Beruflich Dokumente
Kultur Dokumente
Caracterizarea Biochimică Și Moleculară A PDF
Hochgeladen von
Lucan FlorentinaOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Caracterizarea Biochimică Și Moleculară A PDF
Hochgeladen von
Lucan FlorentinaCopyright:
Verfügbare Formate
PPA_753.
fm Page 574 Friday, September 27, 2002 10:25 AM
Plant Pathology (2002) 51, 574 – 584
Biochemical and molecular characterization of Bacillus
Blackwell Science, Ltd
amyloliquefaciens, B. subtilis and B. pumilus isolates with
distinct antagonistic potential against Xanthomonas
campestris pv. campestris
E. G. Wulffa*†, C. M. Mgunib, K. Mansfeld-Giesec, J. Felsd, M. Lübecka and J. Hockenhulla
a
The Royal Veterinary and Agricultural University, Institute of Plant Biology, Plant Pathology Section, Thorvaldsensvej 40, DK-1871,
Frederiksberg C, Copenhagen, Denmark; bDepartment of Research and Special Services, Plant Protection Research Institute, PO Box CY
550, Harare, Zimbabwe; cDanish Institute of Agricultural Sciences, Department of Plant Protection, Flakkebjerg DK-4200, Slagelse,
Denmark; and dNovo Nordisk A /S, Novo Allé, 2880 Bagsværd, Denmark
Fifty-one Bacillus isolates were characterized by fatty acid methyl ester (FAME) analysis; universal primer polymerase
chain reaction (UP-PCR) fingerprinting; production of secondary metabolites and antagonistic activity against
Xanthomonas campestris pv. campestris (causal agent of black rot in cabbage) in vitro and in vivo. Based on FAME analysis
and /or PCR fingerprinting, the isolates were clustered into three different groups, named as Bacillus amyloliquefaciens,
B. subtilis and B. pumilus. Seed treatment with Bacillus spp. generally reduced germination of seeds and incidence of
black rot, but no relationship was found between the results of in vitro and in vivo experiments. The B. amyloliquefaciens
group contained isolates that were generally the most effective at reducing attack of black rot in vivo. The metabolic
profiles of these isolates suggested that they produced surfactin, iturin, bacillomycine and/or azalomycin F. Isolates
belonging to the B. subtilis group were mostly able to synthesize surfactin and arthrobactin. Surfactin, amphomycin,
arthrobactin and valinomycin were generally found in culture extracts of isolates belonging to the B. pumilus group. No
effect on growth of the pathogen was detected when the activity of filtered culture extracts and selected metabolites
produced by the three different Bacillus species was tested in vitro against X. c. pv. campestris. However, inhibition was
seen when bacterial liquid cultures were used. When the ability to colonize cabbage endophytically was examined for
seven selected isolates with different antagonistic potential against black rot, it was found that the ability was related to
the species and not to the antagonistic activity of the isolates.
Keywords: Bacillus spp., biological control, brassica black rot, endophytes, fatty acid methyl esters, secondary metabolites
bacteria (Sasser, 1990; Ash et al., 1991; Stead et al., 1992;
Introduction Alvarez et al., 1994). According to Stead et al. (1992),
The genus Bacillus is characterized by Gram-positive, bacterial species can easily be identified using cellular fatty
aerobic or facultative anaerobic, rod-shaped bacteria acid profiles, and the accuracy of identification at species
that form spores, and contains more than 60 species that level is often 100%. Bacillus species have been identified
have quite different phenotypes (Claus & Berkeley, 1986). and characterized in a study conducted by Ash et al.
Most of the tests conducted for identification of bacteria (1991), where the 16S rRNA gene of several Bacillus
have been based on physiological and nutritional tests spp. was sequenced. The study revealed the presence of five
(Claus & Berkeley, 1986). Today, methods such as fatty acid, highly different lines within the genus; based on sequence
DNA (including PCR fingerprinting) and RNA analysis homologies, B. amyloliquefaciens, B. subtilis and B.
are also useful for identification and classification of pumilus belong to the same group (group 1) (Ash et al.,
1991).
*To whom correspondence should be addressed. Efforts to control plant diseases with antagonistic
bacterial agents have been made successfully (Wei et al.,
†Present address: International Potato Center, PO Box 1558,
1991; Chen et al., 1995). Bacillus species have special
Lima 12, Peru. E-mail: e.wulff@cgiar.org
characteristics that make them good candidates as biolog-
Accepted 2 June 2002 ical control agents. First, they are well known as antibiotic
574 © 2002 BSPP
PPA_753.fm Page 575 Friday, September 27, 2002 10:25 AM
Bacillus spp. antagonistic against X. campestris pv. campestris 575
producers with antagonistic activity against fungal and
some bacterial pathogens (Loeffler et al., 1986; Krebs
Materials and methods
et al., 1998). This ability also appears to contribute to
Bacterial isolates
the establishment and persistence of the antagonist in the
plant (Krebs et al., 1993). Second, they form spores that Fifty-one Bacillus spp. isolates isolated from Brassica
can be easily formulated, and have high viability com- seeds and plants were kept at −80°C in trypticase soy
pared with vegetative cells (Bochow, 1995). Third, they broth (TSB; Difco Laboratories, Detroit, MI, USA)
are commonly found in soils (Stabb et al., 1994). amended with 20% glycerol. Isolates W52 (Bacillus
Several studies have been performed in an attempt to amyloliquefaciens CCUG 28·519), W50 and W51 [Bacillus
elucidate the mechanisms involved in biological control subtilis CCGU 163B (b) and CCUG 163B (a)], and W43
by Bacillus species. The antagonistic activity has often and W44 [Bacillus pumilus CCUG 26016 (b) and CCUG
been associated with production of secondary metabolites 26016 (a)] were identified at the University of Gothe-
with antibiotic properties (Silo-Suh et al., 1994; Stabb burg, Sweden, and used as reference isolates in UP-PCR
et al., 1994; Asaka & Shoda, 1996). Most of the antibiotics fingerprinting.
produced by Bacillus spp. have been characterized as
dipeptides or cyclic peptides with low molecular weight
Fatty acid methyl ester analysis and PCR fingerprinting
(Loeffler et al., 1986; Nakano & Zuber, 1990). The phy-
tosanitary effects provided by the metabolites appear to Identification and grouping of the Bacillus isolates were
be due to the promotion of growth and resistance of the based on fatty acid methyl ester (FAME) analysis results
host plant and direct antibiotic effects against the patho- and UP-PCR fingerprinting profiles for B. amylolique-
gen (Dolej & Bochow, 1996). According to Silo-Suh et al. faciens and B. subtilis. Bacillus pumilus was identified
(1994), the metabolites produced by Bacillus spp. can and grouped only according to the results obtained with
also affect the microflora on the rhizosphere, providing FAME analysis (first name option).
an environment antagonistic to the pathogen, or they can Fatty acid methyl esters were extracted from each
trigger host defence responses. In addition to antibiotic isolate using standard and recommended procedures for
production, the ability to colonize plants endophytically gas chromatographic (GC) FAME analysis (Sasser, 1990).
has also been identified as an important feature of biolog- Analysis was performed with a Hewlett Packard gas
ical control agents intended for use against vascular patho- chromatograph HP5890 CG (Hewlett Packard, Bracknell,
gens (Kloepper et al., 1999). Bacillus species are among UK) and the sherlock Microbial Identification System
the most common bacteria found to colonize plants software (MIDI Inc., Newark, DE, USA) using the MIDI
endophytically (Lilley et al., 1996; Mahaffee & Kloepper, standard method, aerobe Version 3·9. Identification at
1997), and it is likely that their endophytic ability could species level was performed by comparing the fatty acid
play a role in the biocontrol of vascular plant pathogens. profiles to the MIDI standard library, tsba version 3·9.
Black rot is one of the most serious diseases of crucifers Isolates with a similarity index of >60% for best match
(Williams, 1980) and has, for example, become the most were considered to have been identified. The fatty acid
important disease of Brassica spp. in Zimbabwe, causing profiles were clustered using the dendrogram utilities
yield and quality losses of up to 80% (Page et al., 1985). included with the sherlock software.
The disease is caused by the bacterium Xanthomonas DNA extraction was conducted with the Promega
campestris pv. campestris, which is a vascular pathogen. wizard genomic DNA purification kit for Gram-positive
Among Brassica crops, cabbage and cauliflower are bacteria (Promega, Madison, WI, USA). UP-PCR (Bulat &
the most susceptible species to black rot (Mguni, 1996). Minorenko, 1990) amplifications were performed using
Seed contamination is one of the most important means of UP primers that are 15–20 bp long and that target inter-
transmission (Cook et al., 1952), but in Zimbabwe seed genic areas of the genome, which are more variable (Bulat
health testing of Brassica is not carried out and seed et al., 1998). The primer AS15inv (Lübeck et al., 1998),
certification is conducted only by field inspection (Mguni, which is a 17-mer (5′-CATTGCTGGCGAATCGG-3′),
1996). Plants need to be protected from attack as soon was selected from 10 UP primers, based on the ability to
as they germinate. Thus seed treatment with biological distinguish different Bacillus species according to intense
control agents may be useful in an integrated control polymorphic band patterns. UP-PCR was conducted in
programme for black rot. 20 µL volume with 10–100 ng DNA, 4 OD units primer,
This work was carried out (i) to identify and group 51 0·4 units Dynazyme version 2·0 (Finnzymes OY, Espoo,
Bacillus isolates based on FAME analysis and universal Finland), 10 mm Tris–HCl pH 8·8, 3·5 mm MgCl2, 50 mm
primer (UP)-PCR fingerprinting profiles; (ii) to charact- KCl, 0·1% Triton x-100, and 0·4 mm dNTP. PCR was
erize the ability of isolates to produce secondary meta- performed using a MiniCycler, model PTC 150 (MJ
bolites; (iii) to test the antagonistic activity of isolates Research, Watertown, MA, USA) and 0·5 mL standard
(in vitro and in vivo), their culture filtrate extracts (in vitro), PCR tubes (Biozym Diagnostik GmBH, Oldendorf, Ger-
and three selected metabolites (in vitro) against X. c. pv. many). The PCR program had an initial DNA denatura-
campestris; and (iv) to examine the endophytic ability of tion step of 3 min at 94°C, followed by 30 cycles of 50 s
selected isolates (belonging to the three different species) at 92°C, primer annealing for 70 s at 56°C, primer exten-
with different antagonistic potential against black rot. sion for 60 s at 72°C, and the final extension step for
© 2002 BSPP Plant Pathology (2002) 51, 574 –584
PPA_753.fm Page 576 Friday, September 27, 2002 10:25 AM
576 E. G. Wulff et al.
3 min at 72°C. The PCR products were analysed by disinfection by plating 100 seeds per disinfected seed lot
running 2·5 – 4·0 µL of the amplified product on 1·7% on trypticase soy agar (TSA, Difco Laboratories) and
agarose gels at 300 V for 40 min. The gels were stained incubating at 25°C. If no microbial growth was detected
with ethidium bromide and photographed in UV light. on the plates, the seed samples were considered surface-
disinfected and used in further experiments.
Inoculum suspensions were prepared with 24-h-old
Production and analysis of secondary metabolites
bacterial cultures growing on TSA. Sterile saline water
produced by Bacillus isolates
(20 mL, 0·85% NaCl) was added to the bacterial culture,
Fermentation which was suspended with a glass rod. The bacterial
Bacterial cultures 24 h old, growing on trypticase soy agar suspension was homogenized using a vortex mixer, and
(TSA, Difco) at 27°C was suspended with 5 mL sterile the inoculum amount was adjusted with sterile saline to
saline solution (0·9%). Bacterial suspension (2 µL) was OD600 = 0·01 (≈107 CFU mL−1) for X. c. pv. campestris
transferred to 500 mL flasks containing 100 mL boullion and 1·00 (≈109 CFU mL−1) for the Bacillus isolates.
3 (6 g peptone; 4 g pepticase; 3 g yeast extract; 1·5 g meat Disinfected cabbage seeds were inoculated with X. c.
extract; 1000 mL deionized water). The flasks were incu- pv. campestris for 30 min and left to dry in the flow cabinet.
bated for 24 h at 30°C on a rotary shaker at 250 r.p.m. Next day, 0·3 g preinoculated seeds were soaked for 18 h
Flasks (500 mL) containing 100 mL cabbage broth (50 g under agitation (150 r.p.m.) in 10 mL of the inoculum
cabbage, 1000 mL deionized water pH 7·0) or 100 mL suspension made from the respective Bacillus isolates.
half-strength TSB (15 g TSB, 1000 mL) were inoculated Control plants consisted of seeds preinoculated with X. c.
with 3 mL 24-h-old bacterial cultures growing in boullion pv. campestris and untreated seeds, both dipped in 10 mL
3. After inoculation, flasks were incubated at 30°C for sterile saline solution and treated in the same way as those
3 days on a rotary shaker (250 r.p.m.). inoculated with Bacillus isolates. The seeds were then
sown in trays containing pine bark that were watered
Extraction of secondary metabolites twice a day and kept in a glasshouse without controlled
1-butanol (2 µL) was added to 2 mL of the cultured broth conditions. The experiment was designed as a completely
and shaken in 5 mL vials at 250 r.p.m. for 2 h at room randomized block experiment with three blocks and 15
temperature (23°C). Thereafter, the vials were centrifuged repetitions per treatment per block.
(Varifuge RF Inert, Heraeus Sepatech, Hanau, Germany) for Fifteen days after sowing, the effect of the different seed
10 min at 3000 g, −12°C and the supernatant (butanol treatments on germination and the biocontrol effect were
phase) concentrated until dry. The residue was taken up evaluated for the different treatments. The effect on
with 100 µL dimethylsulphoxide (DMSO) and trans- germination was assessed by comparing the number of
ferred to a plastic vial which was kept in the freezer seedlings obtained with the Bacillus-treated seeds, and the
(−20°C) until high performance liquid chromatography number of seedlings obtained with the untreated control
(HPLC) was performed. treatment (100% germination). The effect on black rot
incidence was measured by comparing the number of
HPLC analysis diseased seedlings that developed from seeds treated with
HPLC analysis was conducted with a Hewlett Packard Bacillus isolates with those from seeds treated only with
1090 m Series II with DAD detector. A Novogram column the pathogen (100% black rot incidence). At the time of
[4·0 mm internal diameter (ID), 60 mm long] was used assessment, cabbage seedlings showed two cotyledons
together with a precolumn (4·0 mm ID, 50 mm long) and zero to three true leaves.
containing reverse phase material (Grom, Herrenberg,
Germany). Samples were analysed with gradient elution
Effect of Bacillus isolates and selected metabolites on
from 100% solvent A (0·1% aqueous orthophosphoric
X. c. pv. campestris in vitro
acid) to 100% solvent B (acetonitrile, HPLC grade) in 6 min.
The wavelength of detection was 210 nm. Chromato- The activity of Bacillus isolates, filtered culture extracts
grams were analysed and identification of the metabolites and three selected metabolites [surfactin (Sigma Chemical,
was performed with the Hewlett Packard HPLC 3d St Louis, MO, USA); iturin (kindly provided by Dr
chemstation Software (DOS series). G Winkelmann, University of Tübingen, Germany); and
acivicin (Sigma)] against X. c. pv. campestris was measured
in vitro according to Thornberry (1950), with minor
In vivo screening of Bacillus isolates for antagonistic
modifications. Two different media were used in this
activity against X. c. pv. campestris
experiment. LB medium (yeast extract 5 g; glucose 10 g;
Seeds of cabbage, Brassica oleracea cv. Copenhagen tryptone, 10 g; NaCl, 5 g; agar, 15 g and distilled water,
Market, were surface-disinfected by immersion in 70% 1000 mL) was chosen because antibiotic activity against
ethanol for 1 min, transferred to sodium hypochlorite bacteria was previously detected using this medium
(1% available chlorine) for 3 min, and rinsed three times (Jenny et al., 1991). PDA [39 g potato dextrose agar
consecutively in sterile distilled water. Seeds were then left (Difco), 1000 mL distilled water] was chosen because
to dry in the flow cabinet in Petri dishes containing sterile X. c. pv. campestris grew well on this medium. PDA or LB
filter paper. Sterility control was conducted after seed medium (15 mL) was poured into 9 cm Petri dishes and
© 2002 BSPP Plant Pathology (2002) 51, 574– 584
PPA_753.fm Page 577 Friday, September 27, 2002 10:25 AM
Bacillus spp. antagonistic against X. campestris pv. campestris 577
left to dry for 30 min. Four mL of the liquid agar seeded
Statistical analysis
with bacterial pathogen (107 CFU mL−1) was then spread
uniformly on the previously poured, solidified agar layer anova was conducted using General Linear Models
and left to dry for 10 min. Inoculum suspensions of Bacillus (GLM) of the SAS package (Statistical Analysis Systems
spp. (50 µL; 109 CFU mL−1), filtered culture extracts Institute Inc., Cary, NC, USA). Data on germination and
(concentrated ×20, also used in HPLC analysis) and reduction of black rot incidence were analysed as a com-
antibiotic suspensions (1·0, 0·5, 0·25 and 0·12 mg mL−1) plete randomized block experiment using three repetitions
were transferred to autoclaved filter paper disks 12 mm in (15 replicates per treatment per block). The data on inhi-
diameter, and left to dry for 20 min. The filter paper disks bition zone against X. c. pv. campestris were analysed as
(one per plate) were then placed at the centre of the seeded one-way anova with four repetitions per treatment. Data
PDA or LB plates and incubated at 27°C. Two days after on endophytic ability were analysed as one-way anova
incubation, the inhibition zone around the disks was using seven repetitions. However, before analysis the lat-
measured. Control plates contained disks soaked in sterile ter were transformed to log CFU g−1 plant fresh weight;
water and in 50 µL vancomycin (Eli Lilly, Indianapolis, samples where no bacteria were detected were scored as
IN, USA) at concentrations of 1·0, 0·5, 0·25, 0·12 and zero and included in the average. Means of each treatment
0·0 mg mL−1. Vancomycin was shown to be an active anti- in all three experiments were compared using the Student–
biotic against X. c. pv. campestris in vitro. The experiment Newman–Keuls test (P < 0·05).
was set up with four replicates per treatment.
Results
Endophytic ability of selected Bacillus isolates with
Table 1 shows the group of isolates identified by FAME
different antagonistic potentials against black rot in
analysis and UP-PCR profile as B. amyloliquefaciens.
cabbage
Although the similarity indices were too low for the
Disinfected seeds were inoculated as described above with species to be considered as identified (similarity indices
seven selected Bacillus isolates with different antagonistic <60%), FAME analysis suggested the same species name
potential against black rot. Twenty days after sowing, for the isolates belonging to this group (clusters A and B).
plants were collected and examined for endophytic colo- However, all isolates showed similar UP-PCR profiles
nization. Root (1 cm segment collected from the main when compared with the reference isolate W52 (Fig. 1a).
root just below the soil line); stem (1 cm segment collected The isolates in this group were also generally similar in
just above the soil line); cotyledon and true leaf (latest- relation to their HPLC profiles. Most of the isolates pro-
formed leaf) samples from inoculated and uninoculated duced surfactin (A), iturin (B), bacillomycine (C) and/or
plants were thoroughly washed with tap water, cut with azalomycin F (D) (Tables 1 and 4). The ability to inhibit
a scalpel and transferred to Petri dishes containing 15 mL growth of X. c. pv. campestris in vitro varied from a very
sodium hypochlorite (1% available chlorine) for 2 min. weak (+) to a very strong (++++) effect and generally no
The samples were then washed three times consecutively relationship was seen between in vitro inhibition and
in sterile distilled water, transferred to plastic bags biocontrol effect in vivo (Table 1). All isolates reduced seed
containing 0·5 mL sterile saline solution (0·85%) amended germination, but no significant differences (except for iso-
with glycerol (20%), then crushed with a hammer. To lates 69 and 17C) were found between isolates. Isolates
ensure that the sections were completely surface-disinfected, 29C, 69, 8, 17A, 101, 103, 15, 17C and 71 reduced black
100 µL of the last wash was transferred to vials contain- rot incidence in >60% of seedlings (Table 1).
ing 1 mL TSB and incubated at 27°C. If contamination The group of isolates, shown in Table 2, was identified
was detected, the sample was discarded. Plant extracts by FAME analysis (similarity indices >67%) and UP-PCR
(50 µL) were plated on TSBA [15 g Bacto agar (Difco), profiles as B. subtilis, with all isolates belonging to the
1·5 g TSB, 1000 mL distilled H2O]. After sterilization, same FAME cluster (cluster C). With the exception of
cycloheximide (10 µg mL−1, Sigma) and 2,3,5-triphenyl- isolate 16, all isolates showed UP-PCR profiles very similar
tetrazolium chloride (10 mg mL−1, Merck, Darmstadt, to reference isolates W50 and W51 (Fig. 1b). Most of the
Germany) were added to the agar. Control plates were isolates in this group were also generally found to be
prepared with the inoculum suspension of the reference similar in their HPLC profiles, and produced surfactin (A)
isolates serially diluted, and plated in the same way on the and arthrobactin (G) (Tables 2 and 4). Isolate 16 was the
same medium. All plates were incubated at 27°C. Five only one that produced bacillomycine (Table 2). The
to 7 days after incubation, the plates were examined and ability to inhibit growth of X. c. pv. campestris in vitro was
colonies compared with the reference isolate. Colonies variable, and ranged from a very weak effect (+) to a very
similar in morphology and colour to the reference isolate strong effect (++++). However, no relationship was seen
were picked up with a sterile needle and placed on the between these results and the biocontrol effect in vivo.
TSBA by puncturing the agar. The same was done with Although no significant differences were found in seed
the reference isolate. If test and reference colonies grew germination or reduction of black rot incidence among
similarly and had similar UP-PCR profiles, the test bacte- isolates of group 2, the ability to reduce the incidence
rial colony was considered to be of the same isolate as the of black rot within isolates was generally low, and varied
inoculated one. from 0·0 to 42·1% (Table 2).
© 2002 BSPP Plant Pathology (2002) 51, 574 –584
PPA_753.fm Page 578 Friday, September 27, 2002 10:25 AM
578 E. G. Wulff et al.
Table 1 Characterization of Bacillus amyloliquefaciens isolates (group 1) according to FAME analysis, UP-PCR banding profiles, secondary
metabolite detection in culture extracts, inhibition zone against Xanthomonas campestris pv. campestris in vitro, effect on seed germination (%),
and reduction of black rot incidence (%) in vivo
Reduction of
FAME suggested Secondary Inhibition Seed black rot
Isolate identification [similarity UP-PCR metabolite zone v. germination incidence
number index (%)–clustera] profileb detectionc Xccd (%)e (%)e
55 B. amyloliquefaciens (27·5 –A) 1 A, D & G + 95·5 a 45·5 ab
76 B. amyloliquefaciens (32·1–A) 1 A, C & D + 81·8 a 40·9 ab
29C B. amyloliquefaciens (38·2–A) 1 A, F, G, I & J + 88·6 a 68·2 a
69 B. amyloliquefaciens (38·5 –A) 1 A, D & H + 97·7 a 68·2 a
80 B. amyloliquefaciens (28·0 –A) 1 C, D & M ++ 72·7 ab 36·4 ab
29A B. amyloliquefaciens (35·9 –A) 1 C, D, H & L ++ 93·2 a 31·1 ab
8 B. amyloliquefaciens (37·3 –A) 1 A, B, C, D & G ++ 86·6 a 77·3 a
17A B. amyloliquefaciens (39·0 –A) 1 B ++ 90·9 a 68·2 a
58 B. amyloliquefaciens (49·8 –A) 1 A, B, C & F ++ 86·4 a 40·9 ab
74 B. amyloliquefaciens (43·7–A) 1 A, D & M ++ 93·2 a 27·3 ab
101 B. amyloliquefaciens (47·0 –A) 1 A, B, C, D & H ++ 97·7 a 77·3 a
103 B. amyloliquefaciens (50·0 –A) 1 A&B ++ 93·2 a 65·5 a
68 B. amyloliquefaciens (17·7–A) 1 A, B & I ++++ 95·5 a 0·0 b
15 B. amyloliquefaciens (20·6 –B) 1 A, B, C & D ++ 79·5 ab 68·2 a
17C B. amyloliquefaciens (36·6 –B) 1 B, C, D & H ++ 59·1 b 68·2 a
73 B. amyloliquefaciens (43·9 –B) 1 A, B, D & H ++ 93·2 a 45·5 ab
71 B. amyloliquefaciens (56·7–B) 1 A, B & D ++ 86·4 a 77·3 a
84 B. amyloliquefaciens (34·7–B) 1 A, B, C & D +++ 88·6 a 58·6 ab
100·0 a 0·0 b
(control)f (control)g
a
Cluster grouping according to FAME analysis: Euclidian distance = A, 7·60; B, 4·34.
b
UP-PCR profile 1, see Fig. 1(a).
c
Secondary metabolite identification matches: A, surfactin; B, iturin; C, bacillomycine; D, azalomycin F; F, acivicin; G, arthrobactin; H, rhodutorola
acid; I, valinomycin; J, stenothricin; L, enterochelin; M, nocardamin.
d
Inhibition zone (IZ): + (very weak), 0 –2 mm; ++ (weak), 2 < IZ ≤ 5 mm; +++ (strong), 5 < IZ ≤ 7 mm; ++++ (very strong), 7 < IZ ≤ 10 mm.
e
Means were statistically compared within the bacterial isolates belonging to group 1. Values followed by the same letters were not significantly
different from each other.
f
Seeds dipped in sterile saline water.
g
Preinoculated seeds with Xanthomonas campestris pv. campestris and dipped in sterile saline.
Isolates identified as B. pumilus by FAME analysis generally not related to the ability to reduce the incidence
(similarity indices >60%) were shown to belong to the of black rot in vivo. With the exception of isolate BF3, no
same cluster (cluster D) (Table 3). However, the isolates B. pumilus isolate reduced the germination rate compared
were shown to be very heterogeneous molecularly, with a with the untreated control. Four isolates (60, 64, 102 and
range of different UP-PCR profiles that were distinct from 70) were not able to reduce the incidence of black rot
the reference isolates W43 and W44 (Fig. 1c). Thus the compared with the untreated control (Table 3).
identification of B. pumilus isolates was based only on The general ability of all Bacillus isolates to produce
FAME results (first-named option). Figure 1(c) shows the secondary metabolites is shown in Table 4. All three
UP-PCR profiles obtained for B. pumilus isolates (seven species were shown to be efficient metabolic producers
different UP-PCR profiles). Isolates 52, 56, 62 and 64 when growing in half-strength trypticase soy broth (TSB)
had a similar UP-PCR banding profile (profile 4); while or in cabbage broth (CB). Generally, TSB stimulated
BF2 and BF3 had profile 5; and isolates 163, 163B and 11B greater production of metabolites compared with CB.
had profile 8. Isolates 102, 199, 60, 70 and B7D showed Surfactin appeared to be a very common metabolite
yet other UP-PCR profiles compared with the other produced by Bacillus spp., being detected in culture
isolates in this group (Fig. 1c). Considering their HPLC extracts of all three species when growing in TSB and CB.
profiles, most isolates still produced surfactin (A), amph- Iturin was detected in culture extracts of various isolates
omycin (E), arthrobactin (G) and valinomycin (Is) (Tables 3 of B. amyloliquefaciens and two isolates of B. subtilis. CB
and 4). The ability to inhibit growth of X. c. pv. campestris was shown to be a better substrate for iturin production
in vitro varied from very weak to a very strong effect, compared with TSB (data not shown). Just as iturin was
although most isolates showed a weak effect (Table 3). detected in several isolates of B. amyloliquefaciens, bacil-
As with the other two Bacillus species, this ability was lomycine was detected in culture extracts of nine isolates
© 2002 BSPP Plant Pathology (2002) 51, 574– 584
PPA_753.fm Page 579 Friday, September 27, 2002 10:25 AM
Bacillus spp. antagonistic against X. campestris pv. campestris 579
of B. amyloliquefaciens and one isolate of B. subtilis when
the isolates were growing in CB and TSB. Azalomycin
F was particularly synthesized by B. amyloliquefaciens
isolates (Table 4). This antibiotic was detected only when
the isolates were growing in TSB. Amphomycin and
stenothricin were mainly detected in culture extracts
of B. pumilus isolates, but only a few isolates of B. amylo-
liquefaciens and B. subtilis were also able to produce these
metabolites. Valinomycin and acivicin were detected in
culture extracts of some isolates of B. amyloliquefaciens,
B. subtilis and B. pumilus. Valinomycin was found only
in culture extracts of Bacillus spp. growing in TSB, while
acivicin was detected in culture extracts of isolates
growing in both TSB and CB. Bacillus amyloliquefaciens
and B. subtilis were able to synthesize arthobactin and
rhodutorola acid, which are iron chelators (siderophores),
while B. pumilus synthesized only arthrobactin. Colistin
was produced by three isolates of B. pumilus, and entero-
chelin (siderophore), which was produced only when
Bacillus spp. were cultured in CB, was detected in culture
extracts of B. amyloliquefaciens and B. pumilus (one
isolate of each species). Nocardamin, which is also an iron
chelator, was detected only in culture extracts of two
isolates of B. amyloliquefaciens.
The effects of three selected metabolites tested in vitro
are shown in Table 5. Neither surfactin, iturin nor acivicin
inhibited the growth of X. c. pv. campestris in vitro when
the pathogen was growing on PDA or LB. Surfactin and
acivicin had a slight effect on the growth of the pathogen
on PDA and LB in the form of clump formation (granu-
lated growth), but no growth inhibition was seen on the
plates. Concentrated filtered culture extracts of three
Bacillus isolates (BB, B50A and B7D) were also tested
and showed no inhibition of growth of X. c. pv. campestris
in vitro.
The endophytic ability of seven Bacillus isolates is
shown in Fig. 2. Bacillus pumilus isolates BF3 (high
antagonistic activity) and 60 (no antagonistic activity)
showed the best endophytic ability, as they could be
reisolated from all plant parts 20 days after inoculation.
Bacillus subtilis isolates 77 (high antagonistic activity)
and 7D (low antagonistic activity) colonized mainly roots,
but were also found with less frequency in some stem
sections, and rarely in cotyledons and true leaves. Bacillus
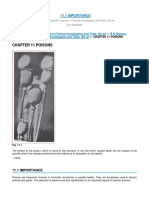
Figure 1 (a) UP-PCR banding profiles for some Bacillus amyloliquefaciens isolates 68 (high antagonistic activity),
amyloliquefaciens isolates (group 1) generated with universal primer 8 (no antagonistic activity), and 101 (high antagonistic
AS15inv. Lanes 2–17: isolates 8, 15, 17A, 17C, 29A, 29C, 55, 58, 68, 73, activity) showed the poorest endophytic ability to colonize
74, 76, 80, 84, 101 and 103, respectively. Lane 18 corresponds to cabbage seedlings compared with the other Bacillus
UP-PCR banding profile of the reference isolate W52. Lanes 1 and 19, species. Thus isolates 8 and 68 were reisolated from roots
molecular weight marker (lambda phage DNA digested with PstI).
and sometimes from stem sections and cotyledons (only
(b) UP-PCR banding profiles (group 2) for some Bacillus subtilis
isolates generated with universal primer AS15inv. Lanes 2–18: isolates
7A (profile 2), 7B (profile 2), 7D (profile 2), 16 (profile 3), 20 (profile 2),
21(profile 2), 28 (profile 2), 77 (profile 2), 78 (profile 2), 89 (profile 2), Lanes 2–15: isolate 52 (profile 4), BF2 (profile 5), BF3 (profile 5), 102
1EF3 (profile 2), 1EL1 (profile 2), 1EL3 (profile 2), 2EL1 (profile 2), 2EL2 (profile 6), 199 (profile 7), 56 (profile 4), 62 (profile 4), 163B (profile 8),
(profile 2), BF4 (profile 2) and BB (profile 2), respectively. Lanes 19 and 64 (profile 4), 60 (profile 9), 163 (profile 8), 70 (profile 10), B7D (profile
20 correspond to UP-PCR banding profile of reference isolates W50 11) and 11B (profile 8), respectively. Lanes 16 and 17 correspond to
and W51, respectively. Lane 1, molecular weight marker (lambda UP-PCR banding profile of reference isolates W43 and W44,
phage DNA digested with PstI). (c) UP-PCR banding profiles (group 3) respectively. Lanes 1 and 18, molecular weight marker (lambda phage
for Bacillus pumilus isolates generated with universal primer AS15inv. DNA digested with PstI).
© 2002 BSPP Plant Pathology (2002) 51, 574 –584
PPA_753.fm Page 580 Friday, September 27, 2002 10:25 AM
580 E. G. Wulff et al.
Table 2 Characterization of Bacillus subtilis isolates (group 2) according to FAME analysis, UP-PCR banding profiles, secondary metabolite
detection in culture extracts, inhibition zone against Xanthomonas campestris pv. campestris in vitro, effect on seed germination (%), and reduction
of black rot incidence (%) in vivo
Reduction of
Secondary Inhibition Seed black rot
Isolate FAME suggested identification UP-PCR metabolite zone v. germination incidence
number [similarity index (%)–clustera] profileb detectionc Xccd (%)e (%)e
78 B. subtilis (67·4 – C) 2 A, G & H + 97·2 a 21·1 a
2EL1 B. subtilis (85·4 – C) 2 F&H + 100·0 a 0·0 a
21 B. subtilis (85·1– C) 2 A&G + 97·2 a 15·8 a
1EK2 B. subtilis (78·1– C) 2 A&F ++ 97·2 a 36·8 a
2EL2 B. subtilis (82·9 – C) 2 G ++ 88·9 a 5·3 a
1EL3 B. subtilis (86·9 – C) 2 A ++ 88·9 a 36·8 a
20 B. subtilis (87·4 – C) 2 A&G ++ 86·1 a 26·3 a
77 B. subtilis (88·1– C) 2 A, G, H & J ++ 66·7 a 42·1 a
7A B. subtilis (88·4 – C) 2 A&G +++ 100·0 a 15·8 a
16 B. subtilis (67·8 – C) 3 A&C +++ 100·0 a 26·3 a
BF4 B. subtilis (84·6 – C) 2 A, I & J +++ 100·0 a 5·3 a
28 B. subtilis (87·5 – C) 2 A, F & G +++ 97·2 a 31·6 a
1EL1 B. subtilis (85·4 – C) 2 A +++ 100·0 a 26·3 a
1EF3 B. subtilis (90·1– C) 2 A&G +++ 100·0 a 31·6 a
7B B. subtilis (77·5 – C) 2 A, G & H ++++ 86·1 a 21·1 a
B7A B. subtilis (81·5 – C) 2 A, B & G ++++ 88·9 a 42·1 a
7D B. subtilis (81·7– C) 2 A, G & H ++++ 97·2 a 0·0 a
BB B. subtilis (87·0 – C) 2 A&G ++++ 100·0 a 36·3 a
89 B. subtilis (89·0–C) 2 A, B, F & G ++++ 83·3 a 36·8 a
100·0 a 0·0 a
(control)f (control)g
a
Cluster grouping according to FAME analysis: C, Euclidian distance = 6·69.
b
UP–PCR profiles 2 and 3, see Fig. 1(b).
c
Secondary metabolite identification matches, see Table 1.
d
Inhibition zone (IZ), see Table 1.
e
Means were statistically compared within the bacterial isolates belonging to group 1. Values followed by the same letters were not
significantly different from each other.
f
See Table 1.
g
See Table 1.
isolate 68), while 101 was found only in a few root samples in vivo. They suggested that Bacillus isolates might not
20 days after inoculation. produce the same quantity or quality of antibiotics in vivo
as in vitro, or that the population of B. subtilis in planta
might not be high enough or located near the pathogenic
Discussion cells (Schreiber et al., 1988). Two isolates belonging to
The use of FAME analysis and UP-PCR fingerprinting group 1 were also shown to inhibit seed germination, and
profiles was generally helpful in the identification of their activity against black rot in vivo was generally higher
Bacillus species, making these features useful for the compared with the isolates of the other Bacillus groups.
classification of the genus at species level. Isolates of group 2, identified as B. subtilis, showed the
Isolates of group 1, mainly identified by UP-PCR analysis highest indices of similarity according to FAME analysis,
as B. amyloliquefaciens, showed an ability to produce with all isolates belonging to the same cluster (Table 2).
secondary metabolites characteristic for this group. Furthermore, with the exception of isolate 16, all isolates
However, the ability to inhibit growth of X. c. pv. campestris showed similar UP-PCR profiles (profile group 2) and
in vitro was generally not relatd to the biocontrol effect ability to produce surfactin and arthrobactin in culture.
in vivo. Utkhede & Gaunce (1983) and Schreiber et al. As with B. amyloliquefaciens, the ability to inhibit growth
(1988) described similar findings. In vitro, antibiosis can of X. c. pv. campestris in vitro generally did not relate to
be influenced by the agar used and order of application the biocontrol effect found in vivo. Reduction of seed
of the bacteria (Bell et al., 1995). Schreiber et al. (1988) germination was lower compared with the other groups.
found that, despite high inhibition of the Dutch elm patho- Also, the ability to reduce black rot was generally low,
gen in vitro by metabolites produced by an endophytic with no significant differences between the isolates and
B. subtilis isolate, no biological control was obtained the untreated control.
© 2002 BSPP Plant Pathology (2002) 51, 574– 584
PPA_753.fm Page 581 Friday, September 27, 2002 10:25 AM
Bacillus spp. antagonistic against X. campestris pv. campestris 581
Table 3 Characterization of Bacillus pumilus isolates (group 3) according to FAME analysis, UP-PCR banding profiles, secondary metabolite
detection in culture extracts, inhibition zone against Xanthomonas campestris pv. campestris in vitro, effect on seed germination (%), and reduction
of black rot incidence (%) in vivo
Reduction of
Secondary Inhibition Seed black rot
Isolate FAME suggested identification UP-PCR metabolite zone v. germination incidence
number [similarity index (%)–clustera] profileb detectionc Xccd (%)e (%)e
163 B. pumilus (86·5 – D) 8 A, E, I & J + 84·1 a 37·9 a
11B B. pumilus (88·7– D) 8 A&G + 75·0 a 48·2 a
60 B. pumilus (91·9 – D) 9 J&K + 86·4 a 17·2 ab
B. pumilus (88·4 – D) 4 A, E, I & J ++ 95·5 a 41·4 a
B. pumilus (68·1– D) 4 A, G & I ++ 93·2 a 41·4 a
B. pumilus (89·6 – D) 4 A, E, G, I & K ++ 79·5 a 31·1 ab
B. pumilus (90·9 – D) 4 A, E, G, I & J ++ 75·0 a 51·7 a
BF3 B. pumilus (85·5 – D) 5 A, E & I ++ 52·7 b 55·1 a
BF2 B. pumilus (83·5 – D) 5 A, E & I ++ 77·3 a 37·9 a
102 B. pumilus (61·7– D) 6 A, E, F, I & K ++ 81·8 a 24·1 ab
199 B. pumilus (91·2– D) 7 G ++ 93·2 a 55·1 a
B. pumilus (81·8 – D) 10 A, E, I & J ++ 88·6 a 31·1 ab
B7D B. pumilus (74·2– D) 11 A, F, G & I ++ 86·4 a 41·4 a
163B B. pumilus (81·5 – D) 8 A, E, G & I ++++ 68·2 a 44·8 a
100·0 a 0·0 b
(control)f (control)g
a
Cluster grouping according to FAME analysis: D, Euclidian distance = 9·39.
b
UP–PCR-profile 4 –11, see Fig. 1(c).
c
Secondary metabolite identification matches, see Table 1 except for E = amphomycin; K = colistin.
d
Inhibition zone (IZ), see Table 1.
e
Means were statistically compared within the bacterial isolates belonging to group 1. Values followed by the same letters were not
significantly different from each other.
f
See Table 1.
g
See Table 1.
Table 4 General ability of Bacillus species to
Bacillus species
Secondary metabolite produce secondary metabolites when growing
matchesa B. amyloliquefaciens B. subtilis B. pumilus in tryptic soy broth and cabbage broth at 30°C
b e f f
Surfactin + (12 : 18) + (17 : 19) + (13 : 14)f
Iturinb + (11 : 18) + (2 : 19) –
Bacillomycineb + (9 : 18) + (1 : 19) –
Azalomycin Fc + (15 : 18) – –
Amphomycinc + (1 : 18) + (1 : 19) + (10 : 14)
Acivicinb + (3 : 18) + (5 : 19) + (2 : 14)
Arthrobactinc + (8 : 18) + (14 : 19) + (8 : 14)
Rhodutorola acidc + (7 : 18) + (5 : 19) –
Valinomycinc + (3 : 18) + (1 : 19) + (12 : 14)
Stenothricinb + (1 : 18) + (2 : 19) + (5 : 14)
Colistinc –g – + (3 : 14)
Enterochelind + (1 : 18) – + (1 : 14)
Nocardaminc + (2 : 18) – –
a
Identified substances (>60% match factor: retention time and UV spectrum).
b
Detected in both half-strength trypticase soy broth and cabbage broth.
c
Detected only in half-strength trypticase soy broth.
d
Detected only in cabbage broth.
e
+, Ability to produce the corresponding metabolite.
f
Numbers of producing isolates : total numbers of tested isolates.
g
–, No ability to produce the corresponding metabolite.
© 2002 BSPP Plant Pathology (2002) 51, 574 –584
PPA_753.fm Page 582 Friday, September 27, 2002 10:25 AM
582 E. G. Wulff et al.
Table 5 Effect in vitro of surfactin, iturin and acivicin on growth of antibiotic has generally been found in culture extracts
Xanthomonas campestris pv. campestris on PDA and LB medium of B. subtilis (Hiraoka et al., 1992; Asaka & Shoda,
1996). In contrast, azalomycin F, amphomycin, acivicin,
Secondary metabolite Inhibition zone (mm) Media valinomycin and stenothricin have not been described
Surfactina
0·0 PDA and LB for Bacillus species before, whereas they have been for
Acivicina 0·0 PDA and LB Streptomyces species. None of the iron chelators (arthro-
Iturinb 0·0 PDA and LB bactin, rhodutorola acid, enterochelin and nocardamin)
Control (vancomycin) (mg mL−1) detected in the present study has been previously reported
1·0 10·7 PDA in the literature on Bacillus spp. However, the identifica-
0·5 8·7 PDA tion of the secondary metabolites in this study should be
0·25 4·3 PDA interpreted with caution, as they are based only on HPLC
0·12 1·3 PDA match profiles suggested by a reference HPLC library.
1·0 9·8 LB Mass spectophotometry is still recommended to confirm
Culture extract (20 × concentrate) the identification of the metabolites detected.
BB 0·0 PDA and LB No antibiotic activity was found in vitro when testing
B7D 0·0 PDA and LB filtered culture extracts of three Bacillus isolates (repre-
B50A 0·0 PDA and LB sentative of the three species), or when surfactin, iturin
and acivicin were tested in different concentrations
a
Tested at four concentrations: 1·0, 0·5, 0·25 and 0·12 mg mL−1. against X. c. pv. campestris. In the literature, the activity
b
Tested at two concentrations: 1·0 and 0·5 mg mL−1.
of antibiotics produced by Bacillus spp. is more commonly
found against fungal than against bacterial pathogens. In
the work conducted by Ebata et al. (1969), three types of
The final group (group 3) consisted of isolates identified subsporins, which are peptide metabolites produced by
by FAME analysis as B. pumilus. Although the isolates B. subtilis, were active against many filamentous fungi
pertained to the same FAME cluster and showed some and yeasts, but no activity was found against most of the
similarity in their HPLC profiles, the group was shown to bacteria tested. Gatavalin, another metabolite produced
be very heterogeneous considering their UP-PCR profiles. by Bacillus, was active against Gram-positive bacteria,
There were eight different UP-PCR profiles for the 14 mycobacteria, yeasts and moulds, but no activity was
isolates tested of this group, and their profiles were shown found for Gram-negative bacteria (Nakajima et al., 1972).
to be different from those of the two reference isolates Swinburne et al. (1975) tested the activity of antibacterial
used. As with B. amyloliquefaciens, some isolates in group components found in culture extracts of B. subtilis, and
3 significantly reduced germination, and the ability to proved that they were rapidly deactivated following
inhibit black rot in vitro was generally not related to the maximum production. However, Loeffler et al. (1986)
ability to reduce incidence of the disease in vivo. reported that bacilysin, an antibiotic produced by B.
The production of secondary metabolites was a feature subtilis, was active against some isolates of B. subtilis at
associated with individual Bacillus isolates, but generally the rate of 1 mg mL−1, and against the Gram-negative
the detection of some metabolites was associated with Escherichia coli at the rate of 10 mg mL−1. The antagonis-
a group of Bacillus species. Bacillus amyloliquefaciens tic activity found for metabolites produced by Bacillus
isolates generally produced surfactin, iturin, bacillomycine spp. has also been associated with the synergistic effect
and /or azalomycin F, while B. subtilis isolates were caused by the combination of antibiotics (Asaka &
mostly able to synthesize surfactin and arthrobactin. Shoda, 1996). Growth inhibition of X. c. pv. campestris
Surfactin, amphomycin, arthrobactin and valinomycin were was seen in the study reported here when using bacterial
generally found in culture extracts of B. pumilus isolates. liquid cultures (containing living cells) of most of the
Surfactin was produced by all three Bacillus species. This Bacillus isolates tested. Perhaps the antibiotic effect is
Figure 2 Endophytic ability of seven Bacillus
isolates with high (BF3, 77 and 101) and low
(60, 7D and 68) antagonistic potential against
Xanthomonas campestris pv. campestris,
20 days after inoculation. Isolates BF3 and 60
(B. pumilus); 77 and 7D (B. subtilis); and 8, 68
and 101 (B. amyloliquefaciens) were reisolated
from different plant parts of cabbage. Error
bars represent standard deviations.
© 2002 BSPP Plant Pathology (2002) 51, 574– 584
PPA_753.fm Page 583 Friday, September 27, 2002 10:25 AM
Bacillus spp. antagonistic against X. campestris pv. campestris 583
transmitted only from bacterial cell to cell; or perhaps Bulat SA, Minorenko NV, 1990. Species identity of the
the Bacillus cells changed the agar composition at the phytopathogenic fungi Pyrenophora teres Dreschler and
inhibition zone, thereby affecting the growth of X. c. pv. P. graminea Ito and Kuribayashi. Mikologiya I Fitopatologia
campestris. 24, 435 –41. [In Russian].
Considering the endophytic ability of seven isolates Bulat SA, Lübeck M, Minorenko N, Jensen DF, Lübeck PS,
with distinct antagonistic potential against black rot, the 1998. UP-PCR analysis and ITS1 ribotyping of strains of
ability to colonize cabbage tissues internally appears to be Trichoderma and Gliocladium. Mycological Research 102,
933 –43.
related to the Bacillus species. No relationship was found
Chen C, Bauske EM, Musson G, Rodríguez-Kábana R,
between their endophytic ability and antagonistic poten-
Kloepper JW, 1995. Biological control of Fusarium wilt on
tial. Both isolates BF3 (highest antagonistic activity
cotton by use of endophytic bacteria. Biological Control
against black rot in vivo in group 3) and 60 (lowest antag-
5, 83–91.
onistic activity) were found in root, stem, cotyledon and Claus D, Berkeley RCW, 1986. Genus Bacillus Cohn 1872. In:
true leaf. Bacillus subtilis isolates differing in antagonistic Sneath PHA, ed. Bergey’s Manual of Systematic Bacteriology,
activity were reisolated with lower frequency compared Section 13, Vol.2 Baltimore, MD, USA: Williams & Wilkins
with the isolates of group 3, but at 20 days after sowing, Co, 1105 –39.
the isolates could be reisolated from all four parts of the Cook AA, Larson RH, Walker JC, 1952. Relation of the black
plant. Bacillus amyloliquefaciens isolates with (isolates 8 rot pathogen to cabbage seeds. Phytopathology 42, 316–20.
and 101) and without (isolate 68) antagonistic potential Dolej S, Bochow H, 1996. Studies of the mode of action of
against black rot showed the poorest ability to colonize Bacillus subtilis culture filtrates in the model pathosystem
cabbage internally. Isolates 8 and 68 were sometimes tomato seedling – Fusarium oxysporum f.sp. radicis-
reisolated from root and stem sections, while isolate 101 lycopersici. Mededelingen Faculteit
was found almost nowhere in any plant part. The results Landbouwwetenschappen Rijksuniversitet (Gent) 61,
presented here suggest that the mechanisms involved 483–9.
in biological control of black rot may be complex, and Ebata M, Miyazaki K, Takahashi Y, 1969. Studies on subsporin.
appear to be dependent on the individual isolate rather I. Isolation and characterization of subsporins A, B and C.
than the species. Journal of Antibiotics 22, 467–72.
Hiraoka H, Osaka O, Ano T, Shoda M, 1992. Characterization
of Bacillus subtilis RB14 coproducer of peptide antibiotics
Acknowledgements iturin A and surfactin. Journal of Applied Microbiology 38,
635 –40.
The present work was funded by the Danish Council
Jenny K, Käppeli O, Fiechter A, 1991. Biosurfactants from
of Development Research, project number 90842. We
Bacillus licheniformis: structural analysis and charcterization.
would like to thank Mr Lovemore Mukwicho for excel-
Applied Microbiology and Biotechnology 36, 5–13.
lent technical assistance during glasshouse experiments Kloepper JW, Rodríguez-Kábana R, Zehnder GW, Murphy JF,
and part of the laboratory work conducted in Zimbabwe. Sikora E, Fernández C, 1999. Plant root–bacterial
We thank Dr G Winkelmann from the University of interactions in biological control of soilborne diseases and
Tübingen in Germany for providing the antibiotic iturin potential extension to systemic and foliar diseases.
used in the in vitro tests. Australasian Plant Pathology 28, 21–6.
Krebs B, Junge H, Ockhardt A, Höding B, Heubner D, Erben U,
1993. Bacillus subtilis – an effective biocontrol agent.
References
Pesticide Science 37, 427–33.
Alvarez AM, Benedict AA, Mizumoto CY, Hunter JE, Gabriel DW, Krebs B, Höding B, Kübart S, Alemayehu Workie M, Junge H,
1994. Serological, pathological, and genetic diversity among Schmiedeknecht G, Grosch R, Bochow H, Hevesi M, 1998.
strains of Xanthomonas campestris pv. campestris infecting Use of Bacillus subtilis as biological control agent. I. Activities
crucifers. Phytopathology 84, 1449–57. and characterization of Bacillus subtilis strains. Journal of
Asaka O, Shoda M, 1996. Biocontrol of Rhizoctonia solani Plant Disease and Protection 105, 181–97.
damping-off of tomato with Bacillus subtilis RB14. Applied Lilley AK, Fry JC, Bailey MJ, Day MJ, 1996. Comparison of
and Environmental Microbiology 62, 4081–5. aerobic heterotrophic taxa isolated from root domains of
Ash C, Farrow JAE, Wallkbanks S, Collins MD, 1991. mature sugar beet. FEMS Microbiological Ecology 21,
Phylogenetic heterogeneity of the genus Bacillus revealed by 231–42.
comparative analysis of small subunit – ribosomal RNA Loeffler W, Tschen S-M, Vanittanakom N, Kugler M, Knorpp E,
sequences. Letters in Applied Microbiology 13, 202–6. Hsieh T-F, Wu T-G, 1986. Antifungal effects of bacilysin
Bell CR, Dickie GA, Chan JWYF, 1995. Variable response of and fengymycin from Bacillus subtilis F-29-3: a comparison
bacteria isolated from grapevine xylem to control grape with activities of other Bacillus antibiotics. Journal of
crown gall disease in plant. American Journal of Enology and Phytopathology 115, 204 –13.
Viticulture 46, 499–508. Lübeck M, Alekhina IA, Lübeck PS, Jensen DF, Bulat SA, 1998.
Bochow H, 1995. Mode of action and practical use of the Development of specific primers for monitoring a biocontrol
Bacillus subtilis as complex acting bioproduct. In: Manka M, strain of Gliocladium roseum in field soils. In: Duffy B,
ed. Environmental Biotic Factors in Integrated Plant Disease Rosenberger U, Défago G, eds. Proceedings of IOBC/EFPP
Control. Poznan, Poland: The Polish Phytopathological Workshop, Molecular Approaches in Biological Control,
Society, 97–104. September 1997, Delémont, Switzerland, 217–23.
© 2002 BSPP Plant Pathology (2002) 51, 574 –584
PPA_753.fm Page 584 Friday, September 27, 2002 10:25 AM
584 E. G. Wulff et al.
Mahaffee WF, Kloepper JW, 1997. Temporal changes in the Silo-Suh LA, Lethbridge BJ, Raffel SJ, He H, Clardy J,
bacterial communities of soil, rhizosphere and endorhiza Handelsman J, 1994. Biological activities of two fungistatic
associated with field-grown cucumber (Cucumis sativus L.). antibiotics produced by Bacillus cereus UW85. Applied and
Microbial Ecology 34, 210–23. Environmental Microbiology 60, 2023–30.
Mguni CM, 1996. Bacterial black rot (Xanthomonas campestris Stabb WV, Jacobson LM, Handelsman J, 1994. Zwittermicin A
pv. campestris) of vegetable brassicas in Zimbabwe. – producing strains of Bacillus cereus from diverse soils.
Copenhagen, Denmark: The Royal Veterinary and Applied and Environmental Microbiology 60, 4404 –12.
Agricultural University/Danish Government Institute of Seed Stead DE, Sellwood JE, Wilson J, Viney I, 1992. Evaluation of a
Pathology for Developing Countries, PhD Thesis. commercial microbial identification system based on fatty
Nakajima N, Chihara S, Koyama Y, 1972. A new antibiotic, acid profiles for rapid, accurate identification of plant
gatavalin. I. Isolation and characterization. Journal of pathogenic bacteria. Journal of Applied Bacteriology 72,
Antibiotics 25, 243–7. 315–21.
Nakano MM, Zuber P, 1990. Molecular biology of antibiotic Swinburne TR, Barr JG, Brown AE, 1975. Production of
production in Bacillus. Critical Reviews in Biotechnology 10, antibiotics by Bacillus subtilis and their effect on fungal
223– 40. colonists of apple leaf scars. Transactions of the British
Page JLS, Mguni CM, Sithole SZ, 1985. Pests and Diseases of Mycological Society 65, 211–7.
Crops in the Communal Areas of Zimbabwe. London, UK: Thornberry HH, 1950. A paper-disk plate method for the
Overseas Development Administration Technical Report, quantitative evaluation of fungicides and bactericides.
203. Phytopathology 40, 419–29.
Sasser M, 1990. Identification of bacteria through fatty acid Utkhede RS, Gaunce AP, 1983. Inhibition of Phytophthora
analysis. In: Klement Z, Rudolph K, Sands DC, eds. Methods cactorum by bacterial antagonists. Canadian Journal of
in Phytobacteriology. Budapest, Hungary: Akademiai Kiado, Botany 61, 3343–8.
199–204. Wei G, Kloepper JW, Tuzun S, 1991. Induction of systemic
Schreiber LR, Gregory GF, Krause CR, Ichida JM, 1988. resistance of cucumber to Colletotrichum orbiculare by select
Production, partial purification, and antimicrobial activity strains of plant growth-promoting rhizobacteria.
of a novel antibiotic produced by a Bacillus subtilis isolated Phytopathology 81, 1508–12.
from Ulmus americana. Canadian Journal of Botany 66, Williams PH, 1980. Black rot: a continuing threat to world
2338– 46. crucifers. Plant Disease 64, 736–7.
© 2002 BSPP Plant Pathology (2002) 51, 574– 584
Das könnte Ihnen auch gefallen
- Authoritarian Regimes: Gabriela Gonzalez VaillantDokument4 SeitenAuthoritarian Regimes: Gabriela Gonzalez VaillantLucan FlorentinaNoch keine Bewertungen
- Intelligence A Brief History PDFDokument18 SeitenIntelligence A Brief History PDFLucan FlorentinaNoch keine Bewertungen
- The Comparisons About Macro Elements Content in Fruits Collected From The Gotlob Locality of Timis CountyDokument7 SeitenThe Comparisons About Macro Elements Content in Fruits Collected From The Gotlob Locality of Timis CountyLucan FlorentinaNoch keine Bewertungen
- Federal Register / Vol. 78, No. 244 / Thursday, December 19, 2013 / Rules and RegulationsDokument2 SeitenFederal Register / Vol. 78, No. 244 / Thursday, December 19, 2013 / Rules and RegulationsLucan FlorentinaNoch keine Bewertungen
- Federal Register / Vol. 77, No. 29 / Monday, February 13, 2012 / Proposed RulesDokument3 SeitenFederal Register / Vol. 77, No. 29 / Monday, February 13, 2012 / Proposed RulesLucan FlorentinaNoch keine Bewertungen
- 2010 29813 PDFDokument13 Seiten2010 29813 PDFLucan Florentina100% (1)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Prominence SIL-20aDokument4 SeitenProminence SIL-20amr_stali2987Noch keine Bewertungen
- 21-2022 - Vieira-Sellai-Green HPLC Lamivudine Zidovudine and NevirapineDokument11 Seiten21-2022 - Vieira-Sellai-Green HPLC Lamivudine Zidovudine and NevirapinemercedesNoch keine Bewertungen
- Development and Validation of HPLC Method For Estimation of Gliclazide in Gliclazide Tablets Prepared Using Natural DisintegrantDokument7 SeitenDevelopment and Validation of HPLC Method For Estimation of Gliclazide in Gliclazide Tablets Prepared Using Natural DisintegrantEditor IJTSRDNoch keine Bewertungen
- 15.10 Methodology LCMS IMPDokument8 Seiten15.10 Methodology LCMS IMPNitish TankNoch keine Bewertungen
- FluvastatinDokument3 SeitenFluvastatinJuan PerezNoch keine Bewertungen
- Zorbax Eclipse PlusDokument2 SeitenZorbax Eclipse PlusluisaugoliniNoch keine Bewertungen
- Coating Failures - Causes, Identification, and AnalysisDokument36 SeitenCoating Failures - Causes, Identification, and AnalysisSUBODH100% (1)
- Simple Titrimetric Analysis For Determination of Pitavastatin Calcium in Bulk and Formulation DosageDokument10 SeitenSimple Titrimetric Analysis For Determination of Pitavastatin Calcium in Bulk and Formulation DosageAndika AndiNoch keine Bewertungen
- HPLC Determination of Caffeine in Coffee BeverageDokument7 SeitenHPLC Determination of Caffeine in Coffee Beveragemuhammad ihklasulNoch keine Bewertungen
- Recovery/bias Evaluation: Ivo Leito University of Tartu Ivo - Leito@ut - EeDokument18 SeitenRecovery/bias Evaluation: Ivo Leito University of Tartu Ivo - Leito@ut - EeRina ErlinaNoch keine Bewertungen
- Development and Validation of LC Method For The Estimation of Lincomycin in Pharmaceutical DosageformsDokument4 SeitenDevelopment and Validation of LC Method For The Estimation of Lincomycin in Pharmaceutical DosageformsLam NguyễnNoch keine Bewertungen
- Oil Debate Chromatography PPT 2Dokument17 SeitenOil Debate Chromatography PPT 2Eridha TriwardhaniNoch keine Bewertungen
- Bacopa HPLC TraceDokument5 SeitenBacopa HPLC TraceThoh Xin MeiNoch keine Bewertungen
- Method 6.6 - C-Molasses: Fructose, Glucose and Sucrose by HPLCDokument5 SeitenMethod 6.6 - C-Molasses: Fructose, Glucose and Sucrose by HPLCVishnuNoch keine Bewertungen
- Isolation, Quantification, and Identification of Rosmarinic Acid, GasDokument7 SeitenIsolation, Quantification, and Identification of Rosmarinic Acid, GasLiliana Papuico SanchezNoch keine Bewertungen
- Revicion de Métodos CromatograficosDokument14 SeitenRevicion de Métodos CromatograficosMirko D'aNoch keine Bewertungen
- Get Empowered - Review Window and The Processing Method - Tip #134, 3D PDA Data - WatersDokument6 SeitenGet Empowered - Review Window and The Processing Method - Tip #134, 3D PDA Data - WatersBruno IndústriaNoch keine Bewertungen
- Dissertation LSD Patrick Dolder 18.09.17Dokument130 SeitenDissertation LSD Patrick Dolder 18.09.17nineeNoch keine Bewertungen
- 11.1 Importance PDFDokument62 Seiten11.1 Importance PDFkuldeep Roy SinghNoch keine Bewertungen
- Industrial Training Thesis RanbaxyDokument38 SeitenIndustrial Training Thesis RanbaxySameer QureshiNoch keine Bewertungen
- Penentuan Kadar Kalium Sorbat Dan Persen Recovery Pada Selai Dengan Metoda High Performance Liquid ChromatographyDokument4 SeitenPenentuan Kadar Kalium Sorbat Dan Persen Recovery Pada Selai Dengan Metoda High Performance Liquid ChromatographySuprianto, M.Si., AptNoch keine Bewertungen
- HPLC ReportDokument19 SeitenHPLC ReportRichard CarizonNoch keine Bewertungen
- Analytical Tasks - Efficiently Solved by HPTLC: Camag Bibliography ServiceDokument68 SeitenAnalytical Tasks - Efficiently Solved by HPTLC: Camag Bibliography ServiceDarian HerascuNoch keine Bewertungen
- Lucr 10 oniscuBTDokument8 SeitenLucr 10 oniscuBTanggunputriviona31Noch keine Bewertungen
- New HPLC System Sys LC 138Dokument3 SeitenNew HPLC System Sys LC 138Sachin S RaneNoch keine Bewertungen
- Chemical Composition of Two Species of TeaDokument8 SeitenChemical Composition of Two Species of TeaMaría Luisa Marcos SánchezNoch keine Bewertungen
- 2016 - Methods For Identification, Quantification and Characterization of PHAsDokument7 Seiten2016 - Methods For Identification, Quantification and Characterization of PHAsVirgi CortésNoch keine Bewertungen
- LC-MS/MS Assay of Methylphenidate: Stability and Pharmacokinetics in HumanDokument6 SeitenLC-MS/MS Assay of Methylphenidate: Stability and Pharmacokinetics in HumanasdgasdfasdfassdfasdfNoch keine Bewertungen
- Extraction of Ce and TH From Monazite Using REE Tolerant Aspergillus NigerDokument10 SeitenExtraction of Ce and TH From Monazite Using REE Tolerant Aspergillus Nigerjay9037Noch keine Bewertungen
- Cannabis Sativa Bioactive Compounds and TheirDokument15 SeitenCannabis Sativa Bioactive Compounds and TheirSORIN AVRAMESCUNoch keine Bewertungen