Beruflich Dokumente
Kultur Dokumente
9 The Ultimate Igcse Guide To Chemistry by Cgpwned
Hochgeladen von
RewanOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
9 The Ultimate Igcse Guide To Chemistry by Cgpwned
Hochgeladen von
RewanCopyright:
Verfügbare Formate
<
Contents
Routemap 6 <
Chapter 1
Atoms – the big idea 8 <
Atoms, elements and compounds 10 <
More about atoms 12 <
Isotops and Ar 14 <
How electrons are arranged 16 <
How ideas of the atom developed 18 <
The atom : the inside story 20 <
Question for chapter 1 22 <
Chapter 2
Bonding 24 <
Why compounds form 26 <
The ionic bond 28 <
Some other ions 30 <
Ionic compounds and their properties 32 <
The covalent bond 34 <
Covalent substances 36 <
Metals: more giant structures 38 <
Questions for chapter 2 40 <
Chapter 3
Acids and bases 42 <
Acids and alkalis 44 <
The reactions of acids 46 <
A closer look at neutralization 48 <
Acids and alkalis in everyday life 50 <
Questions for chapter 3 52 <
Chapter 4
The metals 54 <
Metals and the periodic table 56 <
How the periodic table developed 58 <
Group 1: the alkali metals 60 <
The patterns within Group 1 62 <
The transition metals 64 <
Metals and reactivity 66 <
The reactivity series 68 <
Questions for chapter 4 70 <
Chapter 5
Extracting metals 72 <
Metals in the Earth’s crust 74 <
Extracting metals from their ores 76 <
Making use of metals 78 <
Extracting iron 80 <
Principles of electrolysis 82 <
Extracting aluminum 84 <
Purifying copper by electrolysis 86 <
Corrosion 88 <
Metals, civilization and you 90 <
Questions for chapter 5 92 <
Chapter 6
The non-metals 94 <
The non-metal groups in the periodic table 96 <
Reactions of the halogens 98 <
Compounds of the halogens 100 <
The electrolyses of sodium chloride 102 <
The chlor-alkali industry 104 <
Questions for chapter 6 106 <
Chapter 7
Reactions, equations and amounts 108 <
The masses of atoms 110 <
Percentage composition of a compound 112 <
The formula of a compound 114 <
Equations for chemical reactions 116 <
Calculations from equations 118 <
Calculating the volumes of gases 120 <
Calculations electrolyses 122 <
Questions for chapter 7 124 <
Chapter 8
Getting the rate right 126 <
Rates of reaction 128 <
Measuring the rate of a reaction 130 <
Changing the rate of reaction (1) 132 <
Changing the rate of reaction (2) 134 <
Explaining rates 136 <
More about catalyses 138 <
Enzymes 140 <
Some traditional uses of enzymes 142 <
Some modern uses of enzymes 144 <
Questions for chapter 8 146 <
Chapter 9
Energy changes and reversible reactions 148 <
Exothermic and endothermic reactions 150 <
Explaining energy changes 152 <
Reversible reactions 154 <
Shifting the equilibrium 156 <
Making ammonia in industry 158 <
Fertilizers 160 <
The pros and cons of fertilizers 162 <
Questions for chapter 9 164 <
Chapter 10
Useful materials from crude oil 166 <
Crude oil 168 <
Separating oil into fractions 170 <
Cracking hydrocarbons 172 <
The alkanes and alkenes 174 <
Polymerization and plastics 176 <
Polythene – here to stay 178 <
Oil and the environment 180 <
Global warning 182 <
Questions for chapter 10 184 <
Chapter 11
The Earth 186 <
The atmosphere past and present 188 <
The atmosphere in balance 190 <
What have we done to the ozone layer ? 192 <
The oceans 194 <
The Earth’s structure 196 <
The Earth’s plates 198 <
A closer look at plate movements 200 <
Shaping the Earth’s surface 202 <
Questions for chapter 11 204 <
Chapter 12
Rocks 206 <
From weathering to deposition 208 <
From sediment to sedimentary rock 210 <
Evidence from fossils 212 <
Limestone and its uses 214 <
To quarry or not? 216 <
Igneous rock 218 <
Metamorphic rock 220 <
The rock cycle 222 <
Questions for chapter 12 224 <
Further topics 226 <
Revision: solids, liquids and gases 228 <
A closer look at gases 230 <
Composite materials 232 <
Soft and hard water 234 <
Making hard water soft 236 <
Exam-style questions 238 <
Revision and exam guidance 244 <
Chapter summaries/checklists 246 <
Glossary 252 <
Appendix 1: Separating solids from mixtures 256 <
Appendix 2: Distillation and chromatography 258 <
Appendix 3: Working with gases in the lab 260 <
Appendix 4: Testing for ions in the lab 262 <
Appendix 5: Math’s toolkit 264 <
Appendix 6: Periodic table and atomic masses 266 <
Appendix 7: Safety first! 268 <
Numerical answers 269 <
Index 270 <
Index anti-up 21
acid rain 51, 180 Ar 15, 110
acids 44-51 argon 96
reactions 46-7, 48-9 Aristotle 18
activation energy 138, 153 atmosphere 188-95
addition polymerization 176 atomic mass units 12
addition reaction 175 atomic number 12, 13
aggregate 215, 217, 232 and periodic table 59
air 188 atoms 10,12-13,18-21
alchemists 18 Avogadro 120
algae 163 bacteria 144, 189 Bakelite 177
alkali metals 57, 60-1, 62-3 baking soda 50
compounds 61 balanced equation 116
alkaline earth metals 57 , 63 barium hydroxide 151
alkalis 44-5, 50 barium sulphate 217
reaction with acids 46, 48-9 barytes, quarrying 217
alkanes 174 basalt 197,218,222,223
alkenes 173,175 batch process 145
allotropes, of carbon 37 bauxite 75, 84
alloys 65, 78, 79, 85 Becker 21
alpha particles 20, 21 Becquerel 20
Alps 201 bee sting 50
alumina 84, 85 beer 143
aluminium benzene 180
and acid rain 51 biodegradable 179
anodized 89 biological detergents 144
and corrosion 89 biological weathering 208
in Earth's crust 74 bitumen 171
electrolysis 76, 77 black smokers 144
extraction 76, 84-5 blast furnace 80
ore 75 bleach 99,132
properties 85 blue baby syndrome 163
uses 78, 79 Bohr, Niels 16, 21
aluminium oxide 84, 89 boiling 229
amino acids 189 boiling point 32
ammonia bond energy 152, 153
in atmosphere 189 bone 232
bonding 35 Bothe 21
and cleaners 50 Boyle, Robert 19
decomposition 152 brass 79
diffusion 231 bread 142
dynamic equilibrium 155 breccia 223 brine 104
and fertilizers 158, 161 bromine 97,98-9
manufacture 156-7, 158-9 isotopes 15
and nitric acid manufacture 161 water, test for unsaturation 175
solution 44, 49 bronze 79
ammonium Bronze Age 90
chloride 151, 154 Brown, Robert 19 butane 172
ion 31 cadmium 64
ions, in soil 158, 159 caesium 60-1, 62
nitrate 161 calamine lotion 50
sulphate 161 calcium and bone 232
amylase 140, 144 in Earth's crust 74
anaerobic bacteria 189 properties 63
Andes 201, 203 reaction with water 66, 67
anhydrous copper(lI) sulphate 154 see also chalk, limestone,
anodized aluminium 89 anti-charm 21 marble calcium carbonate 47, 50, 51, 134-5,
anti-strange 21 194, 214-15,220
calcium fluoride 217 test 103
calcium hydrogencarbonate 208, 234-5 water 99
calcium hydroxide 44, 50, 51, 214 chlorofluorocarbons (CFCs) 101, 192-3
calcium oxide 50, 150, 214, 215 chromium 64
calcium silicate 81 chromium plating 87, 89
Calor gas 174 chymosin 143
camping gas 174 citric acid 44, 151
cancer, skin 192, 193 coal 169, 181
carbohydrase 144 collisions 136, 137, 138
carbon combustion 150, 153
allotropes 37 common salt 28
cycle 190 see also salt, sodium chloride
dating 14 composite fabrics 233
isotopes 14 composite materials 232-3
in reactivity series 68 compound 11, 26, 56
as standard atom 110 compound ions 31
carbon dioxide compression 229
in atmosphere 188, 189, 190 concentration, and reaction rate 132, 136
and fermentation 142 concrete 51, 128, 214, 215, 232
and global warming 180, 182-3 condensing 229
and oceans 195 conglomerate 211
in rainwater 51, 208 conservative boundary 201
carbon monoxide 81, 180-1, 188, 189 constructive boundary 200
carbonate ion 31 contact metamorphism 221
carbonates, reaction with acids 47 continental crust 197
carbon-carbon double bond 173, 175, 176 continental plate 201
carbon-fibre-reinforced plastic 233 continuous flow process 145
carbonic acid 51, 208 converging plates 200, 201
Carlisle, William 91 coolants 192
cast iron 81 copper 64-5, 74, 90
catalase 140 displacement reaction 69
catalysts 138--9, 140 extraction 76
in ammonia manufacture 157 in granite 219
and reaction rate 135, 137 ions 31
transition metals as 65 reaction with oxygen 66
catalytic cracking 173 purification 86-7
cathode rays 20 structure 38
cellulase 144 uses 78
cement 51, 214, 215, 232 copper(II) bromide, electrolysis 83
cementation 211, 223 copper(II) oxide, displacement reaction 68
Chadwick, James 21 copper(II) sulphate 69, 154
chain reaction 192 core, Earth 196
chalk 50 corrosion of metals 88
and hard water 234 corrosive 44
charm 21 covalent bond 34
cheese 143 covalent compounds 35, 38
chemical equation 116 covalent substances 36-7
chemical weathering 208 cracking 158, 172-3, 175
chemists 19 cross-bedding 210
chlor-alkali industry 104-5 crude oil 168-73, 180-1
chloride ion, electron shells 27, 28 refining 170-1, 173
chlorides, of metals 67 uses 169, 171
chlorine 97, 98--9 crust, Earth 74, 196, 197
bonding 34 cryolite 85
isotopes 15, 110 crystals 32, 36
and ozone layer 192 in rock 219, 223
production and uses 104-5 cupronickel 79
reactions 26, 61, 98--9, 152 curds 143
Curie, Marie 20 action 141
Dalton, John 19, 112 as catalysts 140-1
Davy, Humphry 91 and food 141, 142-3
decay, radioactive 14 immobilized 145
Democritus 18 new uses 144-5
denatured 141 equation 116
denitrifying bacteria 189 equilibrium 155, 156-7
deposition 209 erosion 208, 222
destructive boundary 201 ethane 172, 173
detergents 234 ethanoic acid 44, 151
deuterium 14 ethanol 142, 143
diamond 10, 37 ethene 173, 175, 176
diesel oil 171 eutrophication 163
diffusion, gases 231 evaporation 32,229
displacement reaction 69, 99 exhaust, car 138
distilled water 236 exothermic reactions 68, 150, 152, 154
diverging plates 200, 203 expansion 228
Dobereiner, Johann 58 extraction of aluminium 84-5
dolomite 234 extraction of metals 76-7
double bond 34, 173, 175 extremophiles 144
drinking water 163 extrusive igneous rock 218, 219, 222
ductile 39, 56 fault 202
dynamic equilibrium 155 fermentation 142
Earth fertilizers 158, 160-3
atmosphere 188-9 field-ion microscope 19
crust 74 flammable 170, 171
structure 196-7 fluorides 101
earthquakes 198, 199, 201, 202 fluorine 97, 98
eka-aluminium 59 fluorspar, quarrying 217
eka-boron 59 fold mountains 203
eka-silicon 59 folds 202
electrode 82 foliation 220
electrolysis 76, 82-3, 151 formula 11, 114
of aluminium oxide 84-5 fossil fuels 168
calculations 122 and global warming 182-3
of copper(lI) bromide 83 fossils 211,212-13
of copper(lI) sulphate 86-7 fractional distillation 170-1
and discovery of metals 91 fractions 170, 172
of lead bromide 82 francium 60-1
of potassium iodide 83 freeze-thaw weathering 208
of sodium chloride 83, 102-3, 104-5 fuel oil 171
electrolyte 82 fuels, from crude oil 169, 171, 180
electron shells 16, 21 galena 91
electronic configuration 17, 63 gallium 90
electrons 10, 12, 20 galvanized iron 89
electroplating 87 gas 169, 171, 174, 228, 230-1
elementary particles 21 gas syringe 130
elements 10, 19, 56 gasoline 171
in Earth's crust 74 genetically modified bacteria 144
metals found as 76 giant covalent structure 36-7
elixir of life 18 giant ionic structure 28, 29
empirical formula 114 giant structure 32
endothermic reactions 151, 152, 154 glacier 208, 209
energy changes in reactions 153 glass 215
energy level diagram 150, 151 glass-fibre-reinforced plastic 233
of electron shells 16, 17 global warming 180, 182-3, 190, 195
environment, and crude oil 180-1 glucose 153, 190
enzymes 135, 140-5 gneiss 221
goitre 101 incomplete combustion 181
gold 66, 74, 76, 88, 90-1 indigestion 50
alchemists 18 Industrial Revolution 90, 182
in granite 219 inert 96
occurrence 75 intermolecular forces 36
properties 56, 64 intrusive igneous rock 218, 222
graded bedding 210 invertase 144
granite 197, 213, 218, 222 iodine 36, 97, 98-9
graphite 37 ion 27, 30-1
gritstone 213 ion exchange, and hard water 236, 237
groundwater 163 ion-exchange membrane 104
group, in periodic table 10, 57 ionic bond 28
Group 1 57, 60-1, 62 ionic compounds 28, 30-3, 38, 61, 83
Group 2 57,63 iron 90, 91
Group 7 97, 98-9 as catalyst 135, 139, 157, 158, 159
Gulf Stream 182 competition reactions 68-9
gypsum 215, 234 corrosion 88
Haber, Fritz 158 in Earth's core 196
Haber process 65, 158, 162 in Earth's crust 74
haematite 80 extraction 76, 80-1
haemoglobin 181 ions 31
half equation 122 ore 76, 80
half-life 14 properties and uses 56, 64-5, 78
halogens 97, 98-9 reactions 66, 67, 119,151
compounds 100-1 in rock
hard water 234-7 iron(m) oxide, reduction 81
Harman,Joe 192 Iron Age 90
helium 96 island arcs 203
high-density polythene 178 isomerase 144
high-grade metamorphic rock 221 isotopes 110, 14-15
Himalayas 201, 203 joule 150
Hofmann voltameter 102-3 kerosene 171
hydrated crystals 215 kilojoule 150
hydriodic acid 100 krypton 96
hydrobromic acid 100 lactase 145
hydrocarbons 169, 170, 172-3, 174-5 lactic acid 143
hydrochloric acid 44, 67, 100 Lactobacillus acidophilus 143
hydrogen and ammonia manufacture 158 lactose 143
bonding 34 laminated materials 233
and energy 153 lattice 36
ions 48 ionic 32
isotopes 14 lava 218,223
production and uses 104-5 Lavoisier, Antoine 191
reaction with chlorine 152 Law of Conservation of Mass 118
in reactivity series 68 Law of Constant Composition 112
test 103 Law of Octaves 58
hydrogen chloride 100, 231 Law of Triads 58
hydrogen halides 100 Le Chatelier's principle 156
hydrogen peroxide 135, 140 lead 74,76,90,91
hydrogenation of vegetable oils 65 in granite 219
hydrogencarbonate ion 31 and pollution 180
hydrothermal solution 219 reaction with hydrochloric acid 67
hydroxide ion 31, 48 uses 78
hydroxides of alkali metals 60 lead bromide, electrolysis 82
of metals 67 lead(ll) chloride 155
reaction with acids 47 life, beginning of 189
igneous rock 197, 218-19 lightning 158
immobilized enzyme 145 limestone 51,214-15
in blast furnace 81 bonding 35
chemical weathering 208 and energy 153
formation in oceans 194 methanoic acid 44
and hard water 234 Midgely, Thomas 192
in Peak District 213 mild steel 78, 81
quarrying 216-17 mining 75
uses 214 model 21
limewater 142 molecular substances 34, 36-7, 38
lipase 140, 144 molecule 34
liquid 228 monatomic 96
lithium 60-1,62 Montreal Protocol 192
lithosphere 196, 218 mountain ranges 198,201,222
litmus 44 M, 111
liver 140 Muller, Erwin 19
low-density polythene 178 naphtha 171, 173
low-grade metamorphic rock 221 National Parks Authority 217
lubricating oil 171 native 74,76
magma 218, 222 natural gas (North Sea Gas) 174,181
magnesium negative ion 27,30
burning 117 neon 96
in Earth's crust 74 neutral 44
properties 63 neutralization 46-7,48-9,150
reaction with acid 46,67,130-1,132, 136-7 neutrons 21
reaction with oxygen 66 Newlands, John 58
reaction with water 66, 67 Nicholson, Anthony 91
magnesium chloride, electron shells 29 nickel 64,65,79,87,196
magnesium oxide 33 nitrate
electron shells 29 ion 31
malleable 39,56 in soil 158,159, 190
manganese, on ocean floor 195 in water 163
manganese steel 79 nitric acid 44
manganese(IV) oxide, as catalyst 135, 140 manufacture 139, 161
mantle 196 nitrifying bacteria 189,190
marble 220 nitrogen
marble chips 134 and ammonia manufacture 158
mass number 12 in atmosphere 188,189,190
mass spectrometer 110 cycle 189
melamine 177 fertilizer 158, 159
melting 228 nitrogen-fixing bacteria 158
melting point 32 nitrogen oxides see oxides of
membrane cell 104 nitrogen nitrogenous fertilizers 158
Mendeleev, Dmitri 59 noble gases 26, 96
mercury 10, 18,64,74 non-metals
metal oxides 47, 76 negative ions 30
metallic bond 38 and periodic table 10
metals properties 96
history 90-1 reaction with halogens 97
and periodic table 10, 56-7 non-renewable resource 76
positive ions 30 nucleus 10, 12, 21
properties 39,56,78 ocean plate 200, 201
reaction with acids 46 ocean ridge 198
reaction with halogens 97 ocean trench 198, 201, 203
in reactivity series 68 oceanic crust 197
structure 38 oceans 194-5
metamorphic rock 197,220-1 oil see crude oil
metamorphism 220 oil trap 169
methane 172, 182 optimum temperature, enzymes 141
in atmosphere 189 optimum yield 159
ores 75, 90, 91 and equilibrium 157,159
organic compounds 169 and gases 230-1
organic farming 163 Priestley, Joseph 191
overturn 202 product 116
oxides of metals 66, 67 propane 172
oxides of nitrogen, and acid rain 51, 180-1 propene 173,175
oxygen in atmosphere 188, 189, 190 protease 140, 144
bonding 34 proteins 140, 189, 190
discovery 191 proton number 12
in Earth's crust 74 protons 12, 21
reaction with alkali metals 61 Proust, Joseph Louis 19
ozone 189,192 quarrying, limestone 216-17
paints 88 quicklime 50, 150,214
paraffin waxes 171 radiation 14
particles 228-9 radioactive decay, in Earth 196
Peak District 213, 216-17 radioactive isotopes (radioisotopes) 14
pentane 181 radioactivity 20, 60
percentage composition 112-13 rate of reaction 128-37
period, in periodic table 10, 57 reactant 116
periodic table 10, 11, 56-7 reactivity series 68
development 58-9 reactivity, of metals 66-7
and electronic configuration 17, 63 recycling
and properties of elements 62-3, 96-7, 98 metals 76
permanent hardness 235 plastics 179
pH scale 44, 50 redox reaction 77,82,99
pH, and enzymes 141, 144, 145 reducing agent 68
phenolphthalein 49 reduction, of metal oxides 76, 81
phosphorus, fertilizer 158 refining crude oil 170-1,173
photochemical reaction 101 regional metamorphism 221
photography 101 I reinforced concrete 232
photosynthesis 151, 189, 190 reinforced plastic 233
physical weathering 208 relative atomic mass 110, 111, 15
phytoplankton 194, 195 relative formula mass 111
plankton 192, 194 rennet 143
plasterboard 233 reservoir rock 168
plastics 176-9 respiration 150
plate tectonics see tectonic plates reversible reactions 154-9
platinum 64, 74, 76 rhodium 138
as catalyst 138 ripening of fruit 141
platinum-rhodium gauze catalyst 139 rock
plywood 233 cycle 222-3
pollution 163, 180-1 formation 197,211,218,220-1
poly(chloroethene) (polyvinyl chloride, PVC) types 197
176, 177 rock salt 75, 100
poly(phenylethene) (poly(styrene)) 176, 177 root nodules 158
poly(propene) 176, 177 rubidium 60-1, 62
polymerization 151, 176-7 rusting 88, 128
polymers 175, 176-9 Rutherford, Ernest 21, 59
polythene 151,176,177,178-9 :) sacrificial protection 89
positive ion 27, 30 safety glass 232
potassium 88 salt 104
in Earth's crust 74 and alchemists 18
fertilizer 158 preparation 49
properties 60-1, 62 see also sodium chloride
reaction with water 67 salts 46
potassium hydroxide solution 44 in oceans 194
potassium iodide 83, 101 San Andreas Fault 201
pressure sandstone 211, 222
saturated hydrocarbons 174 stonewashed denim 144, 145
scale 50, 235 strange 21
scanning electron microscope 19 stratosphere 188
scarce metals 74 strong acids and alkalis 44, 45
Scheele, Karl 191 subduction 201, 203
schist 221 sulphate ion 31
sea floor spreading 200 sulphur 18, 119, 151
sediment 197, 210 sulphur dioxide
sedimentary rock 197, 210--11, 212-13, 222 and acid rain 51,172, 180-1
selenium 90 pollution 188
shale 211, 213, 217 and sulphuric acid manufacture 139
sherbet 151 sulphur trioxide 139
silica 74, 218 sulphuric acid 44, 139
silicon, in Earth's crust 74 Sumner, James 142
siltstone 211 surface area, and reaction rate 134-5, 137
silver 74, 76, 90 surface catalyst 138
in granite 219 ) sustainable development 217
ions 31 symbols, for elements 20
silver chloride, precipitation 128, 150 tannic acid 44
silver halides 101 tectonic plates 198-203, 222
silver nitrate, displacement reaction 69 temperature
skin cancer 192, 193 and enzymes 141, 144
slag 81 and equilibrium 156, 159
slaked lime 50, 51, 214, 215 and gases 230-1
slate 220, 221, 222 and reaction rate 133, 137
smokeless fuel 121 temporary hardness 235
sodium 10 thermal decomposition 154,172
atom 12 Thermit process 68
in Earth's crust 74 thermosetting plastics 177
ion, electron shells 27, 28 thermosoftening plastics 177
properties 56, 60--1, 62, 64 Thomson, J. J. 20
reaction with chlorine 26 tin 74, 90, 91
reaction with oxygen 66 in granite 219
reaction with water 66, 67 plating 87, 89
uses 79 uses 79
sodium carbonate 151, 236 tincture of iodine 99
sodium chloride 61, 100 titanium 79, 90
electrolysis 83, 102-3, 104-5 transform motion 201
ionic bonding 26-8 transition metals
in oceans 194 as catalysts 135
ore 75 ions 31
production and uses 104-5 and periodic table 57
properties 33 properties 64-5
structure 32 salts 65
sodium hydrogencarbonate 50, 151 transport, rock 208, 209, 222
sodium hydroxide 44, 46, 50 tritium 14
sodium oxide 61 troposphere 188
sodium thiosulphate 133 tuff 219
soft water 234-7 ultraviolet radiation 192
solder 79 universal indicator 44
solid 228 unreactive 26
stainless steel 78, 79 unsaturated hydrocar:bons 175, 176 up 21
starch 190 uranium 20, 196
starter culture 143 urease 142
state symbols 116 vanadium pentoxide 139
steel, and corrosion 65, 81, 88 vinegar 44
sterilizing agent 99 viscous 170, 171
Stone Age 90 volatile 170
volcanic ash 219
volcanoes 198, 199, 201, 203, 222, 223
volume, of gases 120, 230-1
washing soda 236
water
bonding 35
of crystallization 154, 232
cycle 190
in electrolysis 83
soft and hard 234-5
vapour, in atmosphere 188, 189, 190, 194
weak acids and alkalis 44, 45
weathering 208, 222, 234
whey 143,145
wine 143
world population 162
wort 143
xenon 96
yeast 142-3
yoghurt 143
zeolites 237
zinc 64, 74, 76
in granite 219
ions 31
reaction with acid 67, 128
reaction with water 67
uses 79
zinc carbonate 50
ZMS-5 139
Das könnte Ihnen auch gefallen
- 9780198308737Dokument10 Seiten9780198308737Menna50% (4)
- Cambridge Igcse Chemistry Revision GuideDokument50 SeitenCambridge Igcse Chemistry Revision GuideReta SahawnehNoch keine Bewertungen
- Cambridge IGCSE Physics Revision GuideDokument347 SeitenCambridge IGCSE Physics Revision Guidefarhad uddin78% (9)
- BiologíaDokument48 SeitenBiologíaLuz Tapia Villarroel50% (2)
- Cambridge Igcse Chemistry Revision Guide - Public PDFDokument50 SeitenCambridge Igcse Chemistry Revision Guide - Public PDFMoarz Galaxy64% (11)
- Cambridge IGCSE Study Guide For PhysicsDokument113 SeitenCambridge IGCSE Study Guide For Physicsmarize medhat91% (23)
- Cambridge IGCSE Physics Coursebook Second Edition (Cambridge University Press) - Pages-DeletedDokument22 SeitenCambridge IGCSE Physics Coursebook Second Edition (Cambridge University Press) - Pages-DeletedMariam ShehabNoch keine Bewertungen
- Complete Science For Cambridge IGCSE PDFDokument3 SeitenComplete Science For Cambridge IGCSE PDFAbu Mariam0% (1)
- Cambridge IGCSE Biology 3rd Edition PDFDokument401 SeitenCambridge IGCSE Biology 3rd Edition PDFSylvia Nyakujawa84% (25)
- Edexcel International GCSE Chemistry Workbook Robert WensleyDokument97 SeitenEdexcel International GCSE Chemistry Workbook Robert WensleyMochiro ZzZ100% (1)
- EdExcel IGCSE Chemistry Past Paper Questions 2013Dokument320 SeitenEdExcel IGCSE Chemistry Past Paper Questions 2013Sadiq Amin67% (6)
- IGCSE Physics-Formula SheetDokument2 SeitenIGCSE Physics-Formula SheetPraveen PeterNoch keine Bewertungen
- Study PackDokument891 SeitenStudy PackMonisaAslamNoch keine Bewertungen
- 4th Form IGCSE Workbook BiologyDokument34 Seiten4th Form IGCSE Workbook BiologySameer Ala'i100% (2)
- Essential Chemistry For Cambridge IGCSE 2nd EditionDokument286 SeitenEssential Chemistry For Cambridge IGCSE 2nd Editionsam wong88% (25)
- Cambridge IGCSE Chemistry Workbook 2nd Edition PDFDokument97 SeitenCambridge IGCSE Chemistry Workbook 2nd Edition PDFfran85% (13)
- Complete Physics For Cambridge IGCSE - Sarah Lyold PDFDokument166 SeitenComplete Physics For Cambridge IGCSE - Sarah Lyold PDFANIKA DHANIKACHALAM100% (2)
- Physics Revision GuideDokument172 SeitenPhysics Revision GuideShazain Qureshi83% (12)
- Cambridge IGCSE Physics Practice Book AnswersDokument22 SeitenCambridge IGCSE Physics Practice Book AnswersRonnie Quek50% (2)
- 9780199138760Dokument16 Seiten9780199138760eibsource0% (1)
- Complete Chemistry Revision Guide IGCSEDokument290 SeitenComplete Chemistry Revision Guide IGCSEsakibsultan_308Noch keine Bewertungen
- Edexcel International GCSE and Certificate Physics Practice BookDokument98 SeitenEdexcel International GCSE and Certificate Physics Practice Bookeltytan100% (1)
- Cambridge IGCSE™ Physics Workbook (Heather Kennett)Dokument82 SeitenCambridge IGCSE™ Physics Workbook (Heather Kennett)sfaezali09Noch keine Bewertungen
- Calculations For Gcse ChemistryDokument69 SeitenCalculations For Gcse ChemistryMuhammad Hamza0% (2)
- Biology-Igcse FULL NotesDokument63 SeitenBiology-Igcse FULL NotesSameer Abu Munshar100% (5)
- Revisions Checklist For IGCSE Biology 0610 FINALDokument31 SeitenRevisions Checklist For IGCSE Biology 0610 FINALDanil Panci100% (1)
- Biology Revision Guide by Ian J.burtonDokument201 SeitenBiology Revision Guide by Ian J.burtonRana Wasif ALi60% (5)
- Biology Notes IGCSE Cambridge 2014 PDFDokument376 SeitenBiology Notes IGCSE Cambridge 2014 PDFSadia haque100% (4)
- Edexcel GCSE Physics SpecificationDokument80 SeitenEdexcel GCSE Physics SpecificationastargroupNoch keine Bewertungen
- Igcse Biology Text Second EditionDokument6 SeitenIgcse Biology Text Second EditionMikha LayastaNoch keine Bewertungen
- Cambridge IGCSE Physcial Science Chemistry Workbook Sample PDFDokument31 SeitenCambridge IGCSE Physcial Science Chemistry Workbook Sample PDFMaria Rajesh0% (1)
- Cambridge IGCSE Physics Workbook 2nd EditionDokument97 SeitenCambridge IGCSE Physics Workbook 2nd EditionEdgardo Leysa80% (54)
- IGCSE Chemistry NotesDokument46 SeitenIGCSE Chemistry NotesXamiya93% (56)
- Complete: PhysicsDokument8 SeitenComplete: PhysicsConcordia Mannargudi100% (2)
- Igcse-Chemistry Revision GuideDokument290 SeitenIgcse-Chemistry Revision GuideFroggieMonster100% (15)
- Gcse PhysicsDokument150 SeitenGcse PhysicsAditya Sakhuja67% (3)
- The Ultimate IGCSE Physics GuideDokument40 SeitenThe Ultimate IGCSE Physics GuideTaleen Sakayan82% (17)
- Pub Chemistry Made Clear Gcse EditionDokument210 SeitenPub Chemistry Made Clear Gcse EditionKasun PererraNoch keine Bewertungen
- Ebook PDF Chemistry Sixth Edition 6th Edition by Thomas R Gilbert PDFDokument41 SeitenEbook PDF Chemistry Sixth Edition 6th Edition by Thomas R Gilbert PDFbruce.roth349100% (36)
- Hodder Edexcel ChemistryDokument281 SeitenHodder Edexcel ChemistryVictoria A.R100% (2)
- Igcse Chemistry Sample ChapterDokument20 SeitenIgcse Chemistry Sample Chapter123123aa100% (1)
- Preview-9781316675496 A27454940Dokument121 SeitenPreview-9781316675496 A27454940HendrijosphNoch keine Bewertungen
- CHM 231 Organi Chem 1Dokument101 SeitenCHM 231 Organi Chem 1Adams DeborahNoch keine Bewertungen
- Chemistry at A GlanceDokument132 SeitenChemistry at A GlanceJames Roy100% (2)
- Illustrated Guide To CHEMISTRY PDFDokument209 SeitenIllustrated Guide To CHEMISTRY PDFAkicaNoch keine Bewertungen
- AQA KS3 Science Activate Student Book 1 Look InsideDokument10 SeitenAQA KS3 Science Activate Student Book 1 Look InsideAneesahk12350% (2)
- Inorganic Chemistry: Butterworths Intermediate ChemistryVon EverandInorganic Chemistry: Butterworths Intermediate ChemistryNoch keine Bewertungen
- NotesDokument231 SeitenNoteseman.abdellatiflearnNoch keine Bewertungen
- IChO2011 Prep Problems With Solutions 110215Dokument113 SeitenIChO2011 Prep Problems With Solutions 110215Afif Zulfikar PamungkasNoch keine Bewertungen
- Adiabatic PrerefDokument47 SeitenAdiabatic Prerefleonard chokNoch keine Bewertungen
- Agarwal S Engineering Chemistry Fundamentals and Application PDFDokument1.343 SeitenAgarwal S Engineering Chemistry Fundamentals and Application PDFJ. Giroto92% (13)
- Ebook PDF Chemistry 4th Edition by Julia BurdgDownload Ebook PDF Chemistry 4th Edition by Julia Burdge PDFDokument41 SeitenEbook PDF Chemistry 4th Edition by Julia BurdgDownload Ebook PDF Chemistry 4th Edition by Julia Burdge PDFkathleen.williams876100% (34)
- Chemistry 1Dokument3 SeitenChemistry 1Anshul KidileNoch keine Bewertungen
- Lecture 1 Computer Organization 2022Dokument44 SeitenLecture 1 Computer Organization 2022RewanNoch keine Bewertungen
- The Importance of Art To SocietiesDokument4 SeitenThe Importance of Art To SocietiesRewanNoch keine Bewertungen
- CH 02Dokument71 SeitenCH 02RewanNoch keine Bewertungen
- CH 01Dokument78 SeitenCH 01RewanNoch keine Bewertungen
- CSE213 - Numerical Analysis Sheet 1 - Error Analysis To Be Submitted On The 25 of FebruaryDokument4 SeitenCSE213 - Numerical Analysis Sheet 1 - Error Analysis To Be Submitted On The 25 of FebruaryRewanNoch keine Bewertungen
- Sheet 1 NumericalDokument3 SeitenSheet 1 NumericalRewanNoch keine Bewertungen
- Lecture 2 Computer Organization 2022Dokument28 SeitenLecture 2 Computer Organization 2022RewanNoch keine Bewertungen
- 123455Dokument3 Seiten123455RewanNoch keine Bewertungen
- Material Identification ReportDokument11 SeitenMaterial Identification ReportRewanNoch keine Bewertungen
- MIT18 05S14 Reading3Dokument12 SeitenMIT18 05S14 Reading3Md CassimNoch keine Bewertungen
- Fundamentals of Materials ScienceDokument21 SeitenFundamentals of Materials ScienceRewanNoch keine Bewertungen
- 123Dokument3 Seiten123RewanNoch keine Bewertungen
- MSE 212 Material Science Lab Experiment (3) : Tensile Test: Submitted byDokument13 SeitenMSE 212 Material Science Lab Experiment (3) : Tensile Test: Submitted byRewanNoch keine Bewertungen
- MSE 212 Material Science Lab Experiment (3) : Tensile Test: Submitted byDokument13 SeitenMSE 212 Material Science Lab Experiment (3) : Tensile Test: Submitted byRewanNoch keine Bewertungen
- Procedure: (Exp. 1) Preparation of A Standard Solution of Sulfamic AcidDokument3 SeitenProcedure: (Exp. 1) Preparation of A Standard Solution of Sulfamic AcidRewanNoch keine Bewertungen
- 20 ML of Water: Watch Glass Folded Filter Paper Funnel Conical BeakerDokument1 Seite20 ML of Water: Watch Glass Folded Filter Paper Funnel Conical BeakerRewanNoch keine Bewertungen
- Synthesis of Methyl OrangeDokument4 SeitenSynthesis of Methyl OrangeRewanNoch keine Bewertungen
- MSE 222 Materials Science LabDokument12 SeitenMSE 222 Materials Science LabRewanNoch keine Bewertungen
- البرشامة في الثقافة اليابانيةDokument9 Seitenالبرشامة في الثقافة اليابانيةRewanNoch keine Bewertungen
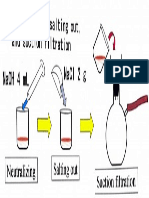
- Naoh 4 ML: Neutralizing, Salting Out, and Suction FiltrationDokument1 SeiteNaoh 4 ML: Neutralizing, Salting Out, and Suction FiltrationRewanNoch keine Bewertungen
- 0452 ChecklistDokument9 Seiten0452 ChecklistRewanNoch keine Bewertungen
- Synthesis of Alum Exp. 1Dokument2 SeitenSynthesis of Alum Exp. 1RewanNoch keine Bewertungen
- BANGTAN NEWS - Google DriveDokument1 SeiteBANGTAN NEWS - Google DriveRewanNoch keine Bewertungen
- Bad DebtsDokument2 SeitenBad DebtsredwanNoch keine Bewertungen
- Chemistry IGCSE Revision GuideDokument138 SeitenChemistry IGCSE Revision GuideMenna Haile95% (57)
- Answers To Workbook EMDokument32 SeitenAnswers To Workbook EMRewanNoch keine Bewertungen
- Chemistry Igcse 1 PDFDokument35 SeitenChemistry Igcse 1 PDFRohit MITTALNoch keine Bewertungen
- The Ultimate Igcse Guide To Chemistry by CgpwnedDokument49 SeitenThe Ultimate Igcse Guide To Chemistry by CgpwnedMaaz RashidNoch keine Bewertungen
- IGCSE Chemistry Work Book by Richard Harwood PDFDokument97 SeitenIGCSE Chemistry Work Book by Richard Harwood PDFRewanNoch keine Bewertungen
- Chapt 19Dokument11 SeitenChapt 19Rebecca Es100% (2)
- Ca12003e-Aerialpolehw Instalación Aérea Chance HubellDokument140 SeitenCa12003e-Aerialpolehw Instalación Aérea Chance HubellMartín AlonsoNoch keine Bewertungen
- Assignment 2Dokument12 SeitenAssignment 2Wâqâr ÂnwârNoch keine Bewertungen
- BarresiDokument348 SeitenBarresijeffaustonNoch keine Bewertungen
- (Open Geosciences) Relationship Between Landform Classification and Vegetation (Case Study Southwest of Fars Province Iran)Dokument8 Seiten(Open Geosciences) Relationship Between Landform Classification and Vegetation (Case Study Southwest of Fars Province Iran)sultanNoch keine Bewertungen
- Tectonics Zagros DehbozorgiDokument13 SeitenTectonics Zagros DehbozorgiMukteshwar MishraNoch keine Bewertungen
- YENNY SZABADICS. Hunters Clovisoids in Paraguana 2015Dokument76 SeitenYENNY SZABADICS. Hunters Clovisoids in Paraguana 2015Jenny_27Noch keine Bewertungen
- Xcavations at Rakhigarhi, 1997-98 To 1999-2000 (Full Text of ASI Report Dr. Amarendra Nath, Former Director (Archaeology), ASIDokument396 SeitenXcavations at Rakhigarhi, 1997-98 To 1999-2000 (Full Text of ASI Report Dr. Amarendra Nath, Former Director (Archaeology), ASISrini Kalyanaraman100% (2)
- Chapter-I Part TwoDokument6 SeitenChapter-I Part TwoTigst Tigst YzachewNoch keine Bewertungen
- Chapter 5: Using Igneous Rocks To Interpret Earth HistoryDokument9 SeitenChapter 5: Using Igneous Rocks To Interpret Earth History林柏儒Noch keine Bewertungen
- Hydrocarbon Detection With AVO: S E I S M I C SDokument9 SeitenHydrocarbon Detection With AVO: S E I S M I C STran Dang SangNoch keine Bewertungen
- Use of Pre-Splitting Technique As An Alternative Approach ToDokument4 SeitenUse of Pre-Splitting Technique As An Alternative Approach Toalvaroaac4Noch keine Bewertungen
- Geology of Trans HimalayasDokument20 SeitenGeology of Trans HimalayasRavindra Pratap SinghNoch keine Bewertungen
- Applied Hydrology Chow Et Al. 1969Dokument3 SeitenApplied Hydrology Chow Et Al. 1969carlosmendezv33% (3)
- Single Phase Flow in Porous Media: Darcy's LawDokument40 SeitenSingle Phase Flow in Porous Media: Darcy's LawSALIM AL MAQBALINoch keine Bewertungen
- Experimental Study of The Strength of Rock Containing Tubular DiscontinuitiesDokument13 SeitenExperimental Study of The Strength of Rock Containing Tubular DiscontinuitiesMahmoud AtiaNoch keine Bewertungen
- 01 - EE and ESDokument21 Seiten01 - EE and ESHerman AucampNoch keine Bewertungen
- Earthquakes and Faults - PowerpointDokument30 SeitenEarthquakes and Faults - PowerpointYour ArniellaNoch keine Bewertungen
- Geología Aplicada A Depósitos MineralesDokument383 SeitenGeología Aplicada A Depósitos MineralesRenato Tribal100% (1)
- Advances in Geosciences Vol 4 Hydro Logical ScienceDokument311 SeitenAdvances in Geosciences Vol 4 Hydro Logical ScienceJohn Manrique100% (1)
- Hydrology Multiple ChoiceDokument7 SeitenHydrology Multiple ChoiceNarayanan RmNoch keine Bewertungen
- BH LogDokument4 SeitenBH LogstylistikNoch keine Bewertungen
- General ScienceDokument147 SeitenGeneral ScienceyabaeveNoch keine Bewertungen
- Activity Sheet 2Dokument3 SeitenActivity Sheet 2Pangangan NHS40% (5)
- Batu BengkungDokument4 SeitenBatu BengkungAndrik HermantoNoch keine Bewertungen
- Dinosaur Safari Hell Creek PDFDokument11 SeitenDinosaur Safari Hell Creek PDFErika PinkNoch keine Bewertungen
- Spe 181629 MS PDFDokument14 SeitenSpe 181629 MS PDFAvininda FitriaNoch keine Bewertungen
- 1-Principles and Practice of Ground Improvement-Wiley (2015) - 3Dokument1 Seite1-Principles and Practice of Ground Improvement-Wiley (2015) - 3Ahmed KhedrNoch keine Bewertungen
- DIY TrilobiteDokument2 SeitenDIY TrilobiteKewl DudzNoch keine Bewertungen