Beruflich Dokumente
Kultur Dokumente
Common MS Fragment Ions
Hochgeladen von
MarianaMuCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Common MS Fragment Ions
Hochgeladen von
MarianaMuCopyright:
Verfügbare Formate
Table of Common Fragment Ions
m/z Ions m/z Ions
15 CH3 63 C5H3 a
16 O 65 C5H5 a
17 OH 67 C5H7
18 H2O 69 C5H9
19 F 70 C5H10
26 CN 71 C5H11, C3H7-C=O
27 C2H3 72 C2H5-CO-CH2+Hb
28 C2H4, CO, N2 73 C3H7OCH2,C2H5O-C=O
C3H7CHOH, C2H5OCHCH3
29 C2H5, CHO 74 CH2-COOCH3+Hb
30 CH2NH2, NO 75 C2H5O-C=O+2Hb, C2H5COO+2Hb
31 CH2OH, OCH3 77 C6H5 a
32 O2 78 C6H5+Hab
33 SH 79 C6H5+2Hab, Br (also 81)
34 H2S 81 C6H9
35, 37 Cl 82 C6H10, CCl2 (also 84&86)
36,38 HCl 83 C6H11, CHCl2 (also 85&87)
39 C3H3a 84 C6H12
40 CH2CN, Ar 85 C6H13, C4H9-C=O
41 C3H5, CH2CN+Hb 86 C3H7-CO-CH2+Hb
42 C3H6 87 Homologs of 59 & 73
43 C3H7, CH3C=O 88 CH2-COOC2H5+Hb
44 CH2CHO+Hb, CO2 89 C3H7-O-C=O+2Hb, C3H7COO+2Hb
CH3CH-NH2
45 CH3CHOH, CH2OCH3, 90 C6H5-CH
CH2CH2OH, COOH
46 NO2 91 C6H5-CH2, C6H5-CH+Hb
47 CH2SH, CH3S 92 C6H5-CH2+Hb
48 CH3S+Hb 93 C7H9, CH2Br (also 95)
49, 51 CH2Cl 94 C6H5O+H
50 C4H2 a 97 C7H13
51 C4H3 a 99 Homologs of 71 & 85
52 C4H4 a 100 Homolog of 72 & 86
53 C4H5 101 Homologs of 59, 73, & 87
54 CH2CH2CN, 102 Homolog of 74 & 88
CH3CH-CN
55 C4H7 103 Homolog of 75 & 89
56 C4H8 105 C6H5C=O,C6H5-CH2CH2
57 C4H9, C2H5-C=O 107 C6H5-CH2O
58 CH3-CO-CH2+Hb 108 C6H5-CH2O+Hb
59 C2H5OCH2, CH3O-C=O 119 C6H5-C(CH3)2
C2H5CHOH, CH3O-CHCH3
60 CH2-COOH+Hb 127 I
61 CH3COO+2Hb, CH3OCO+2Hb
a
Good diagnostic for benzene ring compounds
b
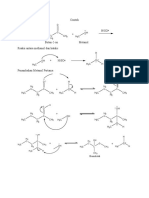
The “+H” notation means that the ion was formed by a rearrangement that involved the
transfer of a hydrogen atom from some other part of the molecule. For example:
H H
C O O
+
C
C CH3 H2C CH3
H2
You will notice that most of these “+H” entries have even mass values. The
rearrangement shown above produces an ion with m/z=58.
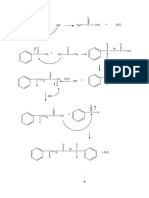
Those ions identified with a “+2H” arise from a double rearrangement. One example,
producing an ion of m/z=61 from an ester, is shown below.
R
H
H H
C O
C O C C
CH2R CH2R =
C H
C C H
O CH3
O CH3 O
O
H3C
H
R
H H
O
C C
+ O
C.
m/z=61
H3C
Das könnte Ihnen auch gefallen
- Chapter 5 HydrocarbonDokument25 SeitenChapter 5 Hydrocarbonmeshal retteryNoch keine Bewertungen
- Aldehyde PDFDokument32 SeitenAldehyde PDFMalti GuptaNoch keine Bewertungen
- Aqa Mechanisms A Level SummaryDokument5 SeitenAqa Mechanisms A Level SummaryRS JNoch keine Bewertungen
- Isomerism WorksheetDokument42 SeitenIsomerism WorksheetChinmay100% (2)
- 4.0 Introduction To Organic Chemistry 2021Dokument119 Seiten4.0 Introduction To Organic Chemistry 2021Khairina NadiahNoch keine Bewertungen
- How To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inDokument2 SeitenHow To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inmyiitchemistry81% (16)
- Alkyl Halides & Aryl Halides-02 - Solved ProblemsDokument13 SeitenAlkyl Halides & Aryl Halides-02 - Solved ProblemsRaju SinghNoch keine Bewertungen
- Mechanism Summary For A-Level AQA Chemistry: BR BRDokument5 SeitenMechanism Summary For A-Level AQA Chemistry: BR BRamrhkmhNoch keine Bewertungen
- Condensed Structures ExplainedDokument6 SeitenCondensed Structures ExplainedkalloliNoch keine Bewertungen
- Qualitative Tests for Elements in Organic Compounds (QTEOCDokument10 SeitenQualitative Tests for Elements in Organic Compounds (QTEOCRovic MelladoNoch keine Bewertungen
- Organic Chemistry NotesDokument24 SeitenOrganic Chemistry NotesSweatNoch keine Bewertungen
- Common MS Fragment Ions PDFDokument2 SeitenCommon MS Fragment Ions PDFSeliaDestianingrumNoch keine Bewertungen
- SPEKTROSKOPI MASSADokument25 SeitenSPEKTROSKOPI MASSAVita Maryam H.Noch keine Bewertungen
- Mekanisme RX DibenzalasetonDokument2 SeitenMekanisme RX DibenzalasetonWulan safitriNoch keine Bewertungen
- Quimica 11 - Didáctica MultimediaDokument197 SeitenQuimica 11 - Didáctica MultimediaviviarcelopezNoch keine Bewertungen
- Hidro KarbonDokument43 SeitenHidro KarbonElisabet NoviantiNoch keine Bewertungen
- Advanced Biochemistry: The Krebs CycleDokument11 SeitenAdvanced Biochemistry: The Krebs CycleMaritsa PerHerNoch keine Bewertungen
- Chemistry PracticeDokument13 SeitenChemistry PracticeSiddharth KrishnamurthyNoch keine Bewertungen
- Topic 17 Exercise 1 - Naming Organic Compounds: C CL ODokument1 SeiteTopic 17 Exercise 1 - Naming Organic Compounds: C CL OAmmaarah PatelNoch keine Bewertungen
- Answers To AssignmentDokument1 SeiteAnswers To AssignmentIgbereyivwe TejiriNoch keine Bewertungen
- CY2102Dokument2 SeitenCY2102Prarabdha SharmaNoch keine Bewertungen
- A) OH B) OH C) OH D) OH E) OH F) OHDokument4 SeitenA) OH B) OH C) OH D) OH E) OH F) OHRodrigo RVNoch keine Bewertungen
- haloalcanos.3Dokument1 Seitehaloalcanos.3MIRIAM SAN FRUTOS GARCÍANoch keine Bewertungen
- 5.111 Principles of Chemical Science: Mit OpencoursewareDokument7 Seiten5.111 Principles of Chemical Science: Mit OpencoursewareAgung SujatmikoNoch keine Bewertungen
- Materi Reaksi KimiaDokument11 SeitenMateri Reaksi KimiaRenn AgenaNoch keine Bewertungen
- ModuleDokument2 SeitenModuleRannabelleNoch keine Bewertungen
- Reaksi antara Metanol dan Butan-2-on menghasilkan Ketal 2,2-dimetoksibutanaDokument2 SeitenReaksi antara Metanol dan Butan-2-on menghasilkan Ketal 2,2-dimetoksibutanaElis TianiNoch keine Bewertungen
- Reaksi antara Metanol dan Butan-2-on menghasilkan Ketal 2,2-dimetoksibutanaDokument2 SeitenReaksi antara Metanol dan Butan-2-on menghasilkan Ketal 2,2-dimetoksibutanaElis TianiNoch keine Bewertungen
- C - Sol - Ch-26 - Aldehydes Ketones and Carboxylic AcidsDokument19 SeitenC - Sol - Ch-26 - Aldehydes Ketones and Carboxylic AcidsHimanshi ChahalNoch keine Bewertungen
- Chapter 7 HaloalkanesDokument11 SeitenChapter 7 HaloalkanesSandra JohnNoch keine Bewertungen
- Problems: Syn To The Methyl GroupDokument1 SeiteProblems: Syn To The Methyl Grouppanda biruNoch keine Bewertungen
- Lipida: Indah Saraswati, M. SCDokument55 SeitenLipida: Indah Saraswati, M. SCPutriNoch keine Bewertungen
- JEE Main Organic Compound Containing Halogens Important QuestionsDokument15 SeitenJEE Main Organic Compound Containing Halogens Important QuestionsRuchitha VNoch keine Bewertungen
- Priority PDFDokument3 SeitenPriority PDFIbin Gooding CloudyNoch keine Bewertungen
- Alcohols, Phenols & Ether - AnswersDokument6 SeitenAlcohols, Phenols & Ether - AnswersK. RupaNoch keine Bewertungen
- Unit 4, CHEMISTRY OF CARBOHYDRATESDokument31 SeitenUnit 4, CHEMISTRY OF CARBOHYDRATESDessalegn Bekele AlemayehuNoch keine Bewertungen
- Problems 11-20-06 Chem231-SSCC: C H H C C C H CH C H CL C C H CH C H + CLDokument6 SeitenProblems 11-20-06 Chem231-SSCC: C H H C C C H CH C H CL C C H CH C H + CLsarahNoch keine Bewertungen
- ALKANE NAMES, Formulas, Properties (Memorize) (Sections 3.2,4)Dokument12 SeitenALKANE NAMES, Formulas, Properties (Memorize) (Sections 3.2,4)Jansenn PastorNoch keine Bewertungen
- Coursebook Answers Chapter 18 Asal ChemistryDokument4 SeitenCoursebook Answers Chapter 18 Asal ChemistryMarin PesicNoch keine Bewertungen
- Reductions PPT 29-08-2020Dokument12 SeitenReductions PPT 29-08-2020jkc collegeNoch keine Bewertungen
- Chemistry Paper - Ii Solution (Code 3)Dokument5 SeitenChemistry Paper - Ii Solution (Code 3)kolodoloNoch keine Bewertungen
- Stereochemistry and chiralityDokument4 SeitenStereochemistry and chiralityPuvaneswary LoganathanNoch keine Bewertungen
- IIT-JEE ChEmistry by N.J. sir - Perkin CondensationDokument1 SeiteIIT-JEE ChEmistry by N.J. sir - Perkin CondensationSDMNoch keine Bewertungen
- Hydrocarbons Pyqs SolnsDokument12 SeitenHydrocarbons Pyqs Solnssaadvik1121Noch keine Bewertungen
- Quiz-Alcohol Ether & Phenols-Rsk - RGVDokument6 SeitenQuiz-Alcohol Ether & Phenols-Rsk - RGVAtharva GanjuNoch keine Bewertungen
- Structural formulae, condensed structural and skeletal formulaeDokument1 SeiteStructural formulae, condensed structural and skeletal formulaekalam lokNoch keine Bewertungen
- 0331 S 05 BioenergyDokument44 Seiten0331 S 05 BioenergyDaisyNoch keine Bewertungen
- Nomenclature of Organic CompoundsDokument80 SeitenNomenclature of Organic CompoundsSajjad MiraniNoch keine Bewertungen
- Peta Minda KimiaDokument36 SeitenPeta Minda KimiaNATASHA 'ALIA BINTI ZULKIFLINoch keine Bewertungen
- MEKANISMEDokument2 SeitenMEKANISMEEry NourikaNoch keine Bewertungen
- One-Step Synthesis of Organic CompoundsDokument13 SeitenOne-Step Synthesis of Organic CompoundsMARITZA QUISPE VILLARREALNoch keine Bewertungen
- IUPAC Exercise-3Dokument6 SeitenIUPAC Exercise-3sumit parasharNoch keine Bewertungen
- Ejercicios ElectroquimicaDokument6 SeitenEjercicios ElectroquimicaEthel Elsa D ́ Arendelle Lan WanJi IPNNoch keine Bewertungen
- Organic HWDokument4 SeitenOrganic HWLawrence KankamNoch keine Bewertungen
- Aldehydes Ketones: Subjective ProblemsDokument16 SeitenAldehydes Ketones: Subjective ProblemsBhaskar AnandNoch keine Bewertungen
- MechanismsDokument5 SeitenMechanismsnajifaahmed223Noch keine Bewertungen
- Assignment 1 2019 AnswersDokument7 SeitenAssignment 1 2019 AnswersDaniel GhiasvandNoch keine Bewertungen
- Alcohol, Ether & PhenolDokument8 SeitenAlcohol, Ether & Phenolshashwat.gupta.707Noch keine Bewertungen
- 100S120 CS19L01Dokument38 Seiten100S120 CS19L01b101112154Noch keine Bewertungen
- Ib Chem Answers 20Dokument4 SeitenIb Chem Answers 20LE ZHAINoch keine Bewertungen
- ND RPT 4 Xii Che Neet Key 08-12-23Dokument6 SeitenND RPT 4 Xii Che Neet Key 08-12-23Deena chemistNoch keine Bewertungen
- Carbohydrates Physical and Chemical PropertiesDokument44 SeitenCarbohydrates Physical and Chemical Propertiesrizal_31Noch keine Bewertungen
- GM Lab Chemical ListDokument8 SeitenGM Lab Chemical ListPrasad Uday BandodkarNoch keine Bewertungen
- US6509503Dokument4 SeitenUS6509503ahmed hargaNoch keine Bewertungen
- AZEOTROPIC DATA (Advances in Chemistry Volume 6) PDFDokument331 SeitenAZEOTROPIC DATA (Advances in Chemistry Volume 6) PDFSachikanta PradhanNoch keine Bewertungen
- Lecture07-09 Alkanes Nomenclature StructureDokument75 SeitenLecture07-09 Alkanes Nomenclature StructureLeslieLooNoch keine Bewertungen
- CHEM 221 Exercise Book Unit II-1Dokument5 SeitenCHEM 221 Exercise Book Unit II-1bacha01Noch keine Bewertungen
- Covalent Bonding MSDokument7 SeitenCovalent Bonding MStasfia2829Noch keine Bewertungen
- Grading Rubric for DNA ModelsDokument1 SeiteGrading Rubric for DNA ModelsCherry Ann MacalmaNoch keine Bewertungen
- Isocyanate PPT BIKASH DUTTA NEWDokument11 SeitenIsocyanate PPT BIKASH DUTTA NEWmita shilNoch keine Bewertungen
- Understanding the Molecular Geometry of Acetylene (C2H2Dokument3 SeitenUnderstanding the Molecular Geometry of Acetylene (C2H2sssNoch keine Bewertungen
- Lab Organic Chemistry UmDokument7 SeitenLab Organic Chemistry UmLinda AidaNoch keine Bewertungen
- 1.6! Drawing Chemical StructuresDokument6 Seiten1.6! Drawing Chemical StructuresSadeeq ArtxzNoch keine Bewertungen
- Solution Formation Electrolytes Acids and Bases Strong and Weak Acids and Bases Concentration Percent Concentration Molarity Molar-Solutions-SolidsDokument71 SeitenSolution Formation Electrolytes Acids and Bases Strong and Weak Acids and Bases Concentration Percent Concentration Molarity Molar-Solutions-SolidsDexter EnthusiastsNoch keine Bewertungen
- Chapter 7 - Chemical Reactions and Equations - Powerpoint Presentation.Dokument16 SeitenChapter 7 - Chemical Reactions and Equations - Powerpoint Presentation.Nicholas KoerbitzNoch keine Bewertungen
- 7 0-AlkenesDokument84 Seiten7 0-AlkenesAj MirandaNoch keine Bewertungen
- 12th Chemistry Slow Learners Study Materials EM Prepared by Mrs. v. BharaniDokument137 Seiten12th Chemistry Slow Learners Study Materials EM Prepared by Mrs. v. Bharani11hariharan06Noch keine Bewertungen
- 8 SaltsDokument44 Seiten8 SaltsGaneshNoch keine Bewertungen
- Preparation of Stains & Solutions Used in The Papnicolaou StainingDokument11 SeitenPreparation of Stains & Solutions Used in The Papnicolaou StainingvivekraghavanmNoch keine Bewertungen
- Chemical Principles The Quest For Insight 7th Edition Atkins Test BankDokument45 SeitenChemical Principles The Quest For Insight 7th Edition Atkins Test Bankwadeperlid9d98k100% (26)
- Ex 13Dokument6 SeitenEx 13Lovelyrabbit26Noch keine Bewertungen
- Chemistry: PH and pOH Calculations: Part 1: Fill in The Missing Information in The Table BelowDokument6 SeitenChemistry: PH and pOH Calculations: Part 1: Fill in The Missing Information in The Table BelowCaryl Ann C. SernadillaNoch keine Bewertungen
- Synthesis of Nitrobenzene ReportDokument6 SeitenSynthesis of Nitrobenzene ReportHasrilia BeskaraNoch keine Bewertungen
- Periodic Table Booklet f22Dokument5 SeitenPeriodic Table Booklet f22QingNoch keine Bewertungen
- Chemsheets AS 1066 Halogen Oxidising PowerDokument19 SeitenChemsheets AS 1066 Halogen Oxidising PowerAyeshaNoch keine Bewertungen
- Organic Chemistry Form 3 Notes: HydrocarbonsDokument18 SeitenOrganic Chemistry Form 3 Notes: HydrocarbonsKevinNoch keine Bewertungen
- TOPIC 3-Physical OrganicDokument8 SeitenTOPIC 3-Physical OrganicNaku MosesNoch keine Bewertungen
- Acids Part 2Dokument4 SeitenAcids Part 2Aljim CarcillarNoch keine Bewertungen
- Chemistry 1 - 11 - Q2 - M5Dokument13 SeitenChemistry 1 - 11 - Q2 - M5sofiamaenopra.comNoch keine Bewertungen