Beruflich Dokumente
Kultur Dokumente
Concept Map - Biology - 2018 - July
Hochgeladen von
Rahique ShuaibOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Concept Map - Biology - 2018 - July
Hochgeladen von
Rahique ShuaibCopyright:
Verfügbare Formate
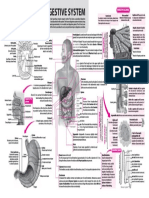
METABOLISM
CONCEPT l Metabolism is a highly coordinated cellular activity in which many multi-enzyme systems (metabolic pathways) cooperate to (i) Obtain chemical energy by
capturing solar energy or degrading energy-rich nutrients from the environment; (ii) Convert nutrient molecules into the cell's own characteristic molecules,
MAP
including precursors of macromolecules; (iii) Polymerise monomeric precursors into macromolecules: (proteins, nucleic acids and polysaccharides) and (iv)
Synthesise and degrade biomolecules required for specialised cellular functions, such as membrane lipids, intracellular messengers and pigments.
l Metabolic pathways fall into three categories: (i) Anabolic pathways which are those involved in the synthesis of larger and more complex compounds from
smaller precursors; (ii) Catabolic pathways which are involved in the breakdown of larger molecules, commonly involving oxidative reactions and are
exothermic and (iii) Amphibolic pathways which occur at the "crossroads" of metabolism, acting as links between the anabolic and catabolic pathways, e.g.,
the citric acid cycle.
Carbohydrate Protein Fat
Digestion and absorption
Metabolism of
Glucose Amino acids
Fatty acids Carbohydrates, Proteins and Lipids
+ Glycerol
The products of digestion of dietary carbohydrates, lipids and proteins are glucose, fatty acid +
l
glycerol and amino acids, respectively.
Transamination l All the products of digestion are metabolised to a common product, acetyl-CoA, which is then oxidised by
Glycolysis the citric acid cycle.
b-Oxidation
l Glucose is metabolised to pyruvate by the pathway of glycolysis. Aerobic tissues metabolise pyruvate to acetyl-CoA,
which can enter the citric acid cycle for complete oxidation to CO2 and H2O, linked to the formation of ATP in the
Acetyl-CoA
process of oxidative phosphorylation. Glycolysis can also occur anaerobically when the end product is lactate.
l Fatty acids may be oxidised to acetyl-CoA by b-oxidation or esterified with glycerol, forming triacylglycerol (fat) as
Citric the body's main fuel reserve. Acetyl-CoA formed by b-oxidation may undergo three fates : (i) It is oxidised to
acid CO2 + H2O via the citric acid cycle ; (ii) It is the precursor for synthesis of cholesterol and other steroids; (iii) In the
cycle 2H ® ATP liver, it is used to form ketone bodies (acetoacetate and 3- hydroxybutyrate) that are important fuels in
prolonged fasting.
l The non-essential amino acids, which are supplied in the diet can also be formed from metabolic
2CO2
Fig.: Outline of pathways for the catabolism of dietary
intermediates by transamination using the amino nitrogen from other amino acids. After
carbohydrate, protein and fat deamination, amino nitrogen is excreted as urea and the carbon skeletons that remain
after transamination may : (i) be oxidised to CO2 via the citric acid cycle; (ii) be used
to synthesise glucose (gluconeogenesis) or; (iii) form ketone bodies
which may be oxidised or be used for synthesis of
fatty acids.
Integration of metabolic
pathways at tissue and organ level
l At tissue and organ level, the nature of substrates entering and Plasma proteins
metabolites leaving tissues and organs is defined.
l Amino acids and glucose resulting from digestion of proteins and carbohydrates,
Liver
respectively are absorbed via hepatic portal vein. Urea Protein
l Excess glucose is converted to glycogen (glycogenesis) or to fatty acids (lipogenesis) in
Amino acids
liver.
l In between the meals, glycogen is broken down to glucose (glycogenolysis) and non- Glucose CO2
carbohydrate metabolites (lactate, glycerol, etc.) are converted to glucose (gluconeogenesis) Amino acids
in liver. Glycogen
Protein
l Liver synthesises major plasma proteins and deaminates amino acids that are in excess, forming urea
which is transported to kidney and excreted. Urea Lactate Amino acids
l Skeletal muscles utilise glucose both aerobically forming CO2 and anaerobically forming lactate.
Alanine
l Lipids in the diet are hydrolysed to monoacylglycerols and fatty acids in the gut, packaged with
Erythrocytes Glucose
protein and secreted into the lymphatic system and then into the bloodstream as chylomicrons. It is phosphate
CO2
first metabolised by tissues that have lipoprotein lipase, which hydrolyses the triacylglycerol, Glucose
Kidney
Urine Glycogen
releasing fatty acids.
Diet
l The other major source of long-chain fatty acids is synthesi from carbohydrate (lipogenesis) in Glucose Carbohydrate
Protein Muscle
adipose tissue and the liver. Blood plasma Amino acids
l Adipose tissue triacylglycerol is hydrolysed (lipolysis) and the fatty acids are transported, Small intestine
bound to serum albumin; they are taken up by most tissues (but not brain or Fig.: Transport and fate of carbohydrate and amino acid substrates and metabolites
erythrocytes) and either esterified to triacylglycerols for storage or oxidised as a fuel.
Free fatty acids
l In the liver, triacylglycerol arising from lipogenesis, free fatty acids and
chylomicron remnants are secreted into the circulation in very low
Glucose CO2
density lipoprotein (VLDL) form. This triacylglycerol undergoes a Fatty Ketone
fate similar to that of chylomicrons. acids bodies
Est
l Partial oxidation of fatty acids in the liver leads to
erification
lysis
ketone body production (ketogenesis).
Lipo
Blood Triacyl-
glycerol
plasma
Liver
Ve lipop LDL)
CO2
ry
Glycogen Cytosol Lipoprotein
low rote
Fatty
lipase
(V
de in
AA Ribosome Protein acids
nsi
Est
ty
erification
olysis
Endoplasmic Lipoprotein
Glucose Reticulum Fatty
TG
Lip
Glucose acids Lipoprotein lipase Triacyl-
Ch
Pentose
Es t
glycerol
ylo
phosphate
e rification
olysis
mi
pathway Muscle
cro
Monoacyl
Lip
ns
Triacyl- glycerol
Triose phosphate Glycerol phosphate Triacylglycerol Fatty acids glycerol
Diet +
Adipose fatty Triacyl-
Triacyl- acids
Glycerol tissue glycerol
glycerol (TG)
Glycolysis
Small intestine
Fig.: Transport and fate of lipid substrates and metabolites
Lactate
Phosphoenolpyruvate
n
Pyruvate
io
at
Gluconeogenesis
xid
O
AA
Integration of metabolic
b-
is
s
ne
CO2
e
pathways at sub-cellular level
og
AA Pyruvate
Lip
Each cell organelle, (e.g., mitochondrion) or compartment (e.g.,
l
cytosol) has specific roles that form part of the sub-cellular pattern of
Ketone
Oxaloacetate Acetyl-CoA bodies metabolic pathways.
l Compartmentation of pathways in separate sub-cellular compartments or
organelles permits integration and regulation of metabolism. There is a central role
AA Fumarate AA Citrate of the mitochondrion, since it acts as the focus of carbohydrate, lipid and amino acid
Citric acid metabolism. It contains the respiratory chain and ATP synthase as well as the
cycle CO2
AA
enzymes of the citric acid cycle, b-oxidation of fatty acids and ketogenesis.
Succinyl-CoA a-Ketoglutarate AA l Glycolysis, the pentose phosphate pathway and fatty acid synthesis all occur in the
cytosol. In gluconeogenesis, substrates such as lactate and pyruvate, which are
formed in the cytosol, enter the mitochondrion to yield oxaloacetate as a
CO2 precursor for the synthesis of glucose in the cytosol.
l The membranes of the endoplasmic reticulum contain the
AA metabolism of one or more
essential amino acids. AA enzyme system for triacylglycerol synthesis and the
AA metabolism of one or more
Mitochondrion AA ribosomes are responsible for protein
non-essential amino acids. synthesis.
AA
Fig.: Overview of major metabolic pathways cell in a liver parenchymal cell
Das könnte Ihnen auch gefallen
- The Leukotrienes: Chemistry and BiologyVon EverandThe Leukotrienes: Chemistry and BiologyLawrence ChakrinNoch keine Bewertungen
- CNS PNS Autonomic Nervous Systems SNS PNS Receptors ResponsesDokument3 SeitenCNS PNS Autonomic Nervous Systems SNS PNS Receptors ResponsesKara Dawn MasonNoch keine Bewertungen
- Chapter 2 - ClassificationDokument59 SeitenChapter 2 - ClassificationgoodmushroomsoupNoch keine Bewertungen
- Biology - November 2017Dokument1 SeiteBiology - November 2017Rahique ShuaibNoch keine Bewertungen
- ACFrOgB4ugyviBu1XK4Lh1UX8Pt64wVQwa2 Exi6l8nAFhE Uu1QLN5OIKowfbqMbZ48dVUAL2yq7eDi4HBBlOHGoCWigzrxxjl305MoZfTvJxd54XHQjc6yi-YzD8c PDFDokument12 SeitenACFrOgB4ugyviBu1XK4Lh1UX8Pt64wVQwa2 Exi6l8nAFhE Uu1QLN5OIKowfbqMbZ48dVUAL2yq7eDi4HBBlOHGoCWigzrxxjl305MoZfTvJxd54XHQjc6yi-YzD8c PDFLpNoch keine Bewertungen
- Slide Drug DeliveryDokument16 SeitenSlide Drug DeliveryatikahNoch keine Bewertungen
- ProteinDokument39 SeitenProteinNICHOLE MOJELLO100% (2)
- Thallophytes 2Dokument32 SeitenThallophytes 2Starnley TemboNoch keine Bewertungen
- Quick Drug Recognition Guide for Nursing StudentsDokument6 SeitenQuick Drug Recognition Guide for Nursing StudentsGenNoch keine Bewertungen
- Living Things Run On BatteriesDokument35 SeitenLiving Things Run On BatteriesAzfar ZackNoch keine Bewertungen
- Biochemistry of Digestive SystemDokument55 SeitenBiochemistry of Digestive SystemSyam UnhasNoch keine Bewertungen
- The Cell Cycle: Powerpoint Lectures ForDokument42 SeitenThe Cell Cycle: Powerpoint Lectures ForWilson HindartoNoch keine Bewertungen
- Membrane Models: Gorter and Grendel's Membrane Theory (1920)Dokument5 SeitenMembrane Models: Gorter and Grendel's Membrane Theory (1920)Hafiz AhmadNoch keine Bewertungen
- Body System ChecklistDokument6 SeitenBody System Checklistapi-422967453Noch keine Bewertungen
- Biochemistry of Kidneys and UrineDokument18 SeitenBiochemistry of Kidneys and UrineAndrias PutriNoch keine Bewertungen
- Protein Notes PDFDokument38 SeitenProtein Notes PDFLUi Anne Mateo LatogNoch keine Bewertungen
- 6.1 Types of NutritionDokument11 Seiten6.1 Types of NutritionNoor Hidayah SambliNoch keine Bewertungen
- Lymphatic SystemDokument70 SeitenLymphatic SystemNang Maizana Megat Yahya100% (1)
- Chemistry & SAR of QuinazolinoneDokument6 SeitenChemistry & SAR of Quinazolinonebooksa2zNoch keine Bewertungen
- Amino Acids and ProteinsDokument1 SeiteAmino Acids and ProteinsQuang OngNoch keine Bewertungen
- BIO 103 Lecture 1Dokument25 SeitenBIO 103 Lecture 1Samiul Hasan Pranto100% (1)
- Plant Morphology and DiversityDokument10 SeitenPlant Morphology and Diversityalyssa mae antonioNoch keine Bewertungen
- Zoology NotesDokument42 SeitenZoology NotesNolour de los SantosNoch keine Bewertungen
- Chapter 3.2-Morphology and Physiology of BacteriaDokument97 SeitenChapter 3.2-Morphology and Physiology of BacteriaTwinkle Parmar100% (1)
- Biological+Macromolecules KalmaDokument40 SeitenBiological+Macromolecules Kalmajudy andradeNoch keine Bewertungen
- Bio Eoc Review PacketDokument29 SeitenBio Eoc Review Packetapi-267855902Noch keine Bewertungen
- Innate ImmunityDokument9 SeitenInnate Immunitynascha dumpNoch keine Bewertungen
- Cellular Respiration - Fermentation Review WorksheetDokument4 SeitenCellular Respiration - Fermentation Review WorksheeterikabeltranNoch keine Bewertungen
- 2.3 Photosynthesis 2.4 Organisms and Their Environment: AQA GCSE Additional Science BiologyDokument28 Seiten2.3 Photosynthesis 2.4 Organisms and Their Environment: AQA GCSE Additional Science BiologySteve BishopNoch keine Bewertungen
- Mar 2018 PDFDokument20 SeitenMar 2018 PDFdanicaNoch keine Bewertungen
- Human Respiratory SystemDokument80 SeitenHuman Respiratory SystemAj MirandaNoch keine Bewertungen
- Pharmacognosy: A Vital Contribution to ScienceDokument39 SeitenPharmacognosy: A Vital Contribution to ScienceWaseem Shabbir AhamadNoch keine Bewertungen
- Ligand Cell Signaling PathwaysDokument117 SeitenLigand Cell Signaling PathwaysShoaib MomenNoch keine Bewertungen
- Drug-Receptor Interactions AND Pharmacodynamics: Ramla KashifDokument36 SeitenDrug-Receptor Interactions AND Pharmacodynamics: Ramla KashifRamla KashifNoch keine Bewertungen
- Bahauddin Zakariya University (BZU) Multan Subject List For BA Private CandidateDokument8 SeitenBahauddin Zakariya University (BZU) Multan Subject List For BA Private CandidateAli ShahidNoch keine Bewertungen
- Carbs LehningerDokument9 SeitenCarbs LehningerElla BangalanNoch keine Bewertungen
- Invertebrate Zoology 5th EditionDokument2 SeitenInvertebrate Zoology 5th EditionAsad Asad100% (1)
- CHPT 4 ENZYMES Lecture Notes (Teacher)Dokument6 SeitenCHPT 4 ENZYMES Lecture Notes (Teacher)api-3728508100% (3)
- Integration of Metabolism Integration of MetabolismDokument10 SeitenIntegration of Metabolism Integration of MetabolismEdison LucianoNoch keine Bewertungen
- AVS 172 Reproductive Physiology: Amin Ahamdzadeh Department of Animal and Veterinary Science University of IdahoDokument41 SeitenAVS 172 Reproductive Physiology: Amin Ahamdzadeh Department of Animal and Veterinary Science University of IdahoCristina CarvalhoNoch keine Bewertungen
- CH 16Dokument45 SeitenCH 16odettereyesNoch keine Bewertungen
- Mr. Faisal's Guide to Biogeochemical CyclesDokument19 SeitenMr. Faisal's Guide to Biogeochemical CyclesMary Jean Asuncion BrocoyNoch keine Bewertungen
- Protein Chemistry: Dr. B.Divya Dharshini Department of BiochemistryDokument57 SeitenProtein Chemistry: Dr. B.Divya Dharshini Department of BiochemistryTasneem AhmedNoch keine Bewertungen
- Pure Biology CHP 8 Transport in HumansDokument94 SeitenPure Biology CHP 8 Transport in Humanshamsterish87% (15)
- Lectures 1 3 Handout For PrintingDokument43 SeitenLectures 1 3 Handout For Printingkriss Wong100% (2)
- Secondary Metabolism Building BlocksDokument11 SeitenSecondary Metabolism Building Blocksleanne_tan_4Noch keine Bewertungen
- CSIR-NET Lifes Sciences (LSA Gwalior) PDFDokument669 SeitenCSIR-NET Lifes Sciences (LSA Gwalior) PDFDr-Priyanka AgarwalNoch keine Bewertungen
- Role of TapetumDokument7 SeitenRole of TapetumPreeti ThakurNoch keine Bewertungen
- Inorganic and Organometallic Reaction MechanismsDokument15 SeitenInorganic and Organometallic Reaction MechanismsKartikeya Singh0% (1)
- Enzymes: Protein Catalysts That Increase The Rate of Reactions Without Being Changed in The Overall ProcessDokument49 SeitenEnzymes: Protein Catalysts That Increase The Rate of Reactions Without Being Changed in The Overall ProcessGhafoor AzamNoch keine Bewertungen
- Functions of Plasma ProteinsDokument10 SeitenFunctions of Plasma ProteinsGaelle Lisette MacatangayNoch keine Bewertungen
- D Pharmacy 1st Year B Pharm First Sem - Anatomy & Physiology Notes - Solved Question PaperDokument28 SeitenD Pharmacy 1st Year B Pharm First Sem - Anatomy & Physiology Notes - Solved Question Papersham100% (2)
- Digestive System NotesDokument10 SeitenDigestive System NotesArchanna VyassNoch keine Bewertungen
- Intermediate FilamentsDokument4 SeitenIntermediate FilamentsSai SridharNoch keine Bewertungen
- Zoology Notes: 004 Chapter 1Dokument9 SeitenZoology Notes: 004 Chapter 1humanupgrade100% (1)
- Medicinal Chemistry I: 4 Year, 7 SemesterDokument58 SeitenMedicinal Chemistry I: 4 Year, 7 SemesterKhan NehalNoch keine Bewertungen
- CephalopodDokument14 SeitenCephalopodAnirudh AcharyaNoch keine Bewertungen
- Medicinal Chemistry—III: Main Lectures Presented at the Third International Symposium on Medicinal ChemistryVon EverandMedicinal Chemistry—III: Main Lectures Presented at the Third International Symposium on Medicinal ChemistryP. PratesiNoch keine Bewertungen
- Biology - October 2016Dokument1 SeiteBiology - October 2016Rahique ShuaibNoch keine Bewertungen
- Lecture 1 Introduction To The TrunkDokument28 SeitenLecture 1 Introduction To The TrunkRahique ShuaibNoch keine Bewertungen
- Lecture 1 Introduction To The TrunkDokument28 SeitenLecture 1 Introduction To The TrunkRahique ShuaibNoch keine Bewertungen
- Nums FLPDokument27 SeitenNums FLPRahique ShuaibNoch keine Bewertungen
- Physics - April 2018Dokument1 SeitePhysics - April 2018Rahique ShuaibNoch keine Bewertungen
- Biology - September 2017Dokument1 SeiteBiology - September 2017Rahique ShuaibNoch keine Bewertungen
- Biology - November 2016Dokument1 SeiteBiology - November 2016Rahique ShuaibNoch keine Bewertungen
- Biology - May 2015Dokument1 SeiteBiology - May 2015Rahique ShuaibNoch keine Bewertungen
- Biology - September 2016Dokument1 SeiteBiology - September 2016Rahique Shuaib0% (1)
- Biology - May 2017Dokument1 SeiteBiology - May 2017Rahique ShuaibNoch keine Bewertungen
- Biology - September 2015Dokument1 SeiteBiology - September 2015Rahique ShuaibNoch keine Bewertungen
- Biology - October 2017Dokument1 SeiteBiology - October 2017Rahique ShuaibNoch keine Bewertungen
- Biology - May 2016Dokument1 SeiteBiology - May 2016Rahique ShuaibNoch keine Bewertungen
- Biology - May 2014Dokument1 SeiteBiology - May 2014Rahique ShuaibNoch keine Bewertungen
- Biology - November 2015Dokument1 SeiteBiology - November 2015Rahique ShuaibNoch keine Bewertungen
- Biology - October 2015Dokument1 SeiteBiology - October 2015Rahique ShuaibNoch keine Bewertungen
- Chemistry - May 2016Dokument1 SeiteChemistry - May 2016Rahique ShuaibNoch keine Bewertungen
- Biology - May 2018Dokument1 SeiteBiology - May 2018Rahique ShuaibNoch keine Bewertungen
- Biology - March 2017Dokument1 SeiteBiology - March 2017Rahique ShuaibNoch keine Bewertungen
- Biology - March 2016Dokument1 SeiteBiology - March 2016Rahique ShuaibNoch keine Bewertungen
- Biology - March 2015Dokument1 SeiteBiology - March 2015Rahique ShuaibNoch keine Bewertungen
- Chemistry - November 2017Dokument1 SeiteChemistry - November 2017Rahique ShuaibNoch keine Bewertungen
- Biology - March 2018Dokument1 SeiteBiology - March 2018Rahique ShuaibNoch keine Bewertungen
- Alicyclic Hydrocarbons + Solid StatesDokument1 SeiteAlicyclic Hydrocarbons + Solid StatesSantanuNoch keine Bewertungen
- ESSENTIALS OF CHEMISTRYDokument1 SeiteESSENTIALS OF CHEMISTRYRahique ShuaibNoch keine Bewertungen
- Chemistry - November 2015Dokument1 SeiteChemistry - November 2015Rahique ShuaibNoch keine Bewertungen
- Chemistry - July 2015Dokument1 SeiteChemistry - July 2015Rahique ShuaibNoch keine Bewertungen
- Chemistry - June 2015Dokument1 SeiteChemistry - June 2015Rahique ShuaibNoch keine Bewertungen
- Chemistry - March 2016Dokument1 SeiteChemistry - March 2016Rahique ShuaibNoch keine Bewertungen
- Pressure Vacuum Valves PDFDokument5 SeitenPressure Vacuum Valves PDFFelipe Correa MahechaNoch keine Bewertungen
- Char-Lynn: Parts InformationDokument4 SeitenChar-Lynn: Parts InformationCarolina López floresNoch keine Bewertungen
- Modern AGE - Companion (GRR6304)Dokument130 SeitenModern AGE - Companion (GRR6304)Kárpáti Géza83% (6)
- A4 - in The Dungeons of The Slave LordsDokument36 SeitenA4 - in The Dungeons of The Slave LordsSturm75% (4)
- Sportaccord, "Sport Betting and Corruption"Dokument94 SeitenSportaccord, "Sport Betting and Corruption"Tifoso BilanciatoNoch keine Bewertungen
- Chapter 5 RotationDokument24 SeitenChapter 5 RotationCHERRY SABASAJENoch keine Bewertungen
- Cel Animation HandoutDokument2 SeitenCel Animation Handoutapi-475274369Noch keine Bewertungen
- Star Ocean 3Dokument9 SeitenStar Ocean 3Erwan YuniardiNoch keine Bewertungen
- Jf016e Jf017e 1 PDFDokument4 SeitenJf016e Jf017e 1 PDFValdir GomieroNoch keine Bewertungen
- Mini Project ReportDokument20 SeitenMini Project ReportBhumika MittapelliNoch keine Bewertungen
- Chess Pseudonyms PDFDokument31 SeitenChess Pseudonyms PDFdon dadaNoch keine Bewertungen
- Volvo d12d Valve AdjustmentDokument7 SeitenVolvo d12d Valve Adjustmentali78% (9)
- Hyper Acc Dragon PDFDokument387 SeitenHyper Acc Dragon PDFmikheil darsavelidze100% (1)
- Chiller Room Chiller Room: Entry Mep Room Entry Mep RoomDokument1 SeiteChiller Room Chiller Room: Entry Mep Room Entry Mep RoomBertha Vinola ChrisanNoch keine Bewertungen
- Everyday To Athlete Nutrition Excel SheetDokument102 SeitenEveryday To Athlete Nutrition Excel SheetmwflusNoch keine Bewertungen
- Surgery - Dychioco - Musculo Skeletal InjuriesDokument79 SeitenSurgery - Dychioco - Musculo Skeletal Injuries3rd yrsNoch keine Bewertungen
- Topic 6 Test 2022Dokument15 SeitenTopic 6 Test 2022Ahmad OmarNoch keine Bewertungen
- Ion ChannelsDokument3 SeitenIon ChannelsMizrab NadeemNoch keine Bewertungen
- The Flower Guarding Bells: Gu LongDokument1.435 SeitenThe Flower Guarding Bells: Gu LongChadalavada Venkata Sai PradeepNoch keine Bewertungen
- Valentine Angel MelanieDokument40 SeitenValentine Angel MelanieSarita E Schz100% (6)
- Planos Electricos T-6Dokument26 SeitenPlanos Electricos T-6jrgonzales24Noch keine Bewertungen
- 3rd IFK U-18 World Tournament WelcomeDokument50 Seiten3rd IFK U-18 World Tournament WelcomeBudo MediaNoch keine Bewertungen
- History of Table TennisDokument2 SeitenHistory of Table TennisGenesis Luigi MagasoNoch keine Bewertungen
- The Runaway PancakeDokument15 SeitenThe Runaway PancakeSean ChangNoch keine Bewertungen
- TGfU ConceptDokument11 SeitenTGfU ConceptRohizamNoch keine Bewertungen
- Narrative Report On The Training of Basketball Coaches and OfficiatingDokument2 SeitenNarrative Report On The Training of Basketball Coaches and Officiating100608Noch keine Bewertungen
- UNit 3 & 4 QuestionsDokument24 SeitenUNit 3 & 4 QuestionsPiyush BhandariNoch keine Bewertungen
- Hand Lasting ProcessDokument8 SeitenHand Lasting ProcessmanojNoch keine Bewertungen
- The Muscular System ExplainedDokument22 SeitenThe Muscular System ExplainedFiraol DiribaNoch keine Bewertungen
- Traditional GamesDokument7 SeitenTraditional GamesAngel Marie CahanapNoch keine Bewertungen