Beruflich Dokumente
Kultur Dokumente
Biology Investigatory Project On Mendelian Disorders
Hochgeladen von
enish0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
76 Ansichten3 Seitenstandard hydrogen
Originaltitel
Kupdf.net Biology Investigatory Project on Mendelian Disorders
Copyright
© © All Rights Reserved
Verfügbare Formate
DOCX, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenstandard hydrogen
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOCX, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
76 Ansichten3 SeitenBiology Investigatory Project On Mendelian Disorders
Hochgeladen von
enishstandard hydrogen
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOCX, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 3
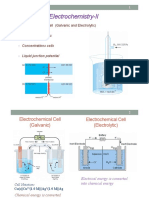
Standard hydrogen electrode
The Standard hydrogen electrode (abbreviated SHE), is a redox electrode which
forms the basis of the thermodynamic scale of oxidation-reduction potentials. Its
absolute electrode potential is estimated to be 4.44 ± 0.02 V at 25 °C, but to form
a basis for comparison with all other electrode reactions, hydrogen's standard
electrode potential (E0) is declared to be zero volts only at 298K.[1] Potentials of
any other electrodes are compared with that of the standard hydrogen electrode
at the same temperature.
Hydrogen electrode is based on the redox half cell:
2 H+(aq) + 2 e− → H2(g)
This redox reaction occurs at a platinized platinum electrode. The electrode is
dipped in an acidic solution and pure hydrogen gas is bubbled through it. The
concentration of both the reduced form and oxidised form is maintained at unity.
That implies that the pressure of hydrogen gas is 1 bar (100 kPa) and the activity
of hydrogen ions in the solution is unity. The activity of hydrogen ions is their
effective concentration, which is equal to the formal concentration times the
activity coefficient. These unit-less activity coefficients are close to 1.00 for very
dilute water solutions, but usually lower for more concentrated solutions.
Construction
The scheme of the standard hydrogen electrode:
1. Platinized platinum electrode
2. hydrogen blow
3. solution of the acid with activity of H+ = 1 mol dm−3
4. hydroseal for prevention of the oxygen interference
5. Reservoir through which the second half-element of the galvanic cell should
be attached. The connection can be direct, through a narrow tube to
reduce mixing, or through a salt bridge, depending on the other electrode
and solution. This creates an ionically conductive path to the working
electrode of interest.
Uses of standard hydrogen electrode
1. It is use to measure the values of standard electrode potentials of some
selected reduction reactions.
Standard
Cathode (Reduction)
Potential
Half-Reaction
E° (volts)
Li+(aq) + e- -> Li(s) -3.04
K+(aq) + e- -> K(s) -2.92
Ca2+(aq) + 2e- -> Ca(s) -2.76
Na+(aq) + e- -> Na(s) -2.71
Mg2+(aq) + 2e- -> Mg(s) -2.38
3+ -
Al (aq) + 3e -> Al(s) -1.66
-
2H2O(l) + 2e -> H2(g) +
-0.83
2OH-(aq)
2. These electrodes are connected to the defibrillator. Adhesive gel
electrodes are commonly used with the automated and semi-automated
units used in ambulance or non-hospital settings due to their ease of
application.
Das könnte Ihnen auch gefallen
- Spiritual Warfare - Mystery Babylon The GreatDokument275 SeitenSpiritual Warfare - Mystery Babylon The GreatBornAgainChristian100% (7)
- RedoxDokument2 SeitenRedoxFiza SakraniNoch keine Bewertungen
- Helen Hodgson - Couple's Massage Handbook Deepen Your Relationship With The Healing Power of TouchDokument268 SeitenHelen Hodgson - Couple's Massage Handbook Deepen Your Relationship With The Healing Power of TouchLuca DatoNoch keine Bewertungen
- PotentiometryDokument27 SeitenPotentiometryShafique Ahmed100% (2)
- Galvanic Cells, The Nernst Equation: Experiment # 2.2Dokument12 SeitenGalvanic Cells, The Nernst Equation: Experiment # 2.2shane escoteNoch keine Bewertungen
- House & Garden - November 2015 AUDokument228 SeitenHouse & Garden - November 2015 AUHussain Elarabi100% (3)
- Electrolytic CellsDokument32 SeitenElectrolytic CellsHendi PratamaNoch keine Bewertungen
- Sample PPP Lesson PlanDokument5 SeitenSample PPP Lesson Planapi-550555211Noch keine Bewertungen
- Sigmund Freud QuotesDokument7 SeitenSigmund Freud Quotesarbeta100% (2)
- Electrochemistry 153strippedDokument65 SeitenElectrochemistry 153strippedJasonTenebrosoNoch keine Bewertungen
- The Magic Limits in Harry PotterDokument14 SeitenThe Magic Limits in Harry Potterdanacream100% (1)
- Practical Research 1 - Quarter 1 - Module 1 - Nature and Inquiry of Research - Version 3Dokument53 SeitenPractical Research 1 - Quarter 1 - Module 1 - Nature and Inquiry of Research - Version 3Iris Rivera-PerezNoch keine Bewertungen
- Standard Hydrogen ElectrodeDokument6 SeitenStandard Hydrogen ElectrodeKks Maths clubNoch keine Bewertungen
- Electrochemistry (Compatibility Mode)Dokument9 SeitenElectrochemistry (Compatibility Mode)Kim Ivan MendozaNoch keine Bewertungen
- Analisis ElektrokimiaDokument108 SeitenAnalisis ElektrokimiaIntan CahyasariNoch keine Bewertungen
- Chapter Five Introduction To Electroanalytical ChemistryDokument16 SeitenChapter Five Introduction To Electroanalytical ChemistryZekarias LibenaNoch keine Bewertungen
- Electrochemistry NotesDokument19 SeitenElectrochemistry NotesLinaNoch keine Bewertungen
- Standard hydrogen electrode (SHEDokument9 SeitenStandard hydrogen electrode (SHEKishore KishoreNoch keine Bewertungen
- Electrochemistry Activity CoefficientsDokument33 SeitenElectrochemistry Activity CoefficientsErnest Nana Yaw AggreyNoch keine Bewertungen
- Electrochemical Cell HLDokument36 SeitenElectrochemical Cell HLRyan BoukaaNoch keine Bewertungen
- Chapter 18 ElectrochemistryDokument19 SeitenChapter 18 ElectrochemistryNagarajan MaradaiyeeNoch keine Bewertungen
- ElectrochemistryDokument42 SeitenElectrochemistryKatarina WuriyaniNoch keine Bewertungen
- Electrochemistry Lesson OverviewDokument11 SeitenElectrochemistry Lesson OverviewBianca VacunawaNoch keine Bewertungen
- Standard Hydrogen ElectrodeDokument15 SeitenStandard Hydrogen ElectrodeHArish RNoch keine Bewertungen
- Electrochemical CellsDokument40 SeitenElectrochemical CellsMohosin MahmudNoch keine Bewertungen
- Electrochemical Energy PotentialDokument50 SeitenElectrochemical Energy PotentialAndrearose Ivy FietasNoch keine Bewertungen
- 2 Electrochemistry (Voltaic Cells)Dokument46 Seiten2 Electrochemistry (Voltaic Cells)Gerald Paul SumagpaoNoch keine Bewertungen
- Standard Hydrogen ElectrodeDokument9 SeitenStandard Hydrogen ElectrodeMuhammad UsamaNoch keine Bewertungen
- Electrochemistry Key ConceptsDokument63 SeitenElectrochemistry Key ConceptsJack WilliamsNoch keine Bewertungen
- ElectroDokument13 SeitenElectrodulalsushant3Noch keine Bewertungen
- Tutorial Sheet7Dokument5 SeitenTutorial Sheet7Lê Anh QuangNoch keine Bewertungen
- MaulikDokument16 SeitenMaulikDevashish JoshiNoch keine Bewertungen
- 6.2 - Standard Electrode Potentials - Chemistry LibreTextsDokument19 Seiten6.2 - Standard Electrode Potentials - Chemistry LibreTextsMildred Mae RodriguezNoch keine Bewertungen
- B. Electrochemistry 2 1Dokument37 SeitenB. Electrochemistry 2 1Dank CoderNoch keine Bewertungen
- Chemical Energy Fuel: Dept of Chemistry, ANITSDokument10 SeitenChemical Energy Fuel: Dept of Chemistry, ANITS320126512165 VSAICHARANGUPTANoch keine Bewertungen
- Electrochemistry NotesDokument32 SeitenElectrochemistry NotesShailendra GargNoch keine Bewertungen
- Lesson 1. ElectrochemistryDokument12 SeitenLesson 1. ElectrochemistryyoureqtNoch keine Bewertungen
- Skcet Class LectureDokument50 SeitenSkcet Class Lecturedineshsilambam2305Noch keine Bewertungen
- Redox Equilibria Revision NotesDokument5 SeitenRedox Equilibria Revision Notes6thuraiNoch keine Bewertungen
- Handout ElectroChemistry BY S.KDokument16 SeitenHandout ElectroChemistry BY S.Katsats815Noch keine Bewertungen
- Basics of electrochemistryDokument41 SeitenBasics of electrochemistryMehul Khimani100% (1)
- Predict the feasibility of redox reactionsDokument22 SeitenPredict the feasibility of redox reactionsGuru temp id-03 for KPM-Guru-TempNoch keine Bewertungen
- ElectrochemistryDokument78 SeitenElectrochemistryGM VillaneaNoch keine Bewertungen
- Electrode PotentialDokument31 SeitenElectrode PotentialseekforheavenNoch keine Bewertungen
- CH.3.14Dokument135 SeitenCH.3.14active learning educationNoch keine Bewertungen
- ElectrochemistryDokument80 SeitenElectrochemistrykunalwahNoch keine Bewertungen
- Reference ElectrodesDokument3 SeitenReference ElectrodesprydeepNoch keine Bewertungen
- Introduction to Electrochemistry: Types of Cells and Half ReactionsDokument7 SeitenIntroduction to Electrochemistry: Types of Cells and Half ReactionsWaien G. WatamamaNoch keine Bewertungen
- ELECTROCHEMISTRY REFERENCESDokument80 SeitenELECTROCHEMISTRY REFERENCESAshish KumarNoch keine Bewertungen
- Standard Hydrogen ElectrodeDokument2 SeitenStandard Hydrogen ElectrodeAmmara Amy100% (1)
- ElectrochemistryDokument27 SeitenElectrochemistry22cs103Noch keine Bewertungen
- Electrochemistry 20222023 (REVIEWED)Dokument101 SeitenElectrochemistry 20222023 (REVIEWED)alyaainsyirah04Noch keine Bewertungen
- Potentiometry: Dr. Kathlia A. de Castro-Cruz Analytical Chemistry For CheDokument59 SeitenPotentiometry: Dr. Kathlia A. de Castro-Cruz Analytical Chemistry For CheCyrus VizonNoch keine Bewertungen
- CHAPTER 2 2023 ElectrochemistryDokument46 SeitenCHAPTER 2 2023 Electrochemistrym.yassinmansor19Noch keine Bewertungen
- SK0014 - Lecture 8 - ElectrochemDokument63 SeitenSK0014 - Lecture 8 - ElectrochemEvaNoch keine Bewertungen
- Oxidation and Reduction (Redox)Dokument56 SeitenOxidation and Reduction (Redox)Abdur RashidNoch keine Bewertungen
- Lec 8bDokument16 SeitenLec 8bdavidolalere7Noch keine Bewertungen
- 3 MergedDokument92 Seiten3 MergedDev LohaniNoch keine Bewertungen
- Chem 17Dokument2 SeitenChem 17keshavNoch keine Bewertungen
- Fourth Hour ContinutaionDokument23 SeitenFourth Hour ContinutaionMegan RiveraNoch keine Bewertungen
- Potentiometry: A Concise Guide to Potentiometric TitrationDokument11 SeitenPotentiometry: A Concise Guide to Potentiometric Titration175-44-Faraz HussainNoch keine Bewertungen
- ElectrochemistryDokument65 SeitenElectrochemistryAhom CK100% (2)
- ElectrozzDokument24 SeitenElectrozzangelaskatsNoch keine Bewertungen
- Sustainable and Green Electrochemical Science and TechnologyVon EverandSustainable and Green Electrochemical Science and TechnologyNoch keine Bewertungen
- Final DSL Under Wire - FinalDokument44 SeitenFinal DSL Under Wire - Finalelect trsNoch keine Bewertungen
- Decision Support System for Online ScholarshipDokument3 SeitenDecision Support System for Online ScholarshipRONALD RIVERANoch keine Bewertungen
- A COIN FOR A BETTER WILDLIFEDokument8 SeitenA COIN FOR A BETTER WILDLIFEDragomir DanielNoch keine Bewertungen
- Hydrocarbon LawDokument48 SeitenHydrocarbon LawParavicoNoch keine Bewertungen
- Multidimensional ScalingDokument25 SeitenMultidimensional ScalingRinkiNoch keine Bewertungen
- Dwnload Full Marriage and Family The Quest For Intimacy 8th Edition Lauer Test Bank PDFDokument35 SeitenDwnload Full Marriage and Family The Quest For Intimacy 8th Edition Lauer Test Bank PDFrainbow.basque1cpq100% (10)
- Mr. Bill: Phone: 086 - 050 - 0379Dokument23 SeitenMr. Bill: Phone: 086 - 050 - 0379teachererika_sjcNoch keine Bewertungen
- Price and Volume Effects of Devaluation of CurrencyDokument3 SeitenPrice and Volume Effects of Devaluation of Currencymutale besaNoch keine Bewertungen
- Daft Presentation 6 EnvironmentDokument18 SeitenDaft Presentation 6 EnvironmentJuan Manuel OvalleNoch keine Bewertungen
- 12 Smart Micro-Habits To Increase Your Daily Productivity by Jari Roomer Better Advice Oct, 2021 MediumDokument9 Seiten12 Smart Micro-Habits To Increase Your Daily Productivity by Jari Roomer Better Advice Oct, 2021 MediumRaja KhanNoch keine Bewertungen
- jvc_kd-av7000_kd-av7001_kd-av7005_kd-av7008_kv-mav7001_kv-mav7002-ma101-Dokument159 Seitenjvc_kd-av7000_kd-av7001_kd-av7005_kd-av7008_kv-mav7001_kv-mav7002-ma101-strelectronicsNoch keine Bewertungen
- MCI FMGE Previous Year Solved Question Paper 2003Dokument0 SeitenMCI FMGE Previous Year Solved Question Paper 2003Sharat Chandra100% (1)
- GPAODokument2 SeitenGPAOZakariaChardoudiNoch keine Bewertungen
- License Key Windows 8Dokument7 SeitenLicense Key Windows 8Juned FahriNoch keine Bewertungen
- Full Download Ebook Ebook PDF Nanomaterials Based Coatings Fundamentals and Applications PDFDokument51 SeitenFull Download Ebook Ebook PDF Nanomaterials Based Coatings Fundamentals and Applications PDFcarolyn.hutchins983100% (43)
- Disability Election ManifestoDokument2 SeitenDisability Election ManifestoDisability Rights AllianceNoch keine Bewertungen
- Inver Powderpaint SpecirficationsDokument2 SeitenInver Powderpaint SpecirficationsArun PadmanabhanNoch keine Bewertungen
- Chengyang Li Archive Cra 23Dokument32 SeitenChengyang Li Archive Cra 23Li ChengyangNoch keine Bewertungen
- The Best Chess BooksDokument3 SeitenThe Best Chess BooksJames Warren100% (1)
- The Butterfly Effect movie review and favorite scenesDokument3 SeitenThe Butterfly Effect movie review and favorite scenesMax Craiven Rulz LeonNoch keine Bewertungen
- Dealer DirectoryDokument83 SeitenDealer DirectorySportivoNoch keine Bewertungen
- Kepler's Law 600 Years Before KeplerDokument7 SeitenKepler's Law 600 Years Before KeplerJoe NahhasNoch keine Bewertungen
- Emergency Order Ratification With AmendmentsDokument4 SeitenEmergency Order Ratification With AmendmentsWestSeattleBlogNoch keine Bewertungen