Beruflich Dokumente
Kultur Dokumente
Lesson Plan: Lesson: Hydroxy Compounds (I)
Hochgeladen von
MarcTnnOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Lesson Plan: Lesson: Hydroxy Compounds (I)
Hochgeladen von
MarcTnnCopyright:
Verfügbare Formate
Lesson Plan
Lesson : Hydroxy Compounds (I)
Aim :
To study the nomenclature and reactions of alcohols.
Learning Outcomes :
By the end of the lesson, students will be able to:
define alcohols
name and draw the structures of alcohols
classify alcohols as primary, secondary or tertiary
describe reactions which involve fission of the RO H bond and fission of the
R OH bond.
Assumed prior knowledge :
Students should already be familiar with :
1. the basic rules in IUPAC nomenclature for alkanes
2. the fact that acids react with reactive metals to liberate hydrogen
3. the general structure of an ester
4. the concept of nucleophilic substitution reaction.
Underlying Principles
1. Making the invisible, visible.
2. Enabling students to know what to look for.
Differentiation
Questions in the student notes are designed to enable all students to complete the activity.
The pop-up answers are provided for the students to view when they have considered their
responses. Worksheet questions include questions that require recall, understanding and
application of the new concepts learned.
© 2004 Ministry of Education Malaysia. All Rights Reserved. Page 1 of 5
Development of Lesson :
No. Steps Strategy Resources
1 Set Induction.
(Ascertaining prior • Teacher to get students to recall the
knowledge and IUPAC names of alkanes.
introducing lesson • Teacher to point out lesson objectives of
topic for the day). the day.
2 Student Activity Teacher to go through Activities 1 - 3 with • Courseware
the students.
Activity 1 : Nomenclature and
classification
Students are shown how to name
and classify alcohols as primary,
secondary or tertiary alcohols. They are
also introduced to two types of polyhydric
alcohols that is, diols and triols.
Activity 2 : Fission of RO H bond
Students are shown how fission of the
RO H bond in an alcohol can occur
due to its polar nature. Students are also
shown a few examples of reactions that
involve this type of bond fission.
Activity 3 : Fission of ROH bond
Students are shown how fission of the
ROH bond can also occur in an alcohol
leading to the formation of haloalkanes
and alkenes.
3 Evaluation • Students to answer questions in the • Worksheet
student worksheet on their own.
4 Extension activity • Students to read up reference materials • References
on their own.
© 2004 Ministry of Education Malaysia. All Rights Reserved. Page 2 of 5
Worksheet Answers
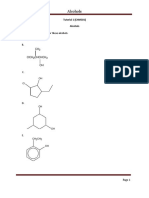
1. Nomenclature and classification
1.1 a. 3-Methyl-2-pentanol
b. 3,3-Dimethyl-1-butanol
c. 2-Methyl-1,2-propanediol
d. 4-Methylphenol
1.2 a. CH3 CH3
CH3 C CH2 CH CH3 3° alcohol
OH
b.
OH
CH3
2° alcohol
CH3
CH2CH3
c. CH3 CH2 C CH2 CH2 CH2 CH2OH 1° alcohol
CH2CH3
d. CH2CH3
CH3 CH2 C CH2 CH2 CH3 3° alcohol
OH
1.3 1. CH3CH2CH2CH2OH 1° alcohol
2. CH3CCH2OH
1° alcohol
CH3
3. CH3CH2CHCH3
2° alcohol
OH
4. CH3
CH2 C CH3 3° alcohol
OH
© 2004 Ministry of Education Malaysia. All Rights Reserved. Page 3 of 5
2. Fission of RO H bond
2.1 A
HOCH2CH2CH2OH + 2Na Na+O−CH2CH2CH2O−Na+ + H2
1 mole HOCH2CH2CH2OH produces 1 mole H2 gas.
2.2 CH3COOH + C2H518OH CH3CO18OC2H5 + H2O
The reaction involves the breaking of the OH bond in C2H518OH.
3. OH bond
Fission of R
3.1 a. (CH3)2CH CH CH3
+ HCI (CH3)2CH CH CH3 + H2O
OH
CI
Cleavage of C OH bond
b.
(CH3)2CH CH CH3 (CH3)2CH CH CH3
1
+ Na + H2
OH −
O Na + 2
Cleavage of O H bond
c. OH Cl
+ SOCI2 + SO2 + HCl
Cleavage of C OH bond
d. CH3CH2CH2OH + CH3COCI CH3COOCH2CH2CH3 + HCI
Cleavage of O H bond
3.2 a. Hydrogen chloride
b. CH3CH2CH2CH2OH + PCl5 CH3CH2CH2CH2Cl + POCI3 + HCI
c. Nucleophilic substitution reaction
© 2004 Ministry of Education Malaysia. All Rights Reserved. Page 4 of 5
3.3 a. Dehydration
b. i. excess concentrated H2SO4 and heat
ii. excess 3-methyl-2-butanol and heat
c. i. (CH3)2C CH2CH3 and (CH3)2CHCH CH2
ii. (CH3)2CHCHOCHCH(CH3)2
CH3 CH3
© 2004 Ministry of Education Malaysia. All Rights Reserved. Page 5 of 5
Das könnte Ihnen auch gefallen
- Lesson Plan: Lesson: Organic Compounds: SummaryDokument4 SeitenLesson Plan: Lesson: Organic Compounds: SummaryMarcTnnNoch keine Bewertungen
- Lesson 13Dokument4 SeitenLesson 13MarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: Hydroxy Compounds (II)Dokument3 SeitenLesson Plan: Lesson: Hydroxy Compounds (II)MarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: Carbonyl Compounds (II)Dokument4 SeitenLesson Plan: Lesson: Carbonyl Compounds (II)MarcTnnNoch keine Bewertungen
- Lesson 24Dokument4 SeitenLesson 24MarcTnnNoch keine Bewertungen
- Leeson 17Dokument5 SeitenLeeson 17MarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: Carbonyl Compounds (I)Dokument4 SeitenLesson Plan: Lesson: Carbonyl Compounds (I)MarcTnnNoch keine Bewertungen
- 25 Alcohols, Phenols and Ethers: SolutionsDokument47 Seiten25 Alcohols, Phenols and Ethers: SolutionsSujalNoch keine Bewertungen
- Chemistry HSSC-II Solution of 2nd Set Model Question PaperDokument15 SeitenChemistry HSSC-II Solution of 2nd Set Model Question PaperIsha KhanNoch keine Bewertungen
- Lesson 14Dokument3 SeitenLesson 14MarcTnnNoch keine Bewertungen
- Alcohols and EthersDokument51 SeitenAlcohols and EthersnanaNoch keine Bewertungen
- Cls Jeead-18-19 Xii Che Target-7 Set-2 Chapter-12Dokument47 SeitenCls Jeead-18-19 Xii Che Target-7 Set-2 Chapter-12DxNoch keine Bewertungen
- CHM 305Dokument260 SeitenCHM 305Ismail AdebanjoNoch keine Bewertungen
- CY2102Dokument3 SeitenCY2102Prarabdha SharmaNoch keine Bewertungen
- Sample MCQ Organic Chemistry Sem II PSCH203 BacklogDokument4 SeitenSample MCQ Organic Chemistry Sem II PSCH203 BacklogganesanneelamuruganNoch keine Bewertungen
- Aldehydes and KetonesDokument6 SeitenAldehydes and KetonesMira JaoNoch keine Bewertungen
- 23 Carboxylic AcidDokument7 Seiten23 Carboxylic Acidizabel50% (2)
- Alcohals Phenols AsDokument44 SeitenAlcohals Phenols AsAmit RoutNoch keine Bewertungen
- Unit 11 - Further Organic Chemistry AnswersDokument11 SeitenUnit 11 - Further Organic Chemistry AnswersSonic EightNoch keine Bewertungen
- Exercise 1 1683183099Dokument27 SeitenExercise 1 1683183099shivam126921Noch keine Bewertungen
- Chapter 16 OH LaNunDokument7 SeitenChapter 16 OH LaNunshehdilanun100% (1)
- Polymer NotesDokument6 SeitenPolymer NotesThe Smart Boy KungFuPawnNoch keine Bewertungen
- Kimia Organik Diskriptif: Daratu Eviana Kusuma Putri Jurusan Kimia FMIPA Universitas Negeri MalangDokument64 SeitenKimia Organik Diskriptif: Daratu Eviana Kusuma Putri Jurusan Kimia FMIPA Universitas Negeri MalangArini SetyaningrumNoch keine Bewertungen
- Lesson Plan: Lesson: Organic Compounds: Physical PropertiesDokument4 SeitenLesson Plan: Lesson: Organic Compounds: Physical PropertiesMarcTnnNoch keine Bewertungen
- Pre Lab 1-4 Module DK024 (Question)Dokument12 SeitenPre Lab 1-4 Module DK024 (Question)Irdina WnNoch keine Bewertungen
- Lesson Plan: Lesson: Carboxylic Acids (II)Dokument3 SeitenLesson Plan: Lesson: Carboxylic Acids (II)MarcTnnNoch keine Bewertungen
- 22 Carbonyl CompoundsDokument6 Seiten22 Carbonyl CompoundsCtNabihahAmilaMarminNoch keine Bewertungen
- Solution of Chemistry HSSC-II (3rd Set)Dokument11 SeitenSolution of Chemistry HSSC-II (3rd Set)Ujala ShahidNoch keine Bewertungen
- The Report of Practicum: Organik Chemistry IiDokument30 SeitenThe Report of Practicum: Organik Chemistry IipranggajatiNoch keine Bewertungen
- 6carboxylic Acids PDFDokument29 Seiten6carboxylic Acids PDFsharmimiameerasanadyNoch keine Bewertungen
- Organic Chemistry 211 PracticeDokument26 SeitenOrganic Chemistry 211 PracticeStephen EshagianNoch keine Bewertungen
- Organic Chemistry II The Report of PracticumDokument8 SeitenOrganic Chemistry II The Report of PracticumpranggajatiNoch keine Bewertungen
- Chapter 5 Hydrocarbon 5.2Dokument112 SeitenChapter 5 Hydrocarbon 5.2AwatifNoch keine Bewertungen
- M-Caps-36: Chemistry: NEET & AIIMS 2018-19Dokument6 SeitenM-Caps-36: Chemistry: NEET & AIIMS 2018-19Vishal SinghNoch keine Bewertungen
- Laboratory Manual For Practical Exercises Properties of Organic CompoundsDokument18 SeitenLaboratory Manual For Practical Exercises Properties of Organic CompoundsSaraNoch keine Bewertungen
- Alcohols, Phenols & EthersDokument12 SeitenAlcohols, Phenols & EtherssiddharthchillapwarNoch keine Bewertungen
- Tutorial 1 - Alcohol PDFDokument5 SeitenTutorial 1 - Alcohol PDFNurul Athirah JainiNoch keine Bewertungen
- Exercise - IV Subjective Type: Solution Slot - 3 (Chemistry)Dokument2 SeitenExercise - IV Subjective Type: Solution Slot - 3 (Chemistry)Priyanshu RajNoch keine Bewertungen
- Chap 6 - AlcoholDokument12 SeitenChap 6 - AlcoholNadhrah AdibahNoch keine Bewertungen
- Block 6 Functional Groups 2Dokument46 SeitenBlock 6 Functional Groups 2Cheng FuNoch keine Bewertungen
- Review On Organic Chemical ReactionsDokument32 SeitenReview On Organic Chemical ReactionsAlice C. RiveraNoch keine Bewertungen
- Exercise C7 - Ans SchemeDokument3 SeitenExercise C7 - Ans Schemeknn233610437Noch keine Bewertungen
- CLS ENG 22 23 XII Che Target 5 Level 1 Chapter 13Dokument62 SeitenCLS ENG 22 23 XII Che Target 5 Level 1 Chapter 13Harsh JakharNoch keine Bewertungen
- Vollhardt Chapter 18 OChem PracticeDokument23 SeitenVollhardt Chapter 18 OChem PracticeDanNoch keine Bewertungen
- 11 - Alcohol Ethers Thiols Wks KeyDokument5 Seiten11 - Alcohol Ethers Thiols Wks KeyMaria Aira Mendoza100% (1)
- CLS Aipmt 19 20 XII Che Study Package 4 Level 1 Chapter 10Dokument36 SeitenCLS Aipmt 19 20 XII Che Study Package 4 Level 1 Chapter 10Utkarsh KumarNoch keine Bewertungen
- 7.0 Haloalkanes 20212022Dokument88 Seiten7.0 Haloalkanes 20212022ZULAIKA BINTI SALLEH A22BE0415Noch keine Bewertungen
- CBSE Class 12 Chemistry 2018Dokument17 SeitenCBSE Class 12 Chemistry 2018parv dhanoteNoch keine Bewertungen
- Alcohal, Phenol and EtherDokument13 SeitenAlcohal, Phenol and EtherVishal TiwariNoch keine Bewertungen
- 27.2 Alcohols Ial Cie Chemistry QPDokument16 Seiten27.2 Alcohols Ial Cie Chemistry QPabdelrahmanNoch keine Bewertungen
- 241 Chem CH-1: AlcoholsDokument21 Seiten241 Chem CH-1: AlcoholsAlanoud AllaNoch keine Bewertungen
- Alcohol 13Dokument19 SeitenAlcohol 13Nor AfidahNoch keine Bewertungen
- Aldehydes and Ketones-02 Solved ProblemsDokument13 SeitenAldehydes and Ketones-02 Solved ProblemsRaju SinghNoch keine Bewertungen
- Alcohols Phenols Thiols and Ethers SimpleDokument58 SeitenAlcohols Phenols Thiols and Ethers Simplevin ocangNoch keine Bewertungen
- Alcohols Phenol S SolutionsDokument11 SeitenAlcohols Phenol S SolutionsagNoch keine Bewertungen
- 8.0 Hydroxy Compounds Pemurniaan by LiyaaaDokument99 Seiten8.0 Hydroxy Compounds Pemurniaan by LiyaaaNURUL HIDAYAH SAIFUL ANUARNoch keine Bewertungen
- CBSE Class 12 Chem Notes Question Bank Alcohols Phenols and Ethers PDFDokument19 SeitenCBSE Class 12 Chem Notes Question Bank Alcohols Phenols and Ethers PDFParam MNoch keine Bewertungen
- AlcoholsDokument82 SeitenAlcoholsDamir BalmassovNoch keine Bewertungen
- CLS JEEAD-19-20 XI Che Target-5 Level-1 Chapter-12Dokument27 SeitenCLS JEEAD-19-20 XI Che Target-5 Level-1 Chapter-12Awan DubeyNoch keine Bewertungen
- Handbook of Coordination Catalysis in Organic ChemistryVon EverandHandbook of Coordination Catalysis in Organic ChemistryNoch keine Bewertungen
- Lesson Plan: Lesson: Heat Energy ChangeDokument4 SeitenLesson Plan: Lesson: Heat Energy ChangeMarcTnnNoch keine Bewertungen
- Lesson PlanDokument4 SeitenLesson PlanMarcTnnNoch keine Bewertungen
- Lesson PlanDokument4 SeitenLesson PlanMarcTnnNoch keine Bewertungen
- Dun DownloadDokument1 SeiteDun DownloadMarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: Uses of ElectrolysisDokument3 SeitenLesson Plan: Lesson: Uses of ElectrolysisMarcTnnNoch keine Bewertungen
- Lesson 50Dokument3 SeitenLesson 50MarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: Standard Cell PotentialDokument4 SeitenLesson Plan: Lesson: Standard Cell PotentialMarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: The Base Dissociation ConstantDokument4 SeitenLesson Plan: Lesson: The Base Dissociation ConstantMarcTnnNoch keine Bewertungen
- Lesson PlanDokument4 SeitenLesson PlanMarcTnnNoch keine Bewertungen
- Lesson 49Dokument3 SeitenLesson 49MarcTnnNoch keine Bewertungen
- Lesson 48Dokument3 SeitenLesson 48MarcTnnNoch keine Bewertungen
- Lesson 45Dokument4 SeitenLesson 45MarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: Colligative Properties of SolutionsDokument3 SeitenLesson Plan: Lesson: Colligative Properties of SolutionsMarcTnnNoch keine Bewertungen
- Lesson 41Dokument4 SeitenLesson 41MarcTnn100% (1)
- Lesson 42Dokument4 SeitenLesson 42MarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: Acid-Base TitrationDokument4 SeitenLesson Plan: Lesson: Acid-Base TitrationMarcTnnNoch keine Bewertungen
- Lesson 40Dokument5 SeitenLesson 40MarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: Introduction To Ionic EquilibriumDokument3 SeitenLesson Plan: Lesson: Introduction To Ionic EquilibriumMarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: K and K For Heterogeneous SystemDokument4 SeitenLesson Plan: Lesson: K and K For Heterogeneous SystemMarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: PH and pOHDokument4 SeitenLesson Plan: Lesson: PH and pOHMarcTnnNoch keine Bewertungen
- Lesson 28Dokument5 SeitenLesson 28MarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: Zero Order ReactionDokument4 SeitenLesson Plan: Lesson: Zero Order ReactionMarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: For Homogeneous SystemsDokument7 SeitenLesson Plan: Lesson: For Homogeneous SystemsMarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: Le Chatelier's Principle (II)Dokument4 SeitenLesson Plan: Lesson: Le Chatelier's Principle (II)MarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: Le Chatelier's Principle (I)Dokument4 SeitenLesson Plan: Lesson: Le Chatelier's Principle (I)MarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: First and Second Order ReactionDokument6 SeitenLesson Plan: Lesson: First and Second Order ReactionMarcTnnNoch keine Bewertungen
- Lesson 27Dokument4 SeitenLesson 27MarcTnnNoch keine Bewertungen
- Lesson 29Dokument3 SeitenLesson 29MarcTnnNoch keine Bewertungen
- Lesson Plan: Lesson: For Homogeneous SystemDokument5 SeitenLesson Plan: Lesson: For Homogeneous SystemMarcTnnNoch keine Bewertungen
- Lesson 21Dokument5 SeitenLesson 21MarcTnnNoch keine Bewertungen
- Carbohydrates 2Dokument81 SeitenCarbohydrates 2smcm11Noch keine Bewertungen
- Bsci330 Lab ReportDokument8 SeitenBsci330 Lab Reportapi-247482516Noch keine Bewertungen
- BCH101 - L1 - The Chemical Basis of LifeDokument52 SeitenBCH101 - L1 - The Chemical Basis of Lifesrabonty.siddikyNoch keine Bewertungen
- BIOCHEMDokument35 SeitenBIOCHEMJULIA AUDREY PERALTANoch keine Bewertungen
- Pharmacognosy - 2 ThakurDokument290 SeitenPharmacognosy - 2 Thakurshoaib1985100% (8)
- Comprehensive Review of Important Analytical Reagents Used in SpectrophotometryDokument29 SeitenComprehensive Review of Important Analytical Reagents Used in SpectrophotometryvarishNoch keine Bewertungen
- 00904828Dokument6 Seiten00904828saau.6771Noch keine Bewertungen
- Chem 10 Ch#9,10,11,13Dokument3 SeitenChem 10 Ch#9,10,11,13Zeeshan AhmadNoch keine Bewertungen
- MMSA Methanol World Supply and Demand Summary Jan 2020Dokument2 SeitenMMSA Methanol World Supply and Demand Summary Jan 2020rifqi98Noch keine Bewertungen
- The Kinetics of Enzyme - Catalyzed ReactionsDokument38 SeitenThe Kinetics of Enzyme - Catalyzed ReactionsRojan Pradhan100% (1)
- DNA As Genetic Material PDFDokument12 SeitenDNA As Genetic Material PDFRudra Narayan Swain 222Noch keine Bewertungen
- GluconeogenesisDokument14 SeitenGluconeogenesisapi-453481795Noch keine Bewertungen
- Nucleic AcidsDokument56 SeitenNucleic AcidsShane G.Noch keine Bewertungen
- Chemistry of COMPOUND Lipids.Dokument60 SeitenChemistry of COMPOUND Lipids.QueenNoch keine Bewertungen
- Inserto Biorad Controles PDFDokument2 SeitenInserto Biorad Controles PDFlenin_villalta67% (3)
- Aerobic RespirationDokument12 SeitenAerobic RespirationShrirang JoshiNoch keine Bewertungen
- The Solution in Energy CuringDokument22 SeitenThe Solution in Energy CuringDr. Suresh ShisodiaNoch keine Bewertungen
- PTA Technology - FundamentalsDokument50 SeitenPTA Technology - FundamentalsSarat Na NiNoch keine Bewertungen
- BIS 102 MT 1 Hilt F10 BlankDokument6 SeitenBIS 102 MT 1 Hilt F10 BlankKimNoch keine Bewertungen
- 6/27/2019 NO NAMA BARANG QTY UOM Batch EDDokument8 Seiten6/27/2019 NO NAMA BARANG QTY UOM Batch EDRedCazorlaNoch keine Bewertungen
- 04 - Spektroskopi UV-Vis - 3Dokument16 Seiten04 - Spektroskopi UV-Vis - 3muktadi-amri-8721Noch keine Bewertungen
- Karakus Ates Keskin Turan Kaya 2023 Mitteilungen KlosterneuburgDokument16 SeitenKarakus Ates Keskin Turan Kaya 2023 Mitteilungen KlosterneuburgfadimeatesNoch keine Bewertungen
- Update Stok 22 Juni 2023Dokument8 SeitenUpdate Stok 22 Juni 2023Lulut Hening PrasetyoNoch keine Bewertungen
- Printable Beers Pocket CardDokument4 SeitenPrintable Beers Pocket CardJames Lindon100% (3)
- A2 CHM 10 Arenes NotesDokument26 SeitenA2 CHM 10 Arenes NotesArishaNoch keine Bewertungen
- General Organic Chemistry: Entry of Vvips - Nomenclature of Organic Compounds With Mono Functional GroupDokument12 SeitenGeneral Organic Chemistry: Entry of Vvips - Nomenclature of Organic Compounds With Mono Functional GroupYaswanth PedapudiNoch keine Bewertungen
- Functional Groups: DefinitionDokument7 SeitenFunctional Groups: DefinitionHamza MughalNoch keine Bewertungen
- Moringa Fresh Leaf Vs Dried LeafDokument3 SeitenMoringa Fresh Leaf Vs Dried LeafAshwani Gaur88% (8)
- Biochemical ParametersDokument39 SeitenBiochemical ParametersdystoNoch keine Bewertungen
- Skeletal Muscle RelaxantDokument18 SeitenSkeletal Muscle Relaxantswaroop ranjan nandaNoch keine Bewertungen