Beruflich Dokumente
Kultur Dokumente
Malignant Parotid Tumors: Introduction and Anatomy
Hochgeladen von
CeriaindriasariOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Malignant Parotid Tumors: Introduction and Anatomy
Hochgeladen von
CeriaindriasariCopyright:
Verfügbare Formate
This site is intended for healthcare professionals
Drugs & Diseases > Plastic Surgery
Malignant Parotid Tumors
Updated: May 18, 2017 | Author: Bardia Amirlak, MD; Chief
Editor: Deepak Narayan, MD, FRCS more...
SECTIONS
Introduction and Anatomy
The parotid glands are the largest salivary glands in
humans and are frequently involved in disease
processes. Approximately 25% of parotid masses are
nonneoplastic; the remaining 75% are neoplastic.
Nonneoplastic causes of parotid enlargement
include cysts, parotitis, lymphoepithelial lesions
associated with AIDS, collagen vascular diseases,
and benign hypertrophy. Benign hypertrophy is
encountered in patients with bulimia, sarcoidosis,
sialosis, actinomycosis infections, and mycobacterial
infections. The vast majority (approximately 80%) of
parotid neoplasms are benign; these are discussed
in detail in the Medscape Drugs & Diseases article
Benign Parotid Tumors.
The paired parotid glands are formed as epithelial
invaginations into the embryological mesoderm and
first appear at approximately 6 weeks gestation. The
glands are roughly pyramidal in shape, with the main
body overlying the masseter muscle.
The glands extend to the zygomatic process and
mastoid tip of the temporal bone and curve around
the angle of the mandible to extend to the
retromandibular and parapharyngeal spaces. The
parotid duct exits the gland medially, crosses the
superficial border of the masseter, pierces the
buccinator, and enters the oral cavity through the
buccal mucosa opposite the second maxillary molar.
The gland is divided into a superficial and deep
portion by the facial nerve, which passes through the
gland. While not truly anatomically discrete, these
"lobes" are important surgically, as neoplasms
involving the deep lobe require sometimes
significant manipulation of the facial nerve to allow
excision. The superficial lobe is the larger of the two
and thereby the location of the majority of parotid
tumors.
The facial nerve exits the cranium via the
stylomastoid foramen and courses through the
substance of the parotid gland. The superficial lobe
of the parotid lies superficial or lateral to the facial
nerve, whereas the deep lobe is deep or medial to
the facial nerve. The facial nerve branches within the
substance of the parotid gland, and the branching
pattern can be highly variable. The main trunk
typically bifurcates in to the zygomaticotemporal
branch and the cervicofacial branch at the pes
anserinus, also known as the goose’s foot (see
images below), and thereafter into the temporal,
zygomatic, buccal, marginal, and cervical branches.
Pes is about 1.3 cm from the stylomastoid foramen.
Extensive anastomoses are usually present between
branches of the zygomatic and buccal branches of
the nerve.
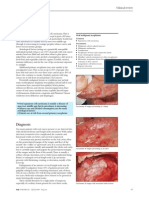
The (Z) zygomaticotemporal branch and the (C)
cervicofacial branch of the facial nerve are
dissected out during resection of a parotid
tumor. The pes (goose's foot) is visible in this
photograph.
View Media Gallery
The surgical anatomy and landmarks of the facial
nerve.
View Media Gallery
Numerous lymph nodes also are present within the
parotid gland itself, subsequently draining to
preauricular, infra-auricular, and deep upper jugular
nodes.
Diagnosis
Evaluation of a patient with a suspected parotid
gland malignancy must begin with a thorough
medical history and physical examination.
The most common presentation is a painless,
asymptomatic mass; >80% of patients present
because of a mass in the posterior cheek region.
Approximately 30% of patients describe pain
associated with the mass, though most parotid
malignancies are painless. Pain most likely indicates
perineural invasion, which greatly increases the
likelihood of malignancy in a patient with a parotid
mass.
Of patients with malignant parotid tumors, 7-20%
present with facial nerve weakness or paralysis,
which almost never accompanies benign lesions and
indicates a poor prognosis. Approximately 80% of
patients with facial nerve paralysis have nodal
metastasis at the time of diagnosis. These patients
have an average survival of 2.7 years and a 10-year
survival of 14-26%.
Other important aspects of the history include length
of time the mass has been present and history of
prior cutaneous lesion or parotid lesion excision.
Slow-growing masses of long-standing duration tend
to be benign. A history of prior squamous cell
carcinoma, malignant melanoma, or malignant
fibrous histiocytoma suggests intraglandular
metastasis or metastasis to parotid lymph nodes.
Prior parotid tumor most likely indicates a recurrence
because of inadequate initial resection.
Trismus often indicates advanced disease with
extension into the masticatory muscles or, less
commonly, invasion of the temporomandibular joint.
Dysphagia or a sensation of a foreign body in the
oropharynx indicates a tumor of the deep lobe of the
gland. A report of ear pain may indicate extension of
the tumor into the auditory canal. The presence of
numbness in the distribution of the second or third
divisions of the trigeminal nerve often indicates
neural invasion.
Physical examination of the head and neck must be
thorough and complete. The entire head and neck
must be examined for cutaneous lesions, which may
represent malignancies that could metastasize to the
parotid gland or parotid nodes.
Palpation of the mass should determine the
degree of firmness. Even benign tumors are
usually firm, but a rock-hard mass generally
denotes malignancy.
Skin fixation, skin ulceration, or fixation to
adjacent structures also indicates malignancy.
The external auditory canal must be visualized
for tumor extension.
All regional nodes must be carefully palpated to
detect nodal metastasis. Examination of the oral
cavity and oropharynx also may yield further
evidence of metastasis or malignant nature of
the lesion.
Blood or pus from the Stenson duct is a sign of
malignancy but is infrequently encountered.
More often, one may see bulging of the lateral
pharyngeal wall or soft palate, indicating tumor
in the deep lobe of the gland.
Bimanual palpation with one finger against the
lateral pharyngeal wall and the other against
the external neck may confirm extent into the
tonsillar fossa and soft palate.
Once a thorough history and physical examination
are complete, perform diagnostic procedures to
confirm the diagnosis and extent of the disease
process.
Fine needle aspiration
See the list below:
Fine needle aspiration of the mass or an
enlarged lymph node may be performed to
obtain a tissue diagnosis. [1] Most surgeons
recommend excision of a parotid mass whether
it is benign or malignant unless a patient's
comorbidity precludes safe surgery. As such,
many surgeons do not routinely perform
cytology before proceeding with surgery.
The sensitivity of this procedure is greater than
95% in experienced hands. However, only a
positive diagnosis should be accepted;
negative results indicate the need for further
attempts at obtaining a histologic diagnosis,
including repeat fine needle aspiration.
The results of the fine needle aspiration provide
a histologic diagnosis and assist in preoperative
planning and patient counseling. It may not
distinguish benign from malignant epithelial
lesions because malignancy of parotid epithelial
cells is related to the behavior of the tumor cells
in relation to tissue planes and surrounding
structures rather than cellular architecture,
which may be rather normal even in malignancy.
Therefore, nonepithelial lesions may be
diagnosed with accuracy, but epithelial lesions
may require further investigation.
If fine needle aspiration is unsuccessful in
obtaining a diagnosis, an incisional biopsy
should not be performed. This procedure has a
high rate of local recurrence and places the
facial nerve at risk for injury from inadequate
visualization.
Some authors advocate large core needle
biopsies, but this procedure is less popular
because of potential facial nerve injury and the
possibility of seeding the needle tract with
tumor cells.
If a core biopsy is performed, the needle should
be inserted so that the tract may be excised
during the definitive operation. When all
attempts at obtaining a histologic diagnosis
have failed, operative exploration should
proceed after appropriate imaging studies have
been obtained.
Intraoperatively, a frozen section of the
specimen should be submitted for diagnosis.
The use of frozen sections has demonstrated
greater than 93% accuracy in the diagnosis of
parotid malignancy.
Imaging studies
Imaging studies may be helpful in staging and for
surgical planning. Sialography may help to
differentiate inflammatory versus neoplastic
processes, but this test is infrequently performed and
is of limited value in the evaluation of parotid
masses. It is mentioned herein for historic interest
only.
Sonography may be very useful. Benign lesions are
of lower density and have smaller caliber blood
vessels. However, determination of a cystic
component may be misleading, because cystic
degeneration may occur as a result of necrosis at the
avascular center of a malignancy.
Computed tomography (CT) scanning and magnetic
resonance imaging (MRI) [2] can be valuable for
evaluation of parotid malignancies. CT scanning
provides better detail of the surrounding tissues,
whereas MRI demonstrates the mass in greater
contrast than a CT scan.
These imaging studies may identify regional lymph
node involvement or extension of the tumor into the
deep lobe or parapharyngeal space. CT scan criteria
for lymph node metastasis include any lymph node
larger than 1-1.5 cm in greatest diameter, multiple
enlarged nodes, and nodes displaying central
necrosis.
Lymph nodes harboring metastasis also may appear
round rather than the normal kidney bean shape, and
evidence of extracapsular extension may be
identified.
A study by Mamlouk et al of pediatric patients with
parotid neoplasms indicated that on MRI scans, the
presence of a hypointense T2 signal, restricted
diffusion, poorly defined borders, and focal necrosis
are suggestive of malignancy, although not specific
for it. The study involved 17 patients, including 11 with
malignant tumors and six with benign neoplasms. [3]
For more information on imaging studies for
malignant parotid tumors, see Medscape Drugs &
Diseases article Malignant Parotid Tumor Imaging.
Pathology
Many types of parotid malignancies exist, most
arising from the epithelial elements of the gland. [4, 5,
6, 7, 8] Classification of these tumors can be quite
confusing. In addition, malignancy may develop in
the secretory element of the gland or malignancy
arising elsewhere may first be noticed as a
metastasis to the gland.
Mucoepidermoid carcinoma
Mucoepidermoid carcinoma is the most common
malignant tumor of the parotid gland, accounting for
30% of parotid malignancies. [9, 10]
Three cell types are found in varying proportions:
mucous, intermediate, and epidermoid cells. High-
grade tumors exhibit cytologic atypia, higher mitotic
frequency, areas of necrosis and more epidermoid
cells. High-grade tumors behave like a squamous
cell carcinoma; low-grade tumors often behave
similar to a benign lesion. [11]
Limited local invasiveness and low metastatic
potential characterize this tumor, particularly when
cytologically low-grade. If metastatic, it is most likely
to metastasize to regional nodal basins rather than to
distant locations.
For patients with low-grade tumors without nodal or
distant metastasis, 5-year survival is 75-95%,
whereas patients with high-grade tumors with lymph
node metastasis at the time of diagnosis have a 5-
year survival of only 5%. Overall 10-year survival is
50%.
Differential diagnosis includes chronic sialoadenitis,
necrotizing sialometaplasia, and other carcinomas.
An association has been reported between
mucoepidermoid carcinoma and myasthenia gravis.
[12]
Adenoid cystic carcinoma
The adenoid cystic carcinoma is characterized by its
unpredictable behavior and propensity to spread
along nerves. It possesses a highly invasive quality
but may remain quiescent for a long time.
This tumor may be present for more than 10 years
and demonstrate little change and then suddenly
infiltrate the adjacent tissues extensively.
The tumor has an affinity for growth along perineural
planes and may demonstrate skip lesions along
involved nerves. Clear margins do not necessarily
mean that the tumor has been eradicated.
Metastasis is more common to distant sites than to
regional nodes; lung metastases are most frequent.
This tumor has the highest incidence of distant
metastasis, occurring in 30-50% of patients.
Three histologic types have been identified: cribrose,
tubular, and solid. The solid form has the worst
prognosis; the cribrose pattern possesses the most
benign behavior and best prognosis. This tumor
requires aggressive initial resection. Overall 5-year
survival is 35%, and 10-year survival is approximately
20%.
Malignant mixed tumors
Malignant mixed tumors arise most commonly as a
focus of malignant degeneration within a preexisting
benign pleomorphic adenoma (carcinoma ex
pleomorphic adenoma).
These tumors also may develop de novo
(carcinosarcoma). The longer pleomorphic adenoma
has been present, the greater the chance of
carcinomatous degeneration.
Carcinosarcomas, true malignant mixed tumors, are
rare. Overall 5-year survival is 56%, and 10-year
survival is 31%.
Acinic cell carcinoma
Acinic cell carcinoma is an intermediate-grade
malignancy with low malignant potential. This tumor
may be bilateral or multicentric and is usually solid,
rarely cystic.
Although this tumor rarely metastasizes, occasional
late distant metastases have been observed. This
tumor also may spread along perineural planes.
Overall 5-year survival is 82%, and 10-year survival is
68%.
Adenocarcinoma
Adenocarcinoma of the parotid develops from the
secretory element of the gland. This is an aggressive
lesion with potential for both local lymphatic and
distant metastases.
Approximately 33% of patients have nodal or distant
metastasis present at the time of initial diagnosis.
Overall 5-year survival is 19-75%, as it is highly
variable and related to grade and stage at
presentation.
A study by Zhan and Lentsch of basal cell
adenocarcinoma of the major salivary glands (509
cases) found that 88% of tumors were in the parotid
glands, with 11.2% in the submandibular glands and
0.8% being sublingual gland lesions. Overall 5- and
10-year survival rates were 79% and 62%,
respectively, while regional and distant metastases
occurred in just 11.9% and 1.8% of cases, respectively.
Older age (65 years or older) and high primary tumor
stage had a significant negative impact on survival; in
patients with a high tumor stage, the survival rate
was significantly better with a combination of surgery
and radiation therapy than with surgery alone. [13]
Primary squamous cell carcinoma
Primary squamous cell carcinoma of the parotid is
rare, and metastasis from other sites must be
excluded. Overall 5-year survival is 21-55%, and 10-
year survival is 10-15%.
Sebaceous carcinoma
Sebaceous carcinoma is a rare parotid malignancy
that often presents as a painful mass. It commonly
involves the overlying skin.
Salivary duct carcinoma
Salivary duct carcinoma is a rare and highly
aggressive tumor. Small cell carcinoma exists as 2
types. The ductal cell origin type is mostly benign
and rarely metastasizes. The neuroendocrine origin
type is often aggressive and has higher metastatic
potential.
Lymphoma
The parotid gland also may be the site of occurrence
of lymphoma, most commonly in elderly males. This
is also observed in approximately 5-10% of patients
with Warthin tumor of the parotid gland, a benign
neoplasm. [14]
The entire parotid is typically enlarged with a rubbery
consistency on palpation. Often, regional nodes also
are enlarged. Biopsy of enlarged regional nodes
avoids unnecessary parotid surgery, as the definitive
treatment consists of chemotherapy or radiation
therapy.
Malignant fibrohistiocytoma
Malignant fibrohistiocytoma is very rare in the parotid
gland. It presents as a slow growing and painless
mass.
Fine needle aspiration and imaging could confuse
this lesion with other kinds of parotid tumors;
therefore, definite diagnosis should be based on
immunohistochemical analysis of the resected tumor.
The tumor should be completely resected. [15]
Parotid metastasis from other sites
The parotid also may be the site of metastasis from
cutaneous, renal, lung, breast, prostate, or GI tract
malignancies.
Operative Management
Generally, therapy for parotid malignancy is complete
surgical resection followed, when indicated, by
radiation therapy. [16] Conservative excisions are
plagued by a high rate of local recurrence. The
extent of resection is based on tumor histology,
tumor size and location, invasion of local structures,
and the status of regional nodal basins.
Most tumors of the parotid (approximately 90%)
originate in the superficial lobe. Superficial parotid
lobectomy is the minimum operation performed in
this situation. This procedure is appropriate for
malignancies confined to the superficial lobe, those
that are low grade, those less than 4 cm in greatest
diameter, tumors without local invasion, and those
without evidence of regional node involvement.
Surgical resection procedure
The most important initial step is identification of the
facial nerve and its course through the substance of
the parotid gland. In order to preserve the facial
nerve, it is important to try to determine the proximity
of the nerve to the capsule of the tumor prior to
surgery. Results of a retrospective review showed
that malignant tumors were likely to have a positive
facial nerve margin. [17] Virtually all surgeons avoid
using paralytic agents, and, to assist finding the
nerve, many surgeons use a nerve stimulator.
Increasingly, surgeons are using intraoperative
continuous facial nerve monitoring any time a
parotidectomy is performed. This is not usually
necessary in the primary setting, but recurrent
resections may be very difficult and probably should
be performed using this device.
Ideally, the dissection of the facial nerve should
be performed without disturbing or violating the
tumor. The facial nerve may be found exiting
the stylomastoid foramen by reflecting the
parotid gland anteriorly and the
sternocleidomastoid muscle posteriorly.
Landmarks include the digastric ridge and the
tympanomastoid suture. Knowledge of the
relationships among these structures allows
more efficient and reproducible identification of
the nerve.
The cartilaginous external auditory canal lies
approximately 5 mm superior to the facial nerve
in this region. The facial nerve is also anterior to
the posterior belly of the digastric muscle and
external to the styloid process.
A second technique for locating the facial nerve
is to identify a distal branch of the nerve and to
dissect retrograde toward the main trunk. This
technique may be more difficult depending on
the ease of identifying the branching pattern. To
perform this maneuver, the buccal branch may
be found just superior to the parotid duct, or the
marginal mandibular branch may be found
crossing over (superficial to) the facial vessels.
These may then be traced back to the origins of
the main facial nerve trunks.
A final way of identifying the nerve in
particularly difficult situations is to drill the
mastoid and to locate the nerve within the
temporal bone. It may then be followed through
the stylomastoid foramen antegrade towards
the parotid.
Once these have been identified, the superficial
lobe of the parotid gland may be removed en
bloc and sent to the pathology laboratory.
If the immediate intraoperative pathologic
examination reveals that the tumor is actually
high-grade or >4 cm in greatest diameter, or
lymph node metastasis is identified within the
specimen, a complete total parotidectomy
should be performed.
If the facial nerve or its branches are adherent
to or directly involved by the tumor, they must
be sacrificed. However, a pathologic diagnosis
of malignancy must be confirmed
intraoperatively prior to sacrificing facial nerve
branches.
All involved local structures should be resected
in continuity with the tumor. This may include
skin, masseter, mandible, temporalis, zygomatic
arch, or temporal bone.
Tumors of the deep lobe are treated by total
parotidectomy. Identification of the facial nerves
and branches is the first and most crucial step.
Total parotidectomy is then performed en bloc,
and the fate of the facial nerve and surrounding
local structures must be decided similar to
superficial lobe tumors. The specimen should
be sent to the pathology laboratory for
immediate examination.
Neck dissection should be performed when
malignancy is detected in the lymph nodes pre-
or intraoperatively.
Other indications for functional neck dissection
include tumors >4 cm in greatest diameter,
tumors that are high-grade, tumors that have
invaded local structures, recurrent tumors when
no neck dissection was performed initially, and
deep lobe tumors.
These recommendations are based on the
higher likelihood of occult, clinically
undetectable nodal disease present at the time
of operation in patients whose tumors display
the above characteristics.
Reconstruction
Following resection of the tumor specimen, most
wounds can be closed primarily. However, the
presence of extension of the tumor to the overlying
skin or surrounding structures may require
reconstructive procedures. The overall goal following
tumor excision is to restore function and achieve the
best possible aesthetic result.
Options for wound closure in the presence of a skin
or soft tissue deficit include skin grafting,
cervicofacial flap, trapezius flap, pectoralis flap,
deltopectoral flap, and microvascular free flap. For
information on various flap procedures, see the Flaps
section of the Medscape Drugs & Diseases Plastic
Surgery journal.
Sacrifice of the facial nerve or one of its branches
also must be managed appropriately. If inadvertently
severed during the operation, the facial nerve should
be immediately repaired under the operating
microscope. If intentionally resected with the tumor
specimen, several options for reconstruction are
available to the surgeon.
The ipsilateral or contralateral great auricular
nerve may be used as an interposition graft,
although this sacrifices sensation to the area
normally supplied by this nerve.
Another option is to anastomose the facial
nerve to the ipsilateral hypoglossal nerve. This
anastomosis may be performed end-to-side to
avoid interfering with normal hypoglossal nerve
function.
During the period of waiting for facial nerve
recovery, maintain corneal protection if the
innervation to the orbicularis oculi has been
interrupted.
Measures include taping the eye closed at night
over ophthalmic ointment and frequent use of
wetting drops during the day. Some authors
recommend a moisture chamber.
If facial nerve recovery is not achieved, certain
measures may be taken to improve form and
function.
A gold weight (0.8-1.2 g) may be inserted in the
upper eyelid to assist with closure. Dynamic
slings of temporalis muscle to the upper and
lower lids and corner of the mouth or masseter
sling to the mouth have proven very successful
in the reconstruction of these patients. Static
slings also have been used and include fascia
lata, tendon, and Mitek anchors.
Following parotidectomy, some patients
develop gustatory sweating or Frey syndrome.
[18] This denotes an aberrant connection of
regenerating parasympathetic salivary fibers to
the sweat glands in the overlying skin flap.
Treatment of this condition has included
irradiation, atropinelike creams, division of the
auriculotemporal nerve (sensory), division of the
glossopharyngeal nerve (parasympathetic),
insertion of synthetic materials (AlloDerm),
fascial grafts, or vascularized tissue flaps
between the parotid bed and overlying skin
flap. Intracutaneous injections of botulinum
toxin A is also an attractive option which has
showed some promise.
Finally, neurovascular free tissue transfer has been
described for facial reanimation for treatment of
established facial paralysis following ablative parotid
surgery. [19]
Vascularized nerve grafts, such as sural nerve
graft, have been described to reestablish facial
nerve continuity.
Functional free muscle transfer with gracilis,
pectoralis minor, or latissimus dorsi muscles are
further options for reconstruction. The ipsilateral
facial nerve stump may be used as the recipient
nerve.
Alternatively, cross facial nerve grafting can be
performed. This is typically performed as a 2-
stage surgery, with anastomosis to a nerve graft
as the first stage and free tissue transfer as the
second stage.
For more information on facial nerve reconstruction
and the treatment of facial nerve paralysis, see the
Medscape Drugs & Diseases articles Facial Nerve
Paralysis, Dynamic Reconstruction for Facial Nerve
Paralysis, and Static Reconstruction for Facial Nerve
Paralysis.
Adjunctive Therapy
Das könnte Ihnen auch gefallen
- Salivary Gland Cancer: From Diagnosis to Tailored TreatmentVon EverandSalivary Gland Cancer: From Diagnosis to Tailored TreatmentLisa LicitraNoch keine Bewertungen
- The Surgical Oncology Review: For the Absite and BoardsVon EverandThe Surgical Oncology Review: For the Absite and BoardsNoch keine Bewertungen
- 6 Neck DissectionDokument9 Seiten6 Neck DissectionAnne MarieNoch keine Bewertungen
- SurgeryDokument47 SeitenSurgerymohamed muhsinNoch keine Bewertungen
- Parotid MassDokument9 SeitenParotid MassAndre Eka Putra PrakosaNoch keine Bewertungen
- Solitary Nodules Are Most Likely To Be Malignant in Patients Older Than 60 Years and in Patients Younger Than 30 YearsDokument5 SeitenSolitary Nodules Are Most Likely To Be Malignant in Patients Older Than 60 Years and in Patients Younger Than 30 YearsAbdurrahman Afa HaridhiNoch keine Bewertungen
- Jurnal Patologi AnatomiDokument5 SeitenJurnal Patologi Anatomiafiqzakieilhami11Noch keine Bewertungen
- Grand Rounds Index UTMB Otolaryngology Home PageDokument9 SeitenGrand Rounds Index UTMB Otolaryngology Home PageandiNoch keine Bewertungen
- Parotidectomy StatPearls NCBIBookshelfDokument16 SeitenParotidectomy StatPearls NCBIBookshelfshehla khanNoch keine Bewertungen
- 50315-72475-1-PB (1) Parotid TumorDokument3 Seiten50315-72475-1-PB (1) Parotid TumorShashank MisraNoch keine Bewertungen
- Recurrent Salivary Gland CancerDokument13 SeitenRecurrent Salivary Gland CancerestantevirtualdosmeuslivrosNoch keine Bewertungen
- Biopsy in Oral SurgeryDokument45 SeitenBiopsy in Oral SurgeryMahamoud IsmailNoch keine Bewertungen
- Salivary Gland MalignanciesDokument13 SeitenSalivary Gland MalignanciesestantevirtualdosmeuslivrosNoch keine Bewertungen
- Hemangiopericytoma of Palate A Rare Case ReportDokument4 SeitenHemangiopericytoma of Palate A Rare Case ReportIJAR JOURNALNoch keine Bewertungen
- 2006 89 Canine and Feline Nasal NeoplasiaDokument6 Seiten2006 89 Canine and Feline Nasal NeoplasiaFelipe Guajardo HeitzerNoch keine Bewertungen
- Normal Mediastinal Anatomy, Pathologies and Diagnostic MethodsDokument9 SeitenNormal Mediastinal Anatomy, Pathologies and Diagnostic MethodsJuma AwarNoch keine Bewertungen
- Adult Neck MassesDokument7 SeitenAdult Neck MassesHanhan90Noch keine Bewertungen
- Solitary Pulmonary Nodule (SPN (Dokument59 SeitenSolitary Pulmonary Nodule (SPN (mahmod omerNoch keine Bewertungen
- August 2013 Ophthalmic PearlsDokument3 SeitenAugust 2013 Ophthalmic PearlsEdi Saputra SNoch keine Bewertungen
- ABC - Oral CancerDokument4 SeitenABC - Oral Cancerdewishinta12Noch keine Bewertungen
- Juvenile Nasopharyngeal Angiofibroma (JNA)Dokument19 SeitenJuvenile Nasopharyngeal Angiofibroma (JNA)YogiHadityaNoch keine Bewertungen
- Principles of Management of Soft Tissue SarcomaDokument33 SeitenPrinciples of Management of Soft Tissue Sarcomabashiruaminu100% (1)
- Cerebellopontine Angle TumoursDokument11 SeitenCerebellopontine Angle TumoursIsmail Sholeh Bahrun MakkaratteNoch keine Bewertungen
- Chapter 146. Management of The Neck in Head and Neck Cancer: David E EiblingDokument3 SeitenChapter 146. Management of The Neck in Head and Neck Cancer: David E EiblingYasin KulaksızNoch keine Bewertungen
- Article Imaging of Salivary Gland TumoursDokument11 SeitenArticle Imaging of Salivary Gland TumourskaryndpNoch keine Bewertungen
- Soft Tissue Tumors of The Neck: David E. Webb, DDS, Brent B. Ward, DDS, MDDokument15 SeitenSoft Tissue Tumors of The Neck: David E. Webb, DDS, Brent B. Ward, DDS, MDCynthia BarrientosNoch keine Bewertungen
- Practice Essentials: View Media GalleryDokument8 SeitenPractice Essentials: View Media GallerywzlNoch keine Bewertungen
- The Evaluation and Management of Neck Masses of Unknown EtiologyDokument38 SeitenThe Evaluation and Management of Neck Masses of Unknown EtiologyShaxawan Mahmood AliNoch keine Bewertungen
- Retro Peritoneal TumorDokument37 SeitenRetro Peritoneal TumorHafizur RashidNoch keine Bewertungen
- Salivary Gland MalignanciesDokument69 SeitenSalivary Gland MalignanciesKessi VikaneswariNoch keine Bewertungen
- Simultaneous Papillary Carcinoma in Thyroglossal Duct Cyst and ThyroidDokument5 SeitenSimultaneous Papillary Carcinoma in Thyroglossal Duct Cyst and ThyroidOncologiaGonzalezBrenes Gonzalez BrenesNoch keine Bewertungen
- Current Controversies in The Management of Malignant Parotid TumorsDokument8 SeitenCurrent Controversies in The Management of Malignant Parotid TumorsDirga Rasyidin LNoch keine Bewertungen
- 2009 0527 rs.1 PDFDokument5 Seiten2009 0527 rs.1 PDFpaolaNoch keine Bewertungen
- Management of Primary Testicular Tumor: Alireza Ghoreifi,, Hooman DjaladatDokument7 SeitenManagement of Primary Testicular Tumor: Alireza Ghoreifi,, Hooman DjaladatfelipeNoch keine Bewertungen
- Adenoid Cystic Carcinoma of Hard Palate: A Case ReportDokument5 SeitenAdenoid Cystic Carcinoma of Hard Palate: A Case ReportHemant GuptaNoch keine Bewertungen
- CR Pa 2 PDFDokument4 SeitenCR Pa 2 PDFmitaNoch keine Bewertungen
- The Role of Usg2Dokument6 SeitenThe Role of Usg2noorhadi.n10Noch keine Bewertungen
- Neck Mass ProtocolDokument8 SeitenNeck Mass ProtocolCharlene FernándezNoch keine Bewertungen
- ScriptDokument8 SeitenScriptChristian Edward MacabaliNoch keine Bewertungen
- Parotid Gland NeoplasmDokument107 SeitenParotid Gland NeoplasmigorNoch keine Bewertungen
- 0 OP Techniques General Surgery Superficial Parotidectomy OLSEN EXCELLENTDokument13 Seiten0 OP Techniques General Surgery Superficial Parotidectomy OLSEN EXCELLENTmuhammad ishaqueNoch keine Bewertungen
- HN 08-2005 Surgical Management of ParapharyngealDokument7 SeitenHN 08-2005 Surgical Management of ParapharyngealJosinaldo ReisNoch keine Bewertungen
- A Rare Huge Myxofibrosarcoma of Chest WallDokument3 SeitenA Rare Huge Myxofibrosarcoma of Chest WallIOSRjournalNoch keine Bewertungen
- 2010 Article 73Dokument12 Seiten2010 Article 73Abhilash AntonyNoch keine Bewertungen
- Carcinoma Maxillary Sinus : of TheDokument6 SeitenCarcinoma Maxillary Sinus : of TheInesNoch keine Bewertungen
- Salivary Gland Tumors: 1. AdenomasDokument18 SeitenSalivary Gland Tumors: 1. AdenomasMahammed Ahmed BadrNoch keine Bewertungen
- Parotidectomia - UpToDate 2022Dokument2 SeitenParotidectomia - UpToDate 2022juanrangoneNoch keine Bewertungen
- Pediatric Desmoid Fibromatosis of TheDokument4 SeitenPediatric Desmoid Fibromatosis of ThevatankhahpooyaNoch keine Bewertungen
- Large CystDokument4 SeitenLarge CystKin DacikinukNoch keine Bewertungen
- Reti No Blast OmaDokument54 SeitenReti No Blast OmaSaviana Tieku100% (1)
- The Patient With Thyroid Nodule. Med Clin of NA. 2010Dokument13 SeitenThe Patient With Thyroid Nodule. Med Clin of NA. 2010pruebaprueba321765Noch keine Bewertungen
- Brain Abscess MimicsDokument16 SeitenBrain Abscess MimicsRikizu HobbiesNoch keine Bewertungen
- Salivary Gland Tumours and Other Lesions BDS 4Dokument38 SeitenSalivary Gland Tumours and Other Lesions BDS 4dunisanijamesonNoch keine Bewertungen
- Contemporary Management of Parapharyngeal TumorsDokument5 SeitenContemporary Management of Parapharyngeal TumorsSilvia SoareNoch keine Bewertungen
- Clinical Implications of The Neck in Salivary Gland DiseaseDokument14 SeitenClinical Implications of The Neck in Salivary Gland DiseaseГулпе АлексейNoch keine Bewertungen
- Primary Chest Wall TumorsDokument13 SeitenPrimary Chest Wall Tumorsmhany12345Noch keine Bewertungen
- Cranio Pha Ryn Gio MaDokument3 SeitenCranio Pha Ryn Gio MaSerious LeoNoch keine Bewertungen
- Meningioma: Tengku Rizky Aditya, MDDokument30 SeitenMeningioma: Tengku Rizky Aditya, MDTengku Rizky AdityaNoch keine Bewertungen
- Case Studies in Advanced Skin Cancer Management: An Osce Viva ResourceVon EverandCase Studies in Advanced Skin Cancer Management: An Osce Viva ResourceNoch keine Bewertungen
- Atlas of Diagnostically Challenging Melanocytic NeoplasmsVon EverandAtlas of Diagnostically Challenging Melanocytic NeoplasmsNoch keine Bewertungen
- Safari - 24 Jul 2018 01.13 PDFDokument1 SeiteSafari - 24 Jul 2018 01.13 PDFCeriaindriasariNoch keine Bewertungen
- Safari - 31 Des 2018 16.12 PDFDokument1 SeiteSafari - 31 Des 2018 16.12 PDFCeriaindriasariNoch keine Bewertungen
- Safari - 25 Agt 2018 01.38 PDFDokument1 SeiteSafari - 25 Agt 2018 01.38 PDFCeriaindriasariNoch keine Bewertungen
- Management of Salivary Gland Tumours: United Kingdom National Multidisciplinary GuidelinesDokument8 SeitenManagement of Salivary Gland Tumours: United Kingdom National Multidisciplinary GuidelinesCeriaindriasariNoch keine Bewertungen
- Malignant Parotid Tumors: Introduction and AnatomyDokument1 SeiteMalignant Parotid Tumors: Introduction and AnatomyCeriaindriasariNoch keine Bewertungen
- Safari - 21 Agt 2018 18.00 PDFDokument1 SeiteSafari - 21 Agt 2018 18.00 PDFCeriaindriasariNoch keine Bewertungen
- Otorhinolaryngology: Parotid Gland Tumors: A Retrospective Study of 154 PatientsDokument6 SeitenOtorhinolaryngology: Parotid Gland Tumors: A Retrospective Study of 154 PatientsCeriaindriasariNoch keine Bewertungen
- Safari - 2 Sep 2018 11.27 PDFDokument1 SeiteSafari - 2 Sep 2018 11.27 PDFCeriaindriasariNoch keine Bewertungen
- Safari - 21 Agt 2018 18.00 PDFDokument1 SeiteSafari - 21 Agt 2018 18.00 PDFCeriaindriasariNoch keine Bewertungen
- Haruskah Diterminas Dengan Bedah SesarDokument1 SeiteHaruskah Diterminas Dengan Bedah SesarCeriaindriasariNoch keine Bewertungen
- Safari - 25 Agt 2018 01.38 PDFDokument1 SeiteSafari - 25 Agt 2018 01.38 PDFCeriaindriasariNoch keine Bewertungen
- Usg in Ra Anaesthesia 2010Dokument12 SeitenUsg in Ra Anaesthesia 2010Ivette M. Carroll D.Noch keine Bewertungen
- Safari - 25 Agt 2018 01.38 PDFDokument1 SeiteSafari - 25 Agt 2018 01.38 PDFCeriaindriasariNoch keine Bewertungen
- c1 PDFDokument16 Seitenc1 PDFCeriaindriasariNoch keine Bewertungen
- Ebook Mata PDFDokument189 SeitenEbook Mata PDFepingNoch keine Bewertungen
- Otorhinolaryngology: Parotid Gland Tumors: A Retrospective Study of 154 PatientsDokument6 SeitenOtorhinolaryngology: Parotid Gland Tumors: A Retrospective Study of 154 PatientsCeriaindriasariNoch keine Bewertungen
- Safari - 2 Sep 2018 11.27 PDFDokument1 SeiteSafari - 2 Sep 2018 11.27 PDFCeriaindriasariNoch keine Bewertungen
- Parotid Glands Tumours: Overview of A 10-Years Experience With 282 Patients, Focusing On 231 Benign Epithelial NeoplasmsDokument5 SeitenParotid Glands Tumours: Overview of A 10-Years Experience With 282 Patients, Focusing On 231 Benign Epithelial NeoplasmsCeriaindriasariNoch keine Bewertungen
- Safari - 2 Sep 2018 11.27 PDFDokument1 SeiteSafari - 2 Sep 2018 11.27 PDFCeriaindriasariNoch keine Bewertungen
- Parotid Mass PDFDokument9 SeitenParotid Mass PDFCeriaindriasariNoch keine Bewertungen
- Parotid Mass PDFDokument9 SeitenParotid Mass PDFCeriaindriasariNoch keine Bewertungen
- c1 PDFDokument16 Seitenc1 PDFCeriaindriasariNoch keine Bewertungen
- Otorhinolaryngology: Parotid Gland Tumors: A Retrospective Study of 154 PatientsDokument6 SeitenOtorhinolaryngology: Parotid Gland Tumors: A Retrospective Study of 154 PatientsCeriaindriasariNoch keine Bewertungen
- Safari - 2 Sep 2018 11.27 PDFDokument1 SeiteSafari - 2 Sep 2018 11.27 PDFCeriaindriasariNoch keine Bewertungen
- Safari - 2 Sep 2018 11.27 PDFDokument1 SeiteSafari - 2 Sep 2018 11.27 PDFCeriaindriasariNoch keine Bewertungen
- Parotid Glands Tumours: Overview of A 10-Years Experience With 282 Patients, Focusing On 231 Benign Epithelial NeoplasmsDokument5 SeitenParotid Glands Tumours: Overview of A 10-Years Experience With 282 Patients, Focusing On 231 Benign Epithelial NeoplasmsCeriaindriasariNoch keine Bewertungen
- Bupivacaine: Review: Charles R. D. D. S. Bert N. M.D. Medical Center, Honolulu, HawaiiDokument5 SeitenBupivacaine: Review: Charles R. D. D. S. Bert N. M.D. Medical Center, Honolulu, HawaiiCeriaindriasariNoch keine Bewertungen
- Surgery v6n1p1 en PDFDokument5 SeitenSurgery v6n1p1 en PDFCeriaindriasariNoch keine Bewertungen
- Safari - 21 Agt 2018 18.00 PDFDokument1 SeiteSafari - 21 Agt 2018 18.00 PDFCeriaindriasariNoch keine Bewertungen
- Bupivacaine: Review: Charles R. D. D. S. Bert N. M.D. Medical Center, Honolulu, HawaiiDokument5 SeitenBupivacaine: Review: Charles R. D. D. S. Bert N. M.D. Medical Center, Honolulu, HawaiiCeriaindriasariNoch keine Bewertungen
- Final SBFR Training PPT 19th April 2022 MoHDokument113 SeitenFinal SBFR Training PPT 19th April 2022 MoHDaniel Firomsa100% (6)
- Soal Bahasa Inggris Paket C Setara SMA/MADokument22 SeitenSoal Bahasa Inggris Paket C Setara SMA/MApkbmalhikmah sukodonoNoch keine Bewertungen
- Mine ScoreDokument7 SeitenMine ScoreCharlys RhandsNoch keine Bewertungen
- Challenges Faced by Roma Women in EuropeDokument29 SeitenChallenges Faced by Roma Women in EuropeaurimiaNoch keine Bewertungen
- Specific Groups EssayDokument1 SeiteSpecific Groups Essaysunilvajshi2873Noch keine Bewertungen
- Iv PrimingDokument16 SeitenIv PrimingZahra jane A.Noch keine Bewertungen
- Special Power of Attorney Chavez 1Dokument2 SeitenSpecial Power of Attorney Chavez 1Atty. R. PerezNoch keine Bewertungen
- Educational TheoryDokument5 SeitenEducational TheoryTejinder SinghNoch keine Bewertungen
- 13 Reasons Why Character DiagnosisDokument9 Seiten13 Reasons Why Character DiagnosisVutagwa JaysonNoch keine Bewertungen
- 03 MSDS BentoniteDokument5 Seiten03 MSDS BentoniteFurqan SiddiquiNoch keine Bewertungen
- OSSSC Diploma in Pharmacy..Dokument8 SeitenOSSSC Diploma in Pharmacy..RAJANI SathuaNoch keine Bewertungen
- Community Health Nursing ExamDokument24 SeitenCommunity Health Nursing ExamClarisse SampangNoch keine Bewertungen
- Electrical Engineering Dissertation SampleDokument4 SeitenElectrical Engineering Dissertation SampleInstantPaperWriterUK100% (1)
- 1 5 4 Diabetes Mellitus - PDF 2Dokument6 Seiten1 5 4 Diabetes Mellitus - PDF 2Maica LectanaNoch keine Bewertungen
- PRACTICAL RESEARCH Q2-W1.editedDokument3 SeitenPRACTICAL RESEARCH Q2-W1.editedRAEJEHL TIMCANGNoch keine Bewertungen
- Final Admission List of Students To Government Health Training CollegesDokument298 SeitenFinal Admission List of Students To Government Health Training CollegesThe New VisionNoch keine Bewertungen
- Wcms - 618575 31 70 28 34Dokument7 SeitenWcms - 618575 31 70 28 34alin grecuNoch keine Bewertungen
- Common Medical AbbreviationsDokument1 SeiteCommon Medical Abbreviationskedwards108Noch keine Bewertungen
- Diass 2ndsem Q2.GDokument8 SeitenDiass 2ndsem Q2.GJohn nhel SinonNoch keine Bewertungen
- Question Text: Clear My ChoiceDokument13 SeitenQuestion Text: Clear My ChoiceLylibette Anne H. CalimlimNoch keine Bewertungen
- Your Clinic Name Here: Feline Acute Pain Scale Canine Acute Pain ScaleDokument1 SeiteYour Clinic Name Here: Feline Acute Pain Scale Canine Acute Pain ScalevetthamilNoch keine Bewertungen
- Make Today Amazing Checklist Printable Guide PDFDokument5 SeitenMake Today Amazing Checklist Printable Guide PDFemna fadliNoch keine Bewertungen
- Archery Tips-Home Training - WAEC - TEC-PHY - ENG - V1 - 0 PDFDokument44 SeitenArchery Tips-Home Training - WAEC - TEC-PHY - ENG - V1 - 0 PDFjenny12Noch keine Bewertungen
- NAMS 2022 Hormone-Therapy-Position-StatementDokument28 SeitenNAMS 2022 Hormone-Therapy-Position-StatementPaul PIETTENoch keine Bewertungen
- Aesthetic Inlays: November 2011Dokument4 SeitenAesthetic Inlays: November 2011Diego SarunNoch keine Bewertungen
- NCP Blurred VisionDokument3 SeitenNCP Blurred VisionRM MoralesNoch keine Bewertungen
- Compensation & Reward ManagementDokument227 SeitenCompensation & Reward ManagementjitendersharmajiNoch keine Bewertungen
- Ingles 2018Dokument12 SeitenIngles 2018Carmen GarciaNoch keine Bewertungen
- Tired Swimmer: A Case StudyDokument4 SeitenTired Swimmer: A Case StudyMorgan KrauseNoch keine Bewertungen
- GEN 013 Day 02 SASDokument5 SeitenGEN 013 Day 02 SASAlbert King CambaNoch keine Bewertungen