Beruflich Dokumente
Kultur Dokumente
6carboxylic Acids
Hochgeladen von
sharmimiameerasanadyOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
6carboxylic Acids
Hochgeladen von
sharmimiameerasanadyCopyright:
Verfügbare Formate
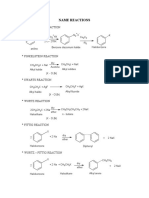
Flow Chart of organic reactions in CHM096
o
Cl2 or Br2/ hv or high heat (300-400 C) Free radical substitution
Alkyl halides/NaOH
3o Amines Quaternary ammonium salt
+
Alkyl halides/NaOH o Con NH3/ NaOH
Mg/dry ether Grignard Dilute H
o 1 Amines
2 Amines e.g CH3CHNH2CH3
Reagent
Alkyl halides/NaOH Nucleophilic
substitution HBr or HCl in ether or H2O
Alkynes 2 mol H2/Pt/Ni/Pd
NaCN/H
+
Nucleophilic hydrohalogenation
Alkyl Halides
Alkane Nitrile OR HCN substitution
e.g CH3CHBrCH3 (major) NaOH/EtOH Elimination 1 mol H2/Pt/Ni/Pd
e.g CH3CHCNCH3 (major) CH3CH2CHBr (minor)
CH3CH2CHCN (minor)
Nucleophilic Alkanes
NaOH/H2O substitution Free redical substitution
Alkoxide e.g CH3CH2CH3
e.g CH3ONa
Metal e.g Na Nucleophilic Alkenes
Carboxylic acid Cold, dil alkaline
+ H2SO4
substitution eg. CH3CH=CH2 hydrogenation
Ester Alcohols PCl3 or PCl5 or PBr3 or SOCl2 SN2 KMnO4 Electrophilic addition
Oxidation 1 mol H2/Pt/Ni/Pd
CH3OH Diol Hot,
1 o 1o eg. CH3CHOHCHOH acidified Br2 or Cl2/CCl4
Nucleophilic HBr or HI / heat
2o substitution
KMnO4
2o HCl/ZnCl2/heat (Lucas reagent)
C=C bond cleavage
Halogenation
3o SN1
3o =C ketoneI
Alcohols Elimination =CH carboxylic acid
e.g Alcohols
CH3CHOHCHBr (major) Conc. H2SO4. (Dehydration)
CH3CHBrCHOH (minor) =CH2 CO2 + H2O
(Elimina Dihalide alkanes
e.g CH3CHBrCHBr
+
H /H2O Hydration
+ + Br2 or Cl2/H2O
Hot KMnO4/H or Hot K2Cr2O7/H
Oxidation
Oxidation Hot KMnO4/H+ or Halohydrin
H2/Pt or LiAlH4 Hot K2Cr2O7/H+ Alcohols + con H2SO4 e.g CH3CHOHCHBr (major)
Aldehydes Carboxylic Acid Ester CH3CHBrCHOH (minor)
H2/Pt or LiAlH4 Tollens reagent +
e.g CH3COOH Dil H /Reflux
Formaldehyde -
Dil OH /Reflux
Reduction
+ Metal e.g Na or alkali e.g NaOH Eg. NaOH
HCN or NaCN/H
H2/Pt or LiAlH4 +
HCN or NaCN/H Alkanoate (salt of
Ketones Cyanohydrin carboxylic acid)
Hot KMnO4/H+ or Oxidation
e.g CH3COONa
Hot K2Cr2O7/H+
Oxidation Iodoform Test : 1) Alcohol with CH3CHOH-
No Reaction 2) Etanal CH3CH=O
3) Ketone with CH3C=O
T. Syed Illah and Dr LHSim
Das könnte Ihnen auch gefallen
- Haloalkanes and Haloarenes1Dokument15 SeitenHaloalkanes and Haloarenes1Poorni RenuNoch keine Bewertungen
- Organic Chemistry ReactionDokument3 SeitenOrganic Chemistry ReactionGAMEPORIUMNoch keine Bewertungen
- Reagent ListDokument9 SeitenReagent ListArka MukhopadhyayNoch keine Bewertungen
- 11 Alcohols Phenols and EthersDokument2 Seiten11 Alcohols Phenols and EthersVarun Sankpal100% (1)
- Name Reactions: Sandmeyer'S ReactionDokument9 SeitenName Reactions: Sandmeyer'S ReactionSai Krishnan100% (1)
- Organic Reagents: 1. Alcoholic KOH 2. Aluminium EthoxideDokument9 SeitenOrganic Reagents: 1. Alcoholic KOH 2. Aluminium EthoxideAarya Nandal100% (1)
- Organic-Chemistry (As Level)Dokument8 SeitenOrganic-Chemistry (As Level)Pirate HunterNoch keine Bewertungen
- Organic Conversion A & B Revise Before NEETDokument2 SeitenOrganic Conversion A & B Revise Before NEETAquib JavedNoch keine Bewertungen
- Name Reactions of Organic ChemistryDokument7 SeitenName Reactions of Organic ChemistryNaynam SharmaNoch keine Bewertungen
- 7 Coordination CompoundsDokument329 Seiten7 Coordination CompoundsArka100% (1)
- Haloalkanes MADDokument31 SeitenHaloalkanes MADggdfjkkvvNoch keine Bewertungen
- NamereactionorganicDokument13 SeitenNamereactionorganicdeykrishna654100% (1)
- Roadmap Problem - 9Dokument1 SeiteRoadmap Problem - 9abhyudaipathwayNoch keine Bewertungen
- Pdf-Haloalkanes and HaloarenesDokument159 SeitenPdf-Haloalkanes and HaloarenesOmkar Singh Shekhawat100% (2)
- X UV Light or Heat: Reactions in Topic XIDokument3 SeitenX UV Light or Heat: Reactions in Topic XImichelsonyip100% (1)
- 18.0 Carbonyl CompoundsDokument9 Seiten18.0 Carbonyl CompoundsKudzayi Tusaumwe100% (1)
- WWW - Crackjee.xyz: Organic ChemistryDokument9 SeitenWWW - Crackjee.xyz: Organic ChemistryRau100% (1)
- Haloalkanes and HaloarenesDokument26 SeitenHaloalkanes and Haloarenesrajputrishi1982Noch keine Bewertungen
- Reaction List v002Dokument5 SeitenReaction List v002cecil3414Noch keine Bewertungen
- CBSE Class-12 Chemistry Quick Revision Notes Chapter-11: Alcohols, Phenols and Ethers Structure of AlcoholsDokument8 SeitenCBSE Class-12 Chemistry Quick Revision Notes Chapter-11: Alcohols, Phenols and Ethers Structure of AlcoholsSAKET TYAGINoch keine Bewertungen
- AIEEE Chemistry Quick ReviewDokument1 SeiteAIEEE Chemistry Quick ReviewYashwanth KalyanNoch keine Bewertungen
- 12 Chemistry Notes ch11 Alcohols Phenols and EthersDokument8 Seiten12 Chemistry Notes ch11 Alcohols Phenols and Ethersmv7602456Noch keine Bewertungen
- SGDGDDDokument33 SeitenSGDGDDyopoboy100% (1)
- Reactions of Aldehydes and Ketones: Oxidation Reduction Nucleophilic AdditionDokument51 SeitenReactions of Aldehydes and Ketones: Oxidation Reduction Nucleophilic AdditionmacybnzNoch keine Bewertungen
- Revision Notes On AlcoholsDokument13 SeitenRevision Notes On AlcoholsMuredzwa MuzendaNoch keine Bewertungen
- Inorganic ChemistryDokument10 SeitenInorganic Chemistrydebraj sethi100% (1)
- Alkyl HalidesDokument20 SeitenAlkyl HalidesShivam Gupta0% (1)
- OQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO DDokument2 SeitenOQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO Dmanya9b32100% (1)
- Hydrocarbons NotesDokument13 SeitenHydrocarbons NotesShivansh Pundir100% (1)
- DPP Percentage - 2 (1) 5321633Dokument2 SeitenDPP Percentage - 2 (1) 5321633netra7222Noch keine Bewertungen
- 100 Organic Reagentspptx - 230327 - 085539 PDFDokument15 Seiten100 Organic Reagentspptx - 230327 - 085539 PDFHeera MeenaNoch keine Bewertungen
- CBSE Class 12 Alcohol Phenol and Ether Study NotesDokument378 SeitenCBSE Class 12 Alcohol Phenol and Ether Study NotesV T PRIYANKANoch keine Bewertungen
- Name ReactionsDokument10 SeitenName ReactionsParam SoniNoch keine Bewertungen
- Organic Chemistry ReagentsDokument7 SeitenOrganic Chemistry ReagentsRishabhNoch keine Bewertungen
- Redox RxnsDokument30 SeitenRedox RxnsJolaine ValloNoch keine Bewertungen
- Aldehydes Ketones and Carboxylic AcidsDokument37 SeitenAldehydes Ketones and Carboxylic Acidsssheeladevi84100% (1)
- Organic Chemistry ChartsDokument84 SeitenOrganic Chemistry ChartsPRIYANSHU KUMARNoch keine Bewertungen
- G R Reduction AlkaneDokument43 SeitenG R Reduction AlkaneManthan HaritashNoch keine Bewertungen
- Reaction Reactants Products Conditions Mechanism Other: AlkanesDokument3 SeitenReaction Reactants Products Conditions Mechanism Other: AlkanesInzamam A HaqueNoch keine Bewertungen
- Organic Named Reactions PDFDokument8 SeitenOrganic Named Reactions PDFAshis BisoyiNoch keine Bewertungen
- Organic 6 CDokument26 SeitenOrganic 6 CDr.Rajarshi PatelNoch keine Bewertungen
- Organic Chemistry ChartsDokument84 SeitenOrganic Chemistry ChartsPRIYANSHU KUMARNoch keine Bewertungen
- Reactions and Preparations of AlkenesDokument9 SeitenReactions and Preparations of AlkenesGolda Meyer VidalNoch keine Bewertungen
- Organic Chemistry - GRDokument52 SeitenOrganic Chemistry - GRPRIYANSHU KUMARNoch keine Bewertungen
- Experiment I1 Preparation of Some Cobaltammine ComplexesDokument7 SeitenExperiment I1 Preparation of Some Cobaltammine ComplexesIftitah HauriyahNoch keine Bewertungen
- Join @iitwale On Telegram: Physical Chemistr y Inorganic Chemistr y Organic Chemistr yDokument51 SeitenJoin @iitwale On Telegram: Physical Chemistr y Inorganic Chemistr y Organic Chemistr yK GhatageNoch keine Bewertungen
- 4.1.1 Protic Vs Aprotic SolventDokument36 Seiten4.1.1 Protic Vs Aprotic SolventDawit BirhanuNoch keine Bewertungen
- 1.aldehydes, Ketones and Carboxylic AcidsDokument117 Seiten1.aldehydes, Ketones and Carboxylic AcidsKRISHNARJUNA NNoch keine Bewertungen
- H.D.A. 2021Dokument54 SeitenH.D.A. 2021Every Time Chemistry [ ETC]Noch keine Bewertungen
- Organic ReagentsDokument3 SeitenOrganic ReagentsKushagra Rai100% (1)
- Mole Concept Solution Practice Set Objective by S.K.sinha See Chemistry Animations atDokument1 SeiteMole Concept Solution Practice Set Objective by S.K.sinha See Chemistry Animations atmyiitchemistry50% (2)
- Bridge Course Practice Test 15 Enova EducationDokument10 SeitenBridge Course Practice Test 15 Enova Educationrhancy77Noch keine Bewertungen
- Alkyl HalideDokument8 SeitenAlkyl HalideMegh Raj BhattNoch keine Bewertungen
- 12 Chemistry Keypoints Revision Questions Chapter 12Dokument20 Seiten12 Chemistry Keypoints Revision Questions Chapter 12sangam patraNoch keine Bewertungen
- Alkene and Alkyne - by Resonance PDFDokument45 SeitenAlkene and Alkyne - by Resonance PDFPrasad Yarra100% (1)
- 1 Roh Carboxylic Acids: H CroDokument15 Seiten1 Roh Carboxylic Acids: H CroandrewwrobleNoch keine Bewertungen
- 04 Reactive IntermediatesDokument115 Seiten04 Reactive IntermediatesMuhammad ArsalanNoch keine Bewertungen
- Reactions of HaloalkanesDokument10 SeitenReactions of Haloalkanesapi-504683923Noch keine Bewertungen
- 4alkyl Halides and AlcoholsDokument85 Seiten4alkyl Halides and AlcoholssharmimiameerasanadyNoch keine Bewertungen
- Imogen SlidesCarnivalDokument29 SeitenImogen SlidesCarnivalZarith Emily Burgoa AguilarNoch keine Bewertungen
- Year 3 EnglishDokument39 SeitenYear 3 EnglishsharmimiameerasanadyNoch keine Bewertungen
- ProposalDokument33 SeitenProposalsharmimiameerasanadyNoch keine Bewertungen
- Learning About Gravity I. Free Fall: A Guide For Teachers and Curriculum DevelopersDokument33 SeitenLearning About Gravity I. Free Fall: A Guide For Teachers and Curriculum DeveloperssharmimiameerasanadyNoch keine Bewertungen
- 6carboxylic Acids PDFDokument29 Seiten6carboxylic Acids PDFsharmimiameerasanadyNoch keine Bewertungen
- 5carbonyl CompoundsDokument25 Seiten5carbonyl CompoundssharmimiameerasanadyNoch keine Bewertungen
- 4alkyl Halides and AlcoholsDokument85 Seiten4alkyl Halides and AlcoholssharmimiameerasanadyNoch keine Bewertungen
- Fieldtrip SafetyDokument5 SeitenFieldtrip SafetysharmimiameerasanadyNoch keine Bewertungen
- Chemical Kinetics PDFDokument162 SeitenChemical Kinetics PDFsharmimiameerasanadyNoch keine Bewertungen
- Intro To Organic Chemistry PDFDokument78 SeitenIntro To Organic Chemistry PDFsharmimiameerasanadyNoch keine Bewertungen
- Storyboard Gerhana Bulan PDFDokument2 SeitenStoryboard Gerhana Bulan PDFsharmimiameerasanadyNoch keine Bewertungen
- Labelling Bilik Sains - For MergeDokument6 SeitenLabelling Bilik Sains - For MergesharmimiameerasanadyNoch keine Bewertungen
- Lecture39-Seismic Response of PilesDokument34 SeitenLecture39-Seismic Response of PilesArun Goyal100% (1)
- Corrosion & Non-Ferrous MetalDokument21 SeitenCorrosion & Non-Ferrous Metalsiraphat.bmNoch keine Bewertungen
- Pick Up The Most Appropriate Statement of The Multiple-Choice Answers by Comment On The Correct AnswersDokument9 SeitenPick Up The Most Appropriate Statement of The Multiple-Choice Answers by Comment On The Correct Answersأحمد إبراهيم شواربNoch keine Bewertungen
- Post Lab FC 2Dokument2 SeitenPost Lab FC 2Adv Sandeep SinghNoch keine Bewertungen
- Delhi Public School Bangalore North ACADEMIC SESSION 2021-2022 Ut2 Revision Work Sheet TOPIC: Sorting Materials Into Group Answer KeyDokument6 SeitenDelhi Public School Bangalore North ACADEMIC SESSION 2021-2022 Ut2 Revision Work Sheet TOPIC: Sorting Materials Into Group Answer KeySumukh MullangiNoch keine Bewertungen
- Chap 7 Ultra Low Power BioelectronicsDokument3 SeitenChap 7 Ultra Low Power BioelectronicsVarun GuptaNoch keine Bewertungen
- Solubility of Acid Oxalic in WaterDokument8 SeitenSolubility of Acid Oxalic in WaterHakim BenNoch keine Bewertungen
- Tabel Astm 53a PDFDokument27 SeitenTabel Astm 53a PDFadmin selamatNoch keine Bewertungen
- Week 9 Chem R Eng (1) 30-01-2023Dokument6 SeitenWeek 9 Chem R Eng (1) 30-01-2023Zain Ul AbedinNoch keine Bewertungen
- ExploreDokument3 SeitenExploreLourdes Joy LibradoNoch keine Bewertungen
- 2015 - Mathematical Modeling and Control of Plate N and Tube Heat ExchangersDokument11 Seiten2015 - Mathematical Modeling and Control of Plate N and Tube Heat ExchangersGanglin CaoNoch keine Bewertungen
- De Broglie PHD Thesis LengthDokument8 SeitenDe Broglie PHD Thesis Lengthfjdqvrcy100% (2)
- (VCE Chemistry) 2008 TSSM Unit 2 Sample ExamDokument5 Seiten(VCE Chemistry) 2008 TSSM Unit 2 Sample ExamJustine LyNoch keine Bewertungen
- 1 Quantum Confinement Effect 1.1: Correlation Between Bohr Radius and BandgapDokument3 Seiten1 Quantum Confinement Effect 1.1: Correlation Between Bohr Radius and Bandgapnirmalya prasun nayakNoch keine Bewertungen
- Sustainable Chemistry and Pharmacy: Tanya Sharma, Vinika Tyagi, Megha BansalDokument6 SeitenSustainable Chemistry and Pharmacy: Tanya Sharma, Vinika Tyagi, Megha BansalLind AguilarNoch keine Bewertungen
- Study Area: 37 Page 2 of 21Dokument1 SeiteStudy Area: 37 Page 2 of 21Hana HananeNoch keine Bewertungen
- EuSalt AS008-2005 Potassium - Flame Atomic Absorption Spectrometric MethodDokument4 SeitenEuSalt AS008-2005 Potassium - Flame Atomic Absorption Spectrometric MethodRuth Patinggi LPNoch keine Bewertungen
- MCNPDokument35 SeitenMCNPFahdila RahmaNoch keine Bewertungen
- Handbook On Biogas UtilizationDokument148 SeitenHandbook On Biogas UtilizationRusty MacCharles100% (3)
- Ongc VidtsaurabhDokument50 SeitenOngc VidtsaurabhAbhishek DevpuraNoch keine Bewertungen
- Analytical Method ValidationDokument19 SeitenAnalytical Method ValidationManasa SgrNoch keine Bewertungen
- Module 1 Lecture 2.1 Band Formation & N Type SemiconductorDokument11 SeitenModule 1 Lecture 2.1 Band Formation & N Type SemiconductorAjay KumarNoch keine Bewertungen
- 2 Resume On Atomic TheoryDokument23 Seiten2 Resume On Atomic TheoryacepNoch keine Bewertungen
- Modern PhysicsDokument74 SeitenModern PhysicsLàXsun ShrèsthàNoch keine Bewertungen
- Chapter 1Dokument6 SeitenChapter 1AhmadNoch keine Bewertungen
- Rubber Bond GuideDokument187 SeitenRubber Bond GuidesaleemasimstarNoch keine Bewertungen
- Wien2k UsersguideDokument219 SeitenWien2k UsersguidebyebyecolonelNoch keine Bewertungen
- IGCSE Student Revision Power Point Topic 5 - 複本Dokument16 SeitenIGCSE Student Revision Power Point Topic 5 - 複本yt kNoch keine Bewertungen
- Fdocuments - in - Edaplan Metolat Guide Formulatio PDFDokument6 SeitenFdocuments - in - Edaplan Metolat Guide Formulatio PDFNoor HafidlullahNoch keine Bewertungen
- MicrophoneDokument19 SeitenMicrophoneRaymond Dela CruzNoch keine Bewertungen