Beruflich Dokumente
Kultur Dokumente
Dry About 1
Hochgeladen von
Paolo Naguit0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
11 Ansichten1 SeiteDry about 1
Originaltitel
Dry about 1
Copyright
© © All Rights Reserved
Verfügbare Formate
DOCX, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenDry about 1
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOCX, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
11 Ansichten1 SeiteDry About 1
Hochgeladen von
Paolo NaguitDry about 1
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOCX, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 1
1. Dry about 1.
6 grams of the acid (potassium hydrogen phthalate –
KHP).
2. Accurately weigh (i.e. using the analytical balance) out ~ 1.5 grams
of the acid and dissolve it in water in a 250 mL volumetric flask.
3. Calibrate your pH meter using the supplied buffers. After
calibration, rinse the electrode very well with distilled water and
blot dry with kimwipes and then rinse in a small amount of your
analyte solution.
4. Transfer 100mL of the acid solution to a 250mL beaker using a
transfer pipet. Add a stir bar and position the setup in a way that
the stir bar does not strike the pH electrode. Make sure that the
pH meter is properly immersed in the solution. Allow the electrode
to equilibrate approximately 1 minute with stirring and measure
and record the initial pH of the solution.
5. Add 3 drops of phenolphthalein indicator and titrate the solution
with standard ~ 0.1 M NaOH. Prepare and standardize your NaOH
solution. Be sure to record the exact molarity and the standard
deviation for this solution as you will need to know these
quantities later.
6. Add base in 1.5 mL increments recording the volume and the pH 30
s after each addition until you are within approximately 4 mL of the
theoretical equivalence point calculated from the NaOH molarity
and the amount of KHP.
7. When you are within approximately 4 mL of the equivalence
volume, add the base in 0.4 mL increments, recording the volume
and pH after each addition until you are within 1 mL of the
equivalence point. After that, add base 1 drop at a time, again
recording volume and pH after each addition until you have passed
the pink endpoint by a few tenths of a mL. Add five more 1 mL
aliquots beyond the pink endpoint. Record the exact volume at
which the pink color is observed.
8. Construct a graph of pH vs Vb (volume of added base). Locate the
equivalence volume (Veest) as the maximum point/slope from your
first derivative plot or the point of zero second derivative from
your second derivative plot. Compare these volumes with the
theoretical equivalence point and the phenolphthalein endpoints.
Das könnte Ihnen auch gefallen
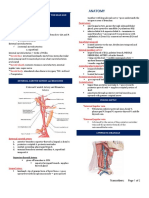
- 1.18 Radiologic Anatomy of The Head and NeckDokument2 Seiten1.18 Radiologic Anatomy of The Head and NeckPaolo NaguitNoch keine Bewertungen
- 1.14 ANATOMY - The Pharynx Landmarks - MusclesDokument3 Seiten1.14 ANATOMY - The Pharynx Landmarks - MusclesPaolo NaguitNoch keine Bewertungen
- 1.17 Anatomy-Neurovascular Structures of Head and NeckDokument2 Seiten1.17 Anatomy-Neurovascular Structures of Head and NeckPaolo NaguitNoch keine Bewertungen
- 1.15 ANATOMY - The LarynxDokument2 Seiten1.15 ANATOMY - The LarynxPaolo NaguitNoch keine Bewertungen
- 12chem301 EDTA Titration PDFDokument17 Seiten12chem301 EDTA Titration PDFPaolo NaguitNoch keine Bewertungen
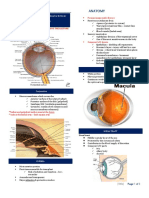
- 1.10 ANATOMY - The Eyeball - Surface Anatomy - Landmarks - Extrinsic - Intrinsic MusclesDokument3 Seiten1.10 ANATOMY - The Eyeball - Surface Anatomy - Landmarks - Extrinsic - Intrinsic MusclesPaolo NaguitNoch keine Bewertungen
- Titration Curve of Amino AcidsDokument3 SeitenTitration Curve of Amino AcidsPaolo NaguitNoch keine Bewertungen
- 1.13 ANATOMY - The Teeth, Palate, Internal Auditory Tube and TonsilsDokument2 Seiten1.13 ANATOMY - The Teeth, Palate, Internal Auditory Tube and TonsilsPaolo NaguitNoch keine Bewertungen
- 1.11 ANATOMY - The Nose and Paransal SinusesDokument4 Seiten1.11 ANATOMY - The Nose and Paransal SinusesPaolo NaguitNoch keine Bewertungen
- 1.16 The TongueDokument2 Seiten1.16 The TonguePaolo NaguitNoch keine Bewertungen
- 11chem301 Argentometric MethodsDokument16 Seiten11chem301 Argentometric MethodsPaolo NaguitNoch keine Bewertungen
- Conductometric Titration: Determination of The Strength of A Solution of Hydrochloric Acid (HCL) by A Standard Solution of Sodium Hydroxide (Naoh)Dokument4 SeitenConductometric Titration: Determination of The Strength of A Solution of Hydrochloric Acid (HCL) by A Standard Solution of Sodium Hydroxide (Naoh)Paolo NaguitNoch keine Bewertungen
- 1.12 ANATOMY - The Ears Surface Anatomy and Landmarks, External, Middle and Inner Portions, Blood Vessels and NervesDokument5 Seiten1.12 ANATOMY - The Ears Surface Anatomy and Landmarks, External, Middle and Inner Portions, Blood Vessels and NervesPaolo NaguitNoch keine Bewertungen
- VectorDokument39 SeitenVectorPaolo Naguit100% (1)
- Vector 3Dokument55 SeitenVector 3Paolo NaguitNoch keine Bewertungen
- Smith1985 PDFDokument10 SeitenSmith1985 PDFLizeth Paola TellezNoch keine Bewertungen
- BradfordDokument7 SeitenBradfordJonatas LopesNoch keine Bewertungen
- Coulometric Titration of Ascorbic Acid With Electrogenerated IodineDokument5 SeitenCoulometric Titration of Ascorbic Acid With Electrogenerated IodinePaolo NaguitNoch keine Bewertungen
- BCA Assay Paper - 1988Dokument7 SeitenBCA Assay Paper - 1988Paolo NaguitNoch keine Bewertungen
- PV92 PCR Kit Manual PDFDokument104 SeitenPV92 PCR Kit Manual PDFPaolo NaguitNoch keine Bewertungen
- PDFDokument124 SeitenPDFratno budiyantoNoch keine Bewertungen
- PDFDokument124 SeitenPDFratno budiyantoNoch keine Bewertungen
- HBB Hemoglobin, Beta (Bos Taurus (Cattle) )Dokument1 SeiteHBB Hemoglobin, Beta (Bos Taurus (Cattle) )Paolo NaguitNoch keine Bewertungen
- Hbb-Ar Hemoglobin, Activating Region (Mus Musculus (House Mouse) )Dokument7 SeitenHbb-Ar Hemoglobin, Activating Region (Mus Musculus (House Mouse) )Paolo NaguitNoch keine Bewertungen
- Transcription: The Path and Control of Gene ExpressionDokument24 SeitenTranscription: The Path and Control of Gene ExpressionPaolo NaguitNoch keine Bewertungen
- HBA Hemoglobin, Alpha 2 (Bos Taurus (Cattle) )Dokument1 SeiteHBA Hemoglobin, Alpha 2 (Bos Taurus (Cattle) )Paolo NaguitNoch keine Bewertungen
- HBZP1 Hemoglobin Subunit Zeta Pseudogene 1 (Homo Sapiens (Human) )Dokument1 SeiteHBZP1 Hemoglobin Subunit Zeta Pseudogene 1 (Homo Sapiens (Human) )Paolo NaguitNoch keine Bewertungen
- Hba-A1 Hemoglobin Alpha, Adult Chain 1 (Mus Musculus (House Mouse) )Dokument1 SeiteHba-A1 Hemoglobin Alpha, Adult Chain 1 (Mus Musculus (House Mouse) )Paolo NaguitNoch keine Bewertungen
- BFG Chapter2 AccessInformation v04Dokument67 SeitenBFG Chapter2 AccessInformation v04Paolo NaguitNoch keine Bewertungen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5782)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (72)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)