Beruflich Dokumente
Kultur Dokumente
Subst - Elim Practice Problems PDF
Hochgeladen von
MaríaOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Subst - Elim Practice Problems PDF
Hochgeladen von
MaríaCopyright:
Verfügbare Formate
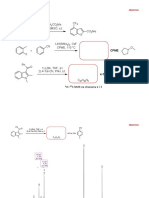
CHM 211 Substitution and Elimination practice problems
Analyze the reactant(s) and reaction conditions, then predict the structure of the major organic
product and indicate the predominant mechanism (SN1, SN2, E1, or E2) of each reaction.
K OC(CH3)3
CH3CH2CH2CH2Br CH3CH2CH CH2 E2 (high T,
(CH3)3COH, 82º C good base)
Na OCH3
CH3CH2CH2CH2Br CH3CH2CH2CH2OCH3 SN2 (low T,
CH3OH, 0º C
good Nu or base)
Cl
SCH2CH3
Na SCH2CH3
SN2 (low T,
DMF, 0º C good Nu)
CH3I + NaNO 2 CH3NO 2 SN2 (low T)
5º C
CH3 CH3
CH3 C OTs H2O SN1 (low T,
CH3 C OH
22º C weak Nu,
CH3 CH3 weak base)
CH3 CH3
CH3OH SN1 (low T,
Br 22º C OCH3 weak Nu,
weak base)
CH3 K OC(CH3)3 CH3
E2, some E1
Br (CH3)3COH, 82º C (high T, good base)
CH3I + CH3SH CH3SCH3 SN 2 (low T)
43º C
Cl
Na OCH2CH3
E2 (high T, good base)
CH3CH2OH, 78º C
CH3 CH3 SN 1 (low T,
H2O weak Nu,
I OH weak base)
22º C
BrBr Na OCH3 OCH3 SN2 (low T,
CH3OH, 0º C allylic RX)
CHM 211 Substitution and Elimination practice problem answers
Analyze the reactant(s) and reaction conditions, then predict the structure of the major organic
product and indicate the predominant mechanism (SN1, SN2, E1, or E2) of each reaction.
K OC(CH3)3
CH3CH2CH2CH2Br CH3CH2CH CH2 E2 (1º RX, high T,
(CH3)3COH, 82º C good base)
Na OCH3
CH3CH2CH2CH2Br CH3CH2CH2CH2OCH3 SN2 (1º RX, low T,
CH3OH, 0º C
good Nu or base)
Cl
SCH2CH3
Na SCH2CH3
SN2 (2º RX, low T,
DMF, 0º C good Nu)
CH3I + NaNO2 CH3NO2 SN2 (Methyl)
5º C
CH3 CH3
H2O SN1 (3º RX, exc. L
CH3 C OTs CH3 C OH
22º C low T, weak Nu
CH3 CH3 weak base)
CH3 CH3
CH3OH SN1 (3º RX, good L
Br 22º C OCH3 low T, weak Nu,
weak base)
CH3 K OC(CH3)3 CH3
E2, some E1 (3º RX, good
Br (CH3)3COH, 82º C L, high T, good base)
CH3I + CH3SH CH3SCH3 SN2 (Methyl)
43º C
Cl
Na OCH2CH3 E2 (2º RX, high T,
CH3CH2OH, 78º C good base)
CH3 CH3
H2O SN1 (3º RX, low T,
I OH v. good L, weak
22º C
Nu, weak base)
BrBr Na OCH3 OCH33
OCH SN2 (allylic, low T,
CH3OH, 0º C allylic RX)
Das könnte Ihnen auch gefallen
- Substitution and Elimination Practice ProblemsDokument2 SeitenSubstitution and Elimination Practice ProblemsImran Khan SulaimanNoch keine Bewertungen
- Kaitocephalin-2 - USDokument2 SeitenKaitocephalin-2 - USPercival GalahadNoch keine Bewertungen
- 1803 Chemistry Paper With Ans Solution MorningDokument7 Seiten1803 Chemistry Paper With Ans Solution MorningDrNoch keine Bewertungen
- Fukuyama Group - Group Meeting Problems 2001/08/22: N N N HDokument2.429 SeitenFukuyama Group - Group Meeting Problems 2001/08/22: N N N HGia PhướcNoch keine Bewertungen
- Stereochemistry of E2 ReactionsDokument79 SeitenStereochemistry of E2 ReactionsSwagata Saha100% (2)
- Substitution and EliminationDokument79 SeitenSubstitution and Eliminationjana srutiNoch keine Bewertungen
- Ch. 9Dokument4 SeitenCh. 9Matthew OshefskyNoch keine Bewertungen
- 118c Practice Synthesis KeyDokument18 Seiten118c Practice Synthesis Keyapi-465421809Noch keine Bewertungen
- For Each of The Following Reactions, Give The Structure of The Product and Indicate Whether The Mechanism Is Likely To Be SN1, SN2, Both or NeitherDokument2 SeitenFor Each of The Following Reactions, Give The Structure of The Product and Indicate Whether The Mechanism Is Likely To Be SN1, SN2, Both or NeitherVarokah VarNoch keine Bewertungen
- Synthesis of Cyclic and Acyclic B-Amino Acids Via Chelation-Controlled 1,3-Dipolar CycloadditionDokument16 SeitenSynthesis of Cyclic and Acyclic B-Amino Acids Via Chelation-Controlled 1,3-Dipolar CycloadditionNguyễn Thái DươngNoch keine Bewertungen
- Reaction of KetonesDokument1 SeiteReaction of KetonesJoko SusiloNoch keine Bewertungen
- CY2101Dokument3 SeitenCY2101Prarabdha SharmaNoch keine Bewertungen
- Synproblems AnDokument1 SeiteSynproblems AnEsther CruzNoch keine Bewertungen
- Laulimalide - USDokument4 SeitenLaulimalide - USPercival GalahadNoch keine Bewertungen
- HPLCDokument20 SeitenHPLCNugroho HartonoNoch keine Bewertungen
- Foto Prosedur PengerjaanDokument29 SeitenFoto Prosedur PengerjaanGalan Rizqi YanuarNoch keine Bewertungen
- Organic Chemistry Summary by Functional Group: Uv Light White Fumes of HCL FormedDokument4 SeitenOrganic Chemistry Summary by Functional Group: Uv Light White Fumes of HCL FormedJong.Gun.KimNoch keine Bewertungen
- Chapter 9-Alkynes 7 Unsaturations 9 Unsaturations 5 UnsaturationsDokument19 SeitenChapter 9-Alkynes 7 Unsaturations 9 Unsaturations 5 Unsaturations張湧浩Noch keine Bewertungen
- TutorialDokument27 SeitenTutorialSiti NuraqidahNoch keine Bewertungen
- Orgo Practice For UA ChemDokument15 SeitenOrgo Practice For UA ChemColin CheNoch keine Bewertungen
- Chemistry of Natural Products: Dr. Mohamed RabieDokument20 SeitenChemistry of Natural Products: Dr. Mohamed RabieKhalid LoveNoch keine Bewertungen
- AminesDokument23 SeitenAminesfhtzzzzzzNoch keine Bewertungen
- Provera Znanja 1209Dokument5 SeitenProvera Znanja 1209ShomiNoch keine Bewertungen
- Peta Minda KimiaDokument36 SeitenPeta Minda KimiaNATASHA 'ALIA BINTI ZULKIFLINoch keine Bewertungen
- Carbanions II312Dokument43 SeitenCarbanions II312ERMIN RISKIANINoch keine Bewertungen
- CCN MHT CET Synopsis PDFDokument7 SeitenCCN MHT CET Synopsis PDFAbhishek Mandlik100% (1)
- Alkene Reactions: CH2N2 to AlcoholsDokument2 SeitenAlkene Reactions: CH2N2 to AlcoholskjjkimkmkNoch keine Bewertungen
- Gryand: Ndhus!Dokument17 SeitenGryand: Ndhus!sunny meenuNoch keine Bewertungen
- Memory Map AromaticsDokument1 SeiteMemory Map AromaticsOCRChemistrySaltersNoch keine Bewertungen
- Alkyl HalidesDokument81 SeitenAlkyl HalideschandramajaviNoch keine Bewertungen
- Benzene Synthesis Page 51-53Dokument3 SeitenBenzene Synthesis Page 51-53Ung HhNoch keine Bewertungen
- Suzuki ReactionDokument2 SeitenSuzuki ReactionKIRAN ALLUNoch keine Bewertungen
- Reaction of Ketone CompleteDokument1 SeiteReaction of Ketone CompleteJoko SusiloNoch keine Bewertungen
- Sn2 MechanismDokument18 SeitenSn2 MechanismDian MustikasariNoch keine Bewertungen
- PH-6 - Mains - Answers - ChemistryDokument17 SeitenPH-6 - Mains - Answers - Chemistrytanu15048Noch keine Bewertungen
- O-TBS For Isq Ref - ArticleDokument15 SeitenO-TBS For Isq Ref - ArticleMutiva YyNoch keine Bewertungen
- Alkyl Halides: R-X (X F, CL, BR, I)Dokument40 SeitenAlkyl Halides: R-X (X F, CL, BR, I)ranjit singh randhawaNoch keine Bewertungen
- Ex 51 - SN1 or SN2? Question OneDokument2 SeitenEx 51 - SN1 or SN2? Question OneVarokah VarNoch keine Bewertungen
- 14Dokument10 Seiten14Hasen umerNoch keine Bewertungen
- Stereochemistry of SN Reactions PPT - Copy - Copy-1Dokument28 SeitenStereochemistry of SN Reactions PPT - Copy - Copy-1Vidya Rani100% (2)
- Halogeno CompoundsDokument28 SeitenHalogeno Compoundsapi-3734333Noch keine Bewertungen
- CH CH H C OK: Classes of S 1, S 2, E1 and E2 ReactionsDokument2 SeitenCH CH H C OK: Classes of S 1, S 2, E1 and E2 ReactionsBereket ShimelisNoch keine Bewertungen
- Adobe Scan Apr 05, 2024 (1)Dokument4 SeitenAdobe Scan Apr 05, 2024 (1)allamjacyNoch keine Bewertungen
- UntitledDokument101 SeitenUntitledAbdelrahim SalehNoch keine Bewertungen
- Amines, Reactions: Basic NucleophilicDokument37 SeitenAmines, Reactions: Basic NucleophilicM. MoizNoch keine Bewertungen
- Organic Short NotesDokument67 SeitenOrganic Short Notespushkariit2006Noch keine Bewertungen
- Group Meeting Problems - Organic ReactionsDokument5 SeitenGroup Meeting Problems - Organic Reactionsdicky wongNoch keine Bewertungen
- Class-XII Amines - Properties and ReactionsDokument8 SeitenClass-XII Amines - Properties and ReactionsAshok PradhanNoch keine Bewertungen
- CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CHDokument3 SeitenCH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CH CHStamati DanNoch keine Bewertungen
- Chemical reactions and structuresDokument22 SeitenChemical reactions and structuresStormy StudiosNoch keine Bewertungen
- Synthesis of Conine via Ziegler Alkylation and ReductionDokument2 SeitenSynthesis of Conine via Ziegler Alkylation and ReductionBhavaniNoch keine Bewertungen
- Chapter 7Dokument189 SeitenChapter 7Eshita SharmaNoch keine Bewertungen
- Functional Class General Formula Functional Group Example: Classification of Organic CompoundsDokument4 SeitenFunctional Class General Formula Functional Group Example: Classification of Organic CompoundsMelvina MikaelaNoch keine Bewertungen
- RXN Summary 09Dokument1 SeiteRXN Summary 09trash303Noch keine Bewertungen
- Ef0c00890 Si 001Dokument6 SeitenEf0c00890 Si 001Austin SmithNoch keine Bewertungen
- R-Che: DMF (Mecc - E-H)Dokument9 SeitenR-Che: DMF (Mecc - E-H)Janardhan BhowmikNoch keine Bewertungen
- Solution Manual for The Elements of Polymer Science and EngineeringVon EverandSolution Manual for The Elements of Polymer Science and EngineeringBewertung: 4 von 5 Sternen4/5 (3)
- Pharm D POC QuestionsDokument16 SeitenPharm D POC Questionspradeep36Noch keine Bewertungen
- Curriculum Target PDFDokument16 SeitenCurriculum Target PDFayaanNoch keine Bewertungen
- Important ConversionDokument3 SeitenImportant ConversionAKVanugrahNoch keine Bewertungen
- STK 1233 Organic Chemistry 1: LU 5.1: Aromatic CompoundsDokument37 SeitenSTK 1233 Organic Chemistry 1: LU 5.1: Aromatic CompoundsArllen Joy AlbertNoch keine Bewertungen
- Fosfa Cargo OilDokument34 SeitenFosfa Cargo OilAlfonso RecioNoch keine Bewertungen
- M.M. : 35 DPP # 04 TIME : 30 MINDokument2 SeitenM.M. : 35 DPP # 04 TIME : 30 MINArjun SabnisNoch keine Bewertungen
- 9701 s16 Ms 22Dokument7 Seiten9701 s16 Ms 22Thaarvena RetinaNoch keine Bewertungen
- Biochem Post Lab 4bDokument7 SeitenBiochem Post Lab 4bJessica Lorenz PablicoNoch keine Bewertungen
- Tds Gaa - India - BP MalaysiaDokument1 SeiteTds Gaa - India - BP MalaysiaErik YerzyNoch keine Bewertungen
- Chemistry Lab #1Dokument5 SeitenChemistry Lab #1Beckham DalchanNoch keine Bewertungen
- Complete List of Licensable ChemicalsDokument14 SeitenComplete List of Licensable ChemicalsLukmannNoch keine Bewertungen
- Chem 233 Aldol Lecture 12Dokument7 SeitenChem 233 Aldol Lecture 12niroanloinNoch keine Bewertungen
- Annexure-78Dokument95 SeitenAnnexure-78Deepak DwivediNoch keine Bewertungen
- Borazine WPSDokument16 SeitenBorazine WPSrohNoch keine Bewertungen
- Carboxylic Acids and EstersDokument31 SeitenCarboxylic Acids and EstersleanneNoch keine Bewertungen
- Branches of ChemistryDokument3 SeitenBranches of ChemistryEmmanuel BartolomeNoch keine Bewertungen
- Naming Hydrocarbons Worksheet and Key: Write The Name of Each of The Hydrocarbon Molecules Shown Below: 1) 8)Dokument2 SeitenNaming Hydrocarbons Worksheet and Key: Write The Name of Each of The Hydrocarbon Molecules Shown Below: 1) 8)Mo NassifNoch keine Bewertungen
- Chemistry Tips For IIT PreparationDokument82 SeitenChemistry Tips For IIT PreparationParas Thakur75% (4)
- Reactions P-Hydroxybenzyl Alcohol Derivatives and Their Methyl Ethers With Molecular Chlorine'Dokument6 SeitenReactions P-Hydroxybenzyl Alcohol Derivatives and Their Methyl Ethers With Molecular Chlorine'Sandipan SahaNoch keine Bewertungen
- HydrocarbonsDokument76 SeitenHydrocarbonsAyush KumarNoch keine Bewertungen
- Laboratory 26: Carbohydrates: GeneralDokument19 SeitenLaboratory 26: Carbohydrates: GeneralShafera ArbiNoch keine Bewertungen
- Preparation of Benzy L Acetate Post LabDokument3 SeitenPreparation of Benzy L Acetate Post LabNina PobleteNoch keine Bewertungen
- Benzene MilanaDokument61 SeitenBenzene MilanaMilana WalujoNoch keine Bewertungen
- Gandini Et Al-2018-European Journal of Lipid Science and TechnologyDokument20 SeitenGandini Et Al-2018-European Journal of Lipid Science and TechnologyEsteban ArayaNoch keine Bewertungen
- Elsevier 1 N08Dokument270 SeitenElsevier 1 N08criticald0% (1)
- Hydroxyl at I OnDokument60 SeitenHydroxyl at I OngbgbkrishnaNoch keine Bewertungen
- Carbohydrates Classification and ReactionsDokument31 SeitenCarbohydrates Classification and ReactionsAlviro CossemeNoch keine Bewertungen
- Stereochemistry Chiral MoleculesDokument95 SeitenStereochemistry Chiral MoleculesRoby PadillaNoch keine Bewertungen
- Science Grade 10: Quarter 4 - Biomolecules: Protein S & Nucleic AcidsDokument14 SeitenScience Grade 10: Quarter 4 - Biomolecules: Protein S & Nucleic AcidsMernalyn Deximo InotNoch keine Bewertungen
- Sharpless Asymmetric Dihydroxylation of OlefinsDokument6 SeitenSharpless Asymmetric Dihydroxylation of OlefinsluoftNoch keine Bewertungen