaDokument46 SeitenKimiaErvina RetnaningtyasNoch keine Bewertungen
- C.N.M. School & N.D. Parekh Pre-Primary School Class Xi Chemistry Worksheet (2017 - 2018) Aldehydes and KetonesDokument2 SeitenC.N.M. School & N.D. Parekh Pre-Primary School Class Xi Chemistry Worksheet (2017 - 2018) Aldehydes and KetonesAfza MukaddamNoch keine Bewertungen
- Conclusion Org ChemDokument1 SeiteConclusion Org ChemAngelica Joyce SinnacoNoch keine Bewertungen
- UOP I-210 CatDokument3 SeitenUOP I-210 Cathml2827Noch keine Bewertungen
- Chemistry ks4 Lesson CHM Y11 U1 l11Dokument18 SeitenChemistry ks4 Lesson CHM Y11 U1 l11Khalid MehmoodNoch keine Bewertungen
- by NVS TEACHER Aldehydes Ketones and Acids Part 3Dokument35 Seitenby NVS TEACHER Aldehydes Ketones and Acids Part 3Srushti GorasiyaNoch keine Bewertungen
- ChemistryDokument9 SeitenChemistrypiyushkanwat123Noch keine Bewertungen
- OxygenDokument10 SeitenOxygenFaheem HaiderNoch keine Bewertungen
- Ch0 Course S Introductionto BkelDokument2 SeitenCh0 Course S Introductionto BkelHoài ThươngNoch keine Bewertungen
- Halogenoalkanes TestDokument5 SeitenHalogenoalkanes TestDr.CharinNoch keine Bewertungen
- Chemistry ScienceDokument63 SeitenChemistry ScienceKingdavid Waitolo100% (2)
- SCIENCE 10 Q4 Module 5 Balancing Chemical EquationsDokument25 SeitenSCIENCE 10 Q4 Module 5 Balancing Chemical EquationsPatrick GalmanNoch keine Bewertungen