Beruflich Dokumente
Kultur Dokumente
Selección de Tecnologías para El Tratamiento de Aguas Residuales Municipales
Hochgeladen von
mario0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
7 Ansichten2 Seitenelectrodeposición del zinc
Originaltitel
Selección de Tecnologías Para El Tratamiento de Aguas Residuales Municipales
Copyright
© © All Rights Reserved
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenelectrodeposición del zinc
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
7 Ansichten2 SeitenSelección de Tecnologías para El Tratamiento de Aguas Residuales Municipales
Hochgeladen von
marioelectrodeposición del zinc
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 2
Patented July 8, 1952 & 2,602,775
UNITED STATES PATENT OFFICEpromotion or one . . . . . .
Eldon Irwin Isherwood, Elin. Flon, Manitoba,
Canada, assignior to Hudson Bay Mining and
Smelting Co., Limited, Winnipeg, Manitoba,
Canada, a corporation of Canada. . .
NoSerial
Drawing. Application September 22, 1950,
No. 186,323. In Canada August 10, 1950
6 Claims. (CI. 204-55)
2
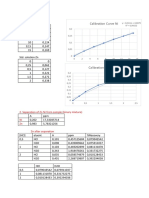
This invention relates to the electrodeposition Silver, about 0.25% copper and the balance lead.
of zinc from zinc sulphate solutions using lead According to the invention, the electrodeposi
base alloy anodes. By lead base alloy anodes is tion of zinc from a zinc sulphate solution may
meant anodes formed of an alloy consisting also be carried out to advantage with a lead base
chiefly of lead. alloy anode consisting essentially of lead alloyed
As is well known, it is desirable in the electro With Small amounts of silver, copper, cobalt and
deposition of zinc from zinc sulphate solutions manganese, the percentages falling within the
to use anodes consisting of lead or consisting ranges of from about 0.4% to about 1.45% silver,
chiefly of that metal, and indeed it is generally from about 0.15% to about 0.45% copper, from
considered that such anodes are the Only Ones 0 about 0.01% to about 0.02% cobalt and from
suitable. Such anodes are, however, Subject to about 0.01% to about 0.15% manganese. It has
the disadvantage that, during the operation of been found that the best proportions for an alloy
the electrolytic cell, some of the lead from the anode of this composition are about 1.0% silver,
anode is transferred to the cathode zinc deposit, about 0.5% copper, about 0.02% cobalt, and about
thereby contaminating it, and decreasing the 15 0.1% manganese.
value of the zinc produced. For certain purposes, The effect of carrying out the electrodeposition
such as pressure die casting, it is essential that with the lead-silver-copper and lead-silver-cop
zinc be almost free from certain impurities, One per-cobalt-manganese alloy anodes in reducing
of the most objectionable of which is lead. the transfer of lead from anode to cathode, as
Many attempts have been made to decrease 20 Compared With a lead-silver anode, is illustrated
this lead transfer from anode to cathode and by the following table showing the results of
various lead base alloys have been proposed for laboratory tests. The percentages given are by
use as anode material. Some of these lead base Weight and, in each case, the remainder of the
alloys have been partially successful, the most anode is lead.
popular lead base alloy anode in use at present 25
in the electrolytic zinc industry consisting of lead Assays of Anodes Percentage
and a small amount of silver varying up to A.SSay 0ff Operation
Of E.
about 2.0% by weight. Other investigators have Test SEE Cathode E. lead
No. fit A Cu Co Mn Zn Pb in cathode
found that the addition of tin or tin and cobalt Per Per Per Per Per Cent zinc
to this basic lead-silver alloy gives good results. 30 Cent Cent Cent Cent 0.00.0%
or less.
It has now been found that the use of a lead
base alloy anode containing small amounts of 140 1.0 Nil Nil Nil 0.0038 2
copper as well as small amounts of silver, has a 78 0.28 0.12 Ni Ni 0.0022 12
very pronounced and surprising effect in reducing 78 0.93
78 1.12
0.25
0.21
Ni Ni
Nil Nil
0.0015
0.003
46
48
the transfer of lead from anode to Cathode, 78 1.45 0, 40 Nil Nil 0.0014 40
Actual tests have shown a reduction in the 78 0.36 0.15 .01 .01 0.0020 19
78 0.75 0.20 .01 .06 0.0014 3.
amount of lead found in the cathode zinc of as 78 1.03
78.45
0.35
0, 45
.01 0.1
.01 0.14
0.002
0.001
63
57
much as about two-thirds when a lead base alloy 84 0.98 0.75 .02 .06 0.0012 79
anode containing Small amounts of Copper as 84 0.47 0.32 .01 .02 0.0018 63
well as small amounts of silver is used in place
of the usual lead base alloy anode, consisting of What I claim as my invention is:
lead and silver. It has also been found that there 1. A process for the electrodeposition of zinc
is an even greater improvement over lead-silver from a zinc Sulphate solution, which comprises
anodes when there is used a lead base alloy anode, causing deposition of zinc from the solution with
consisting of lead and Small amounts of silver, 45 an alloy anode consisting essentially of lead
Copper, cobalt and manganese. alloyed with Small amounts of silver and copper.
Thus, according to the invention, zinc is de 2. A process for the electrodeposition of zinc
posited from a zinc sulphate solution with an from a zinc sulphate solution, which comprises
alloy anode consisting essentially of lead alloyed causing deposition of zinc from the solution with
with small amounts of silver and copper, the per an alloy anode consisting essentially of lead
centage of silver being advantageously within alloyed with Small amounts of silver, copper,
the range of about 0.3% to about 1.45%, and of Cobalt and manganese.
copper being about 0.1% to about 0.4%. It has 3. A process for the electrodeposition of zinc
been found that the best results are obtained
with such an alloy anode containing about 1% from a zinc Sulphate solution as defined in claim
2,602,775 as:
3 4.
1, in which the amounts of silver and Copper 6. A process for the electrodeposition of Zinc
respectively present in the alloy anode are within from a zinc sulphate solution as defined in claim
the ranges of from about 0.3% to about 1.4% sil 5, in which the amount of silver present in the
wer and from about 0.1% to about 0.4% copper. alloy anode is about 1.0%, of copper is about
4. A process for the electrodeposition of zinc 5 0.5%, of cobalt is about 0.02% and of manganese
from a zinc sulphate solution as defined in claim is about 0.1%.
3, in which the amount of silver present in the
alloy anode is about 1.0% and of copper is about ELDON IRWIN ISHERWOOD,
0.25%.
5. A process for the electrodeposition of zinc REFERENCES CITED
from a zinc sulphate solution as defined in claim he following references are of record in the
2, in which the amounts of silver, copper, cobalt file of this patent:
and manganese respectively present in the alloy UNITED STATES PATENTS
anode are Within the ranges of from about 0.4%
to about 1.45% silver, from about 0.15% to about 15 Number Name Date
0.45% copper, from about 0.01% to about 0.15% 1681,272 Yoshikawa --------- Aug. 21, 1928
manganese, and from about 0.01% to about 0.02% 1,699,761 Silberstein --------- Jan. 22, 1929
cobalt. 2,419,722 TOWe et al. --------- Apr. 29, 1947
Das könnte Ihnen auch gefallen
- 1993 - Recycled LeadDokument9 Seiten1993 - Recycled LeadgutobegaNoch keine Bewertungen
- Chemical composition limits for steel gradesDokument8 SeitenChemical composition limits for steel gradesrobin regoNoch keine Bewertungen
- Exepermint 1Dokument4 SeitenExepermint 1Jhone SaaimonNoch keine Bewertungen
- Adsorption of Acetic Acid by Activated CarbonDokument11 SeitenAdsorption of Acetic Acid by Activated CarbonHayden Chappelear-RobbinsNoch keine Bewertungen
- CFD Analysis: Project Presentation byDokument19 SeitenCFD Analysis: Project Presentation byAkshayBhutadaNoch keine Bewertungen
- CIPW Norm Hollacher Norm4Dokument2 SeitenCIPW Norm Hollacher Norm4Lucas CabelierNoch keine Bewertungen
- Dimetilosulfoxido PanreacDokument2 SeitenDimetilosulfoxido Panreaccarolina molano OrjuelaNoch keine Bewertungen
- Mapping Mineral Systems With SWIRDokument45 SeitenMapping Mineral Systems With SWIRHugo Poma FernándezNoch keine Bewertungen
- Calculation of The Compounds in Portland Cement': Analytical EditionDokument6 SeitenCalculation of The Compounds in Portland Cement': Analytical EditionyassinzeNoch keine Bewertungen
- Ion Exchange CalculationDokument3 SeitenIon Exchange CalculationAfra AlethianaNoch keine Bewertungen
- PKM 18Dokument2 SeitenPKM 18Ranjith kumarNoch keine Bewertungen
- Awal Pembagian Debit Pipa Jaringan Distribusi AirDokument6 SeitenAwal Pembagian Debit Pipa Jaringan Distribusi AirTitik A.Noch keine Bewertungen
- CIPW Norm HollacherDokument1 SeiteCIPW Norm HollacherBrandon Nils Calderón PomaNoch keine Bewertungen
- Simultaneous Determination of The Concentrations of Cobalt ( - ) and Nickel ( - ) by UV/Vis SpectrosDokument8 SeitenSimultaneous Determination of The Concentrations of Cobalt ( - ) and Nickel ( - ) by UV/Vis SpectrosJassim123 SabtNoch keine Bewertungen
- CaffeieneDokument8 SeitenCaffeieneHawta AbdullaNoch keine Bewertungen
- STD PreparationDokument4 SeitenSTD PreparationgesecNoch keine Bewertungen
- Catalyst-Aided Amine Regeneration for Improved CO2 CaptureDokument15 SeitenCatalyst-Aided Amine Regeneration for Improved CO2 CaptureMakarand PatilNoch keine Bewertungen
- SSI TechReport AAICP PDFDokument8 SeitenSSI TechReport AAICP PDFAgung GpNoch keine Bewertungen
- Experiment 5 - Data TreatmentDokument6 SeitenExperiment 5 - Data TreatmentShawn Ann SilanNoch keine Bewertungen
- Comparison Between SA516 Gr. 70 and SA612Dokument2 SeitenComparison Between SA516 Gr. 70 and SA612Jaison JoseNoch keine Bewertungen
- CIPW NormDokument1 SeiteCIPW NormGARY ERLAND QUIROZ FLORESNoch keine Bewertungen
- Spectrocheck SpectrometerDokument5 SeitenSpectrocheck SpectrometerChandra SekarNoch keine Bewertungen
- Exp 4result Discussion For FaDokument4 SeitenExp 4result Discussion For FanasuhaNoch keine Bewertungen
- OREAS 22f: Primary Quartz Blank (Grey Pigmented Quartz, Australia) Certified Reference MaterialDokument10 SeitenOREAS 22f: Primary Quartz Blank (Grey Pigmented Quartz, Australia) Certified Reference MaterialsabisamoNoch keine Bewertungen
- Brammer Standard Company, Inc.: Provisional Certificate of AnalysisDokument1 SeiteBrammer Standard Company, Inc.: Provisional Certificate of AnalysisrahulNoch keine Bewertungen
- עקומת כיול 3Dokument6 Seitenעקומת כיול 3Sydney KharmanNoch keine Bewertungen
- KME CuNI Welding ProcedureDokument11 SeitenKME CuNI Welding ProcedureJoaoNoch keine Bewertungen
- Zhu Resumen CongresoDokument1 SeiteZhu Resumen CongresoRenatoNoch keine Bewertungen
- Technical Documents on Radiant Infrared Heating Theory and PrinciplesDokument8 SeitenTechnical Documents on Radiant Infrared Heating Theory and PrinciplesSeptian HarryNoch keine Bewertungen
- Copie de CIPW Norm HollacherDokument1 SeiteCopie de CIPW Norm HollacherLouis NahimanaNoch keine Bewertungen
- Konsentrasi Naoh Terhadap Waktu Konsentrasi Na2Co3 Terhadap WaktuDokument4 SeitenKonsentrasi Naoh Terhadap Waktu Konsentrasi Na2Co3 Terhadap WaktuRudihenNoch keine Bewertungen
- PHYS 252 Lab E3 - Ohm's Law: Coil #4 DataDokument8 SeitenPHYS 252 Lab E3 - Ohm's Law: Coil #4 DataOndra LabíkNoch keine Bewertungen
- PKM 1Dokument2 SeitenPKM 1Ranjith kumarNoch keine Bewertungen
- Electric Arc Furnace SimulationDokument38 SeitenElectric Arc Furnace SimulationGilang Hermawan100% (1)
- Final Assays Confirm High Nickel Sulphide Discovery at Corcel ProjectDokument2 SeitenFinal Assays Confirm High Nickel Sulphide Discovery at Corcel ProjectBassirouNoch keine Bewertungen
- Technical Data Sheet - AK34 Caustic Soda FlakesDokument1 SeiteTechnical Data Sheet - AK34 Caustic Soda FlakesArnoldo SanchezNoch keine Bewertungen
- Compare S235JR, E250BRDokument6 SeitenCompare S235JR, E250BRGANESHNoch keine Bewertungen
- Daniell Cell (Wet Cell) First Trial: Current (I)Dokument3 SeitenDaniell Cell (Wet Cell) First Trial: Current (I)Karl BelasaNoch keine Bewertungen
- Ash Analysis by Atomic Absorption SpectrofotometricDokument4 SeitenAsh Analysis by Atomic Absorption SpectrofotometricErnhy ErnhyNoch keine Bewertungen
- Data Praktikum LM1Dokument3 SeitenData Praktikum LM1Fakhrusy RizqyNoch keine Bewertungen
- IR Thermometers & Emissivity Metal Emissivity TableDokument3 SeitenIR Thermometers & Emissivity Metal Emissivity Tableboba78Noch keine Bewertungen
- I-V and C-V Characteristics of SemiconductorDokument2 SeitenI-V and C-V Characteristics of SemiconductorOscar Alam GuzmánNoch keine Bewertungen
- Electrochemical Cell VoltagesDokument3 SeitenElectrochemical Cell VoltagesjuanNoch keine Bewertungen
- Chromium and SulphateDokument9 SeitenChromium and SulphateDRACO SpadileNoch keine Bewertungen
- Technical & Cost Comparison of Laterite Treatment Processes Part 2Dokument19 SeitenTechnical & Cost Comparison of Laterite Treatment Processes Part 2klshfyusbdfkNoch keine Bewertungen
- Optimization of Desulphurization of Hot Metal and Liquid Steel For RAILDokument16 SeitenOptimization of Desulphurization of Hot Metal and Liquid Steel For RAILratul_sarkarNoch keine Bewertungen
- CIPW Norm HollacherDokument2 SeitenCIPW Norm HollacherAchmad Noviari AkbarNoch keine Bewertungen
- Technical Specifications - Pipes API 5L X65 PSL 2Dokument2 SeitenTechnical Specifications - Pipes API 5L X65 PSL 2isan.structural TjsvgalavanNoch keine Bewertungen
- Nico Young Cobalt-Nickel Laterite: Updated Mineral ResourceDokument23 SeitenNico Young Cobalt-Nickel Laterite: Updated Mineral Resourceflysch_ukNoch keine Bewertungen
- Practica Calificada N°5 - Siderurgia NOMBRE: Magdalena Lucero Gómez Cabrera CODIGO: 20150203IDokument2 SeitenPractica Calificada N°5 - Siderurgia NOMBRE: Magdalena Lucero Gómez Cabrera CODIGO: 20150203ILucero Gomez CabreraNoch keine Bewertungen
- Certified Reference Material for Lithium Ore AnalysisDokument17 SeitenCertified Reference Material for Lithium Ore AnalysisRidouane RidNoch keine Bewertungen
- Tube Catalogue AnitaDokument64 SeitenTube Catalogue AnitaChinnaraja GandhiNoch keine Bewertungen
- Singh, Ramesh Prasad-Applied Welding Engineering, Second Edition - Processes, Codes, and Standards-Butterworth Heinemann (2016) PDFDokument2 SeitenSingh, Ramesh Prasad-Applied Welding Engineering, Second Edition - Processes, Codes, and Standards-Butterworth Heinemann (2016) PDFBraulio AtacusíNoch keine Bewertungen
- Partition and Bulk Dis. Coeff. Igneous Petrology Practical (GLC514) Laboratory Manual (1)Dokument34 SeitenPartition and Bulk Dis. Coeff. Igneous Petrology Practical (GLC514) Laboratory Manual (1)figesab482Noch keine Bewertungen
- THERMO-OPTICAL PROPERTIES OF MATERIALSDokument2 SeitenTHERMO-OPTICAL PROPERTIES OF MATERIALSMariela BaigorriaNoch keine Bewertungen
- Ohmic Devices LabDokument2 SeitenOhmic Devices Laboofnivlak5Noch keine Bewertungen
- HINGED 42MM SERIES PROFILESDokument120 SeitenHINGED 42MM SERIES PROFILESViswanathan Kannoor67% (3)
- Atomic-Absorption Spectrophotometry: International Series of Monographs in Analytical ChemistryVon EverandAtomic-Absorption Spectrophotometry: International Series of Monographs in Analytical ChemistryNoch keine Bewertungen
- Izoterico Berlino 2012Dokument2 SeitenIzoterico Berlino 2012marioNoch keine Bewertungen
- Tech WipoDokument5.375 SeitenTech Wipohoàng đình sơnNoch keine Bewertungen
- Wa TERDokument95 SeitenWa TERarjunansyahNoch keine Bewertungen
- Wa TERDokument95 SeitenWa TERarjunansyahNoch keine Bewertungen
- Trapzilla-Single-Fixture-Sizing (PROMEDIO COMUN DE GPM DE TRAMPA DE ACEITE) PDFDokument1 SeiteTrapzilla-Single-Fixture-Sizing (PROMEDIO COMUN DE GPM DE TRAMPA DE ACEITE) PDFmarioNoch keine Bewertungen
- Izoterico Berlino 2012Dokument2 SeitenIzoterico Berlino 2012marioNoch keine Bewertungen
- Trapzilla-Single-Fixture-Sizing (PROMEDIO COMUN DE GPM DE TRAMPA DE ACEITE) PDFDokument1 SeiteTrapzilla-Single-Fixture-Sizing (PROMEDIO COMUN DE GPM DE TRAMPA DE ACEITE) PDFmarioNoch keine Bewertungen
- Grease Trap SizingDokument2 SeitenGrease Trap SizingmarioNoch keine Bewertungen
- Trapzilla-Single-Fixture-Sizing (PROMEDIO COMUN DE GPM DE TRAMPA DE ACEITE) PDFDokument1 SeiteTrapzilla-Single-Fixture-Sizing (PROMEDIO COMUN DE GPM DE TRAMPA DE ACEITE) PDFmarioNoch keine Bewertungen
- Sulfuric Acid Material BalanceDokument9 SeitenSulfuric Acid Material BalanceBernie_Garcia__9886100% (2)
- Izoterico Berlino 2012Dokument2 SeitenIzoterico Berlino 2012marioNoch keine Bewertungen
- Izoterico Berlino 2012Dokument2 SeitenIzoterico Berlino 2012marioNoch keine Bewertungen
- Faculty of Chemical Engineering (Fche) : LecturerDokument102 SeitenFaculty of Chemical Engineering (Fche) : LecturermarioNoch keine Bewertungen
- Sulfuric Acid Material BalanceDokument9 SeitenSulfuric Acid Material BalanceBernie_Garcia__9886100% (2)
- Tabla T de StudentDokument2 SeitenTabla T de Studentyrisnoemi100% (3)
- Drinking BirdDokument7 SeitenDrinking BirdSourav KhannaNoch keine Bewertungen
- Integrales Tabla CompletaDokument6 SeitenIntegrales Tabla CompletaArias Miguel100% (2)
- AnimalsK 2FreeSamples PDFDokument36 SeitenAnimalsK 2FreeSamples PDFcatarinaferreirafreiNoch keine Bewertungen
- End of Unit Test: Name Class DateDokument4 SeitenEnd of Unit Test: Name Class DateVictor Barber Sanchis100% (1)
- International Results of The 6th IGODokument22 SeitenInternational Results of The 6th IGOsameer chaharNoch keine Bewertungen
- Cbse Tenth ClassDokument10 SeitenCbse Tenth ClassDEVARAJU K GNoch keine Bewertungen
- Stoichiometry Formulas, Equations and CalculationsDokument33 SeitenStoichiometry Formulas, Equations and CalculationsAhmadNoch keine Bewertungen
- 4024q1 Specimen PaperdocxDokument12 Seiten4024q1 Specimen PaperdocxLeses MayNoch keine Bewertungen
- PUMA RSL V - 06 - 14 Final PDFDokument2 SeitenPUMA RSL V - 06 - 14 Final PDFTrinhTruongNoch keine Bewertungen
- Composition and Formulation of Mineral Mixture For Dairy Animals.Dokument3 SeitenComposition and Formulation of Mineral Mixture For Dairy Animals.Dr. MANOJ SHARMA80% (15)
- A29 05 PDFDokument16 SeitenA29 05 PDFFernando Palacios MaldonadoNoch keine Bewertungen
- CoA of EgcgDokument1 SeiteCoA of EgcgMirna Candra RNoch keine Bewertungen
- UltraWAVE Application GuidelinesDokument37 SeitenUltraWAVE Application GuidelinesAngela MoraNoch keine Bewertungen
- 6i TitaniumDokument28 Seiten6i TitaniumRajesh PatelNoch keine Bewertungen
- iGCSE Chemistry Extraction of MetalsDokument57 SeiteniGCSE Chemistry Extraction of MetalsJuman AlbuhaisiNoch keine Bewertungen
- Revision - 1 - On ElectrochemistryDokument12 SeitenRevision - 1 - On ElectrochemistryKiro RemonNoch keine Bewertungen
- Health Lab No. Lab Date Sampled Date Model Asset ID Asset SerialDokument29 SeitenHealth Lab No. Lab Date Sampled Date Model Asset ID Asset SerialjohnNoch keine Bewertungen
- Comparatii Si Echivalente Branduri Sarma TubularaDokument4 SeitenComparatii Si Echivalente Branduri Sarma TubularaAnonymous LpKY4pNoch keine Bewertungen
- Chlorine GasDokument4 SeitenChlorine Gaselvergonzalez1Noch keine Bewertungen
- Sodium Nitroprusside, Na (Fe (CN) NO) - 2H O: Assignment 1: Coordination Complex in Daily LifeDokument8 SeitenSodium Nitroprusside, Na (Fe (CN) NO) - 2H O: Assignment 1: Coordination Complex in Daily LifeFatimah NazliaNoch keine Bewertungen
- Laporan Unit 1 Anorganik Mutia Salsabila PDFDokument47 SeitenLaporan Unit 1 Anorganik Mutia Salsabila PDFMutia SalsabilaNoch keine Bewertungen
- Workbook - Oxidation and Reduction ReactionsDokument113 SeitenWorkbook - Oxidation and Reduction Reactionscharwill1234100% (1)
- Basic Ideas in ChemistryDokument16 SeitenBasic Ideas in ChemistryLucianaAcostaNoch keine Bewertungen
- Fdocuments - in Chemistry Investigatory Project Study of Constituent of AlloysDokument19 SeitenFdocuments - in Chemistry Investigatory Project Study of Constituent of AlloysLogesh BalamuruganNoch keine Bewertungen
- Elements and CompoundsDokument10 SeitenElements and CompoundsMaryEunice RodriguezNoch keine Bewertungen
- X45NiCrMo4 - 1.2767Dokument34 SeitenX45NiCrMo4 - 1.2767patiltushar79Noch keine Bewertungen
- AP Chemistry Chapter 4 TestDokument3 SeitenAP Chemistry Chapter 4 Testphysteach1216100% (2)
- Electrochemistry (Paper-01) WYDokument12 SeitenElectrochemistry (Paper-01) WYgreedy AsunaNoch keine Bewertungen
- PVD HardCoating PDFDokument20 SeitenPVD HardCoating PDFsatanjrNoch keine Bewertungen
- Analysis of Alloy ConstituentsDokument17 SeitenAnalysis of Alloy ConstituentsParth ChakrabortyNoch keine Bewertungen
- Cambridge International Examinations Cambridge International General Certificate of Secondary EducationDokument16 SeitenCambridge International Examinations Cambridge International General Certificate of Secondary EducationDenver DemisNoch keine Bewertungen
- Grade 8 ExamDokument2 SeitenGrade 8 ExamFerna Joy LapinigNoch keine Bewertungen
- Directions: Fill Out The Missing Information Below: Write Your Answer in A Separate Sheet of Paper. 3. GraphiteDokument5 SeitenDirections: Fill Out The Missing Information Below: Write Your Answer in A Separate Sheet of Paper. 3. GraphiteFain AloyanNoch keine Bewertungen