Beruflich Dokumente
Kultur Dokumente
Duhok Polytechnique University-Petrochemical Department 2018 / 2019 Catalysis DR Farhad M. Ali 2018/2019
Hochgeladen von
MUHAMMAD AKRAMOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Duhok Polytechnique University-Petrochemical Department 2018 / 2019 Catalysis DR Farhad M. Ali 2018/2019
Hochgeladen von
MUHAMMAD AKRAMCopyright:
Verfügbare Formate
4th Year Stage Catalyst Science and Technology Lecture: 03
Duhok Polytechnique University-Petrochemical Department
2018 / 2019
Catalysis
Dr Farhad M. Ali
2018/2019
The term catalysis (from the Greek kata-, “down,” and lyein, “loosen”) was first
employed by the great Swedish chemist Jöns Jacob Berzelius in 1835
1
Page
DR FARHAD M. ALI L03-CATALYSIS-2018-2019
4th Year Stage Catalyst Science and Technology Lecture: 03
Catalysis
What is meant by catalysis?
The phenomenon using catalyst is known as catalysis. It is the process of using catalyst.
Catalysis is the key to chemical transformations. Most industrial syntheses and nearly all
biological reactions require catalysts. Furthermore, catalysis is the most important
technology in environmental protection, i. e., the prevention of emissions, a well-known
example is the catalytic converter for automobiles.
Catalytic reactions were already used in antiquity, although the underlying principle of
catalysis was not recognized at the time. For example, the fermentation of sugar to ethanol
and the conversion of ethanol to ethanoic acid are catalyzed by enzymes (biocatalysts).
However, the systematic scientific development of catalysis only began about 200 years
ago, and its importance has grown up to the present day.
What is a catalyst?
A catalyst is a substance which enhances the rate of a chemical reaction without itself
getting used up in the reaction. A catalyst offers an alternative, energetically favourable
mechanism to the non-catalytic reaction, thus enabling processes to be carried out under
industrially feasible conditions of pressure and temperature.
For an active catalyst, the number of molecules transformed per minute by one molecule
of catalyst may be as large as several million. Where a given substance or a combination
of substances undergoes two or more simultaneous reactions that yield different products,
2
Page
DR FARHAD M. ALI L03-CATALYSIS-2018-2019
4th Year Stage Catalyst Science and Technology Lecture: 03
the distribution of products may be influenced by the use of a catalyst that selectively
accelerates one reaction relative to the other(s).
Does the catalyst affect the chemical equilibrium position?
The catalyst for a given reaction accelerates the reaction in both directions equally.
Therefore, a catalyst does not affect the position of equilibrium of a chemical reaction; it
affects only the rate at which equilibrium is attained. Apparent exceptions to this
generalization are those reactions in which one of the products is also a catalyst for the
reaction. Such reactions are termed autocatalytic
What is the advantage of using catalyst or catalysis?
The main advantages of catalysis is that we get the desired product faster, using fewer
resources, generating less waste, using less energy
▪ A catalyst lowers the activation energy (Ea) by providing a different 'pathway' or
mechanism that makes the bond breaking processes (or other electronic changes in the
reactants) occur more readily.
3
Page
DR FARHAD M. ALI L03-CATALYSIS-2018-2019
4th Year Stage Catalyst Science and Technology Lecture: 03
Catalysis also involves the formation of intermediates, not just a matter of an 'activated
complex' or 'transition state'. e.g. for a transition metal the reactant molecules may be
adsorbed and their bonds weakened, or, for a transition metal compound, it may involve a
change in ligand or oxidation state or other bonding re–arrangement, but will return to its
original state often via a 2–3 stage 'catalytic cycle'.
A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants (or
preceding intermediates) and reacts further to give the directly observed products of
a chemical reaction. Most chemical reactions are stepwise, that is they take more than one
elementary step to complete
Why are the catalysts important?
Many important chemical reactions require inputs of energy to proceed. If a catalyst is
present less energy will be required to complete the reaction.
What the catalyst does?
It provides an alternative reaction pathway of lower activation energy Ea, compared to
4
Page
the uncatalysed reaction.
DR FARHAD M. ALI L03-CATALYSIS-2018-2019
4th Year Stage Catalyst Science and Technology Lecture: 03
Some examples of comparing the activation energies of uncatalysed and catalysed reactions.
Example Ea for Uncatalysed Ea for Catalysed Catalyst
The decomposition of 75 kJmol-1 49 kJmol-1 colloidal platinum
hydrogen peroxide:
23 kJmol enzyme
2H2O2(aq) → 2H2O(l) + O2(g)
The decomposition of 183 kJmol-1 105 kJmol-1 Au
hydrogen iodide:
2HI(g) → H2(g) + I2(g) 58 kJmol-1 Pt
The synthesis of ammonia: 350 kJmol-1 162 kJmol-1 W
N2(g) + 3H2(g) → 2NH3(g)
What is Activation Energy?
Activation energy is the minimum amount of energy that must be available for a chemical
reaction to occur. In order for reactions to proceed we must provide energy to break bonds.
This energy is known as the activation energy for a particular reaction.
Hyperlink for tutor: L03-CATALYSIS-ACTIVATION ENERGY.pptx
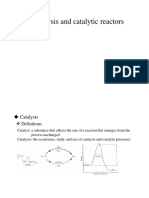
Based on Maxwell -
Boltzmann distribution
description, shows
how reducing the
activation energy
considerably increases
the proportion of particles
with sufficient kinetic
energy to overcome the
barrier of the activation
energy.
5
Page
DR FARHAD M. ALI L03-CATALYSIS-2018-2019
4th Year Stage Catalyst Science and Technology Lecture: 03
A catalyst can be changed physically e.g. the granules can end up more powdery or the
surface become roughened. This may be due to a heat effect from exothermic reactions
or just side effect of regeneration in the catalytic cycle.
e.g. in the laboratory preparation of oxygen from the MnO2(s) catalysed decomposition of
hydrogen peroxide solution, the residual water seems stained brown due to very fine
particles of MnO2(s).
6
Page
DR FARHAD M. ALI L03-CATALYSIS-2018-2019
Das könnte Ihnen auch gefallen
- Green ChemistryDokument3 SeitenGreen Chemistrymanbirsshowdown48Noch keine Bewertungen
- CREII-Module-I - Lecture 4 PDFDokument34 SeitenCREII-Module-I - Lecture 4 PDFshubhamNoch keine Bewertungen
- 9A23401 Mass Transfer OperationsDokument8 Seiten9A23401 Mass Transfer OperationssivabharathamurthyNoch keine Bewertungen
- CP303 - Packed bed reactor & catalytic kineticsDokument7 SeitenCP303 - Packed bed reactor & catalytic kineticsEmanoel FrazãoNoch keine Bewertungen
- Chemical Reaction Engineering 2 (CRE-2Dokument26 SeitenChemical Reaction Engineering 2 (CRE-2AgithaNoch keine Bewertungen
- Catalysis and Catalytic ReactorsDokument59 SeitenCatalysis and Catalytic ReactorssyedmuhammadtariqueNoch keine Bewertungen
- 05 Characterization of Heterogeneous Catalyst (256512)Dokument86 Seiten05 Characterization of Heterogeneous Catalyst (256512)kitchaya UHVNoch keine Bewertungen
- 06 Chapter 37 (Complete)Dokument61 Seiten06 Chapter 37 (Complete)Jian JieNoch keine Bewertungen
- Energy Balnce For Unsteady State SystemsDokument39 SeitenEnergy Balnce For Unsteady State SystemsAbdulRehman VirkNoch keine Bewertungen
- Mass Transfer Mass Transfer Coefficients Notes 10-11-2015Dokument28 SeitenMass Transfer Mass Transfer Coefficients Notes 10-11-2015John OliverNoch keine Bewertungen
- Solution Tutorial 3 Q1-Q10Dokument7 SeitenSolution Tutorial 3 Q1-Q10hoboslayer97Noch keine Bewertungen
- 8 3 Packed Bed ReactorsDokument20 Seiten8 3 Packed Bed ReactorsridhajamelNoch keine Bewertungen
- Membrane Lecture 1Dokument16 SeitenMembrane Lecture 1writtingtuNoch keine Bewertungen
- CHME 314 Lecture 11 Isothermal Reactor Design 2Dokument28 SeitenCHME 314 Lecture 11 Isothermal Reactor Design 2AmroKashtNoch keine Bewertungen
- Chapter 2 - Steady Heat Conduction PDFDokument62 SeitenChapter 2 - Steady Heat Conduction PDFAroon KumarNoch keine Bewertungen
- Stoichiometric TableDokument22 SeitenStoichiometric TableMark Antony LevineNoch keine Bewertungen
- AdsorptionDokument307 SeitenAdsorptionΟδυσσεας ΚοψιδαςNoch keine Bewertungen
- ChE422 Topic 8Dokument40 SeitenChE422 Topic 8Elle EmmNoch keine Bewertungen
- Lecture 6Dokument48 SeitenLecture 6tkjingNoch keine Bewertungen
- Tutorial 1 SolutionDokument3 SeitenTutorial 1 Solutionpleco4meNoch keine Bewertungen
- MEMBRANE FILTRATION WATER TREATMENT PROCESSDokument12 SeitenMEMBRANE FILTRATION WATER TREATMENT PROCESSTanishq GuptaNoch keine Bewertungen
- Adsorption & Ion Exchange ChapterDokument10 SeitenAdsorption & Ion Exchange ChapterDeepak KanjwaniNoch keine Bewertungen
- Cassava FAO I9181EN PDFDokument296 SeitenCassava FAO I9181EN PDFgiambi-1Noch keine Bewertungen
- 6.multiple ReactionsDokument22 Seiten6.multiple ReactionsFarah Talib Al-sudaniNoch keine Bewertungen
- Steady-State Nonisothermal Reactor Design Energy BalanceDokument130 SeitenSteady-State Nonisothermal Reactor Design Energy BalanceYesid Tapiero MartínezNoch keine Bewertungen
- Lecture Notes-Bioreactor Design and Operation-1Dokument19 SeitenLecture Notes-Bioreactor Design and Operation-1lazytinku100% (1)
- CREII-Module-I - Lecture 2 PDFDokument28 SeitenCREII-Module-I - Lecture 2 PDFshubhamNoch keine Bewertungen
- General Introduction: Chapter Four ExtractionDokument19 SeitenGeneral Introduction: Chapter Four ExtractionMujahid HaddadNoch keine Bewertungen
- CHE 304 Problem Set 8 SolutionsDokument5 SeitenCHE 304 Problem Set 8 SolutionsAgustina Evania DewiNoch keine Bewertungen
- Chemical Reaction Engineering10 - 2010Dokument33 SeitenChemical Reaction Engineering10 - 2010Ingrid Claudia ElianneNoch keine Bewertungen
- Ideal Reactor Operations: Vivek RDokument92 SeitenIdeal Reactor Operations: Vivek RHritik LalNoch keine Bewertungen
- ADSORPTION Lect 01Dokument35 SeitenADSORPTION Lect 01MarwahNoch keine Bewertungen
- Enzyme Kinetics ExplainedDokument43 SeitenEnzyme Kinetics ExplainedBlessings Chawinga100% (1)
- Tutorial For Chapter 23Dokument9 SeitenTutorial For Chapter 23Thurgah VshinyNoch keine Bewertungen
- Membrane Separations: Microfiltration ReviewDokument69 SeitenMembrane Separations: Microfiltration ReviewCristina SilvaNoch keine Bewertungen
- Theories of Reaction RatesDokument28 SeitenTheories of Reaction RatesMay AlmogNoch keine Bewertungen
- Adsorption: By: Zhraa Abas 3ed StageDokument18 SeitenAdsorption: By: Zhraa Abas 3ed Stageado cNoch keine Bewertungen
- Thermo-020H Decanter DownLoadLy - IrDokument6 SeitenThermo-020H Decanter DownLoadLy - IrLuis Rodriguez GonzalesNoch keine Bewertungen
- 04 - Catalyst PreparationDokument52 Seiten04 - Catalyst PreparationArdhito SetiawanNoch keine Bewertungen
- Principles of Catalysis: Part 1c: DiffusionDokument29 SeitenPrinciples of Catalysis: Part 1c: DiffusionSyahminazuhan PipimontelNoch keine Bewertungen
- Process Flowsheeting BasicsDokument93 SeitenProcess Flowsheeting BasicsYunaida YusoffNoch keine Bewertungen
- Chemical Reaction Engineering FundamentalsDokument46 SeitenChemical Reaction Engineering FundamentalsDha OstrIxNoch keine Bewertungen
- L2b Reactor Mole Balance Example ProblemsDokument20 SeitenL2b Reactor Mole Balance Example ProblemsDaniel Eduardo AguirreNoch keine Bewertungen
- Mechanical Design (Compile Draft 1)Dokument87 SeitenMechanical Design (Compile Draft 1)IRIZREENNoch keine Bewertungen
- CHE411 Fall 2010-Chemical Reaction Engineeirng-Ahmed A AbdalaDokument206 SeitenCHE411 Fall 2010-Chemical Reaction Engineeirng-Ahmed A AbdalaYayan IndrayaniNoch keine Bewertungen
- 9A23401 Mass Transfer OperationsDokument8 Seiten9A23401 Mass Transfer OperationssivabharathamurthyNoch keine Bewertungen
- Acetone Reactor Design Complete ProjectDokument29 SeitenAcetone Reactor Design Complete ProjectDeni Yudha PermanaNoch keine Bewertungen
- 8.3 - Packed-Bed ReactorsDokument20 Seiten8.3 - Packed-Bed ReactorscarolinacmleiteNoch keine Bewertungen
- Fundamentals of Convection: Heat and Mass Transfer: Fundamentals & ApplicationsDokument58 SeitenFundamentals of Convection: Heat and Mass Transfer: Fundamentals & ApplicationsWalter Nuas100% (2)
- Recovery of Ammonia As Struvite From Anaerobic Digester EffluentsDokument11 SeitenRecovery of Ammonia As Struvite From Anaerobic Digester EffluentsYuni Hapsari Nyit IINoch keine Bewertungen
- Plasma Technology For Solid Waste ManagementDokument12 SeitenPlasma Technology For Solid Waste Managementup4allNoch keine Bewertungen
- Chapter 1 Reaction and Reactor FundamentalsDokument28 SeitenChapter 1 Reaction and Reactor FundamentalsAndy Tan WXNoch keine Bewertungen
- Lithium-Ion Battery Materials Safety Data SheetDokument3 SeitenLithium-Ion Battery Materials Safety Data SheetIqbal FirdausNoch keine Bewertungen
- Biochemical Oxygen Demand (BOD) Chemical Oxygen Demand (COD)Dokument35 SeitenBiochemical Oxygen Demand (BOD) Chemical Oxygen Demand (COD)wahyu hidayatNoch keine Bewertungen
- Vector CalculusDokument37 SeitenVector CalculuserNoch keine Bewertungen
- Che516 Lecture NotesDokument69 SeitenChe516 Lecture NotesifiokNoch keine Bewertungen
- Reactor Design InsightsDokument9 SeitenReactor Design InsightsHanjin SeoNoch keine Bewertungen
- Thermocouple CallibrationDokument6 SeitenThermocouple CallibrationMUHAMMAD AKRAMNoch keine Bewertungen
- Closed System Continous - ExpDokument7 SeitenClosed System Continous - ExpMUHAMMAD AKRAMNoch keine Bewertungen
- Smoke PointDokument7 SeitenSmoke Pointfaizfrasat123Noch keine Bewertungen
- Catalyst ActivityDokument14 SeitenCatalyst ActivityMUHAMMAD AKRAMNoch keine Bewertungen
- Archimedes Principle ExperimentDokument8 SeitenArchimedes Principle ExperimentMUHAMMAD AKRAMNoch keine Bewertungen
- Determine Gas Diffusion CoefficientDokument4 SeitenDetermine Gas Diffusion CoefficientMUHAMMAD AKRAMNoch keine Bewertungen
- Viscosity BwarDokument11 SeitenViscosity BwarMUHAMMAD AKRAM100% (1)
- Losses in Pipe Systems and FittingsDokument15 SeitenLosses in Pipe Systems and FittingsMUHAMMAD AKRAMNoch keine Bewertungen
- Catalysts and Oxygen ExperimentDokument12 SeitenCatalysts and Oxygen ExperimentMUHAMMAD AKRAMNoch keine Bewertungen
- Experment 2Dokument4 SeitenExperment 2MUHAMMAD AKRAMNoch keine Bewertungen
- Transport PhenomenaDokument9 SeitenTransport PhenomenaMUHAMMAD AKRAMNoch keine Bewertungen
- Duhok Polytechnic UniversityDokument6 SeitenDuhok Polytechnic UniversityMUHAMMAD AKRAMNoch keine Bewertungen
- DPU Catalyst Science & Technology Lab ReportDokument12 SeitenDPU Catalyst Science & Technology Lab ReportMUHAMMAD AKRAMNoch keine Bewertungen
- PhotosynthesisDokument16 SeitenPhotosynthesisMUHAMMAD AKRAMNoch keine Bewertungen
- Experiment Measures Pressure Losses in Pipes & FittingsDokument5 SeitenExperiment Measures Pressure Losses in Pipes & FittingsMUHAMMAD AKRAMNoch keine Bewertungen
- Energy Engineering: Fluid Mechanic (Lab)Dokument8 SeitenEnergy Engineering: Fluid Mechanic (Lab)MUHAMMAD AKRAMNoch keine Bewertungen
- Lect 1 Pavement Design1928210425021488949Dokument27 SeitenLect 1 Pavement Design1928210425021488949MUHAMMAD AKRAMNoch keine Bewertungen
- Catalytic Hydrocracking Process OverviewDokument7 SeitenCatalytic Hydrocracking Process OverviewMUHAMMAD AKRAMNoch keine Bewertungen
- 1Dokument3 Seiten1MUHAMMAD AKRAMNoch keine Bewertungen
- Click To Edit Master Subtitle StyleDokument16 SeitenClick To Edit Master Subtitle StyleMUHAMMAD AKRAMNoch keine Bewertungen
- Duhok Polytechnic University: Unit Operation Laboratory of Unit OperationDokument7 SeitenDuhok Polytechnic University: Unit Operation Laboratory of Unit OperationMUHAMMAD AKRAMNoch keine Bewertungen
- Name: Muhammad Akram Group:A Difference and Definition of (Free Radical and Ions (Cation & Anion) )Dokument1 SeiteName: Muhammad Akram Group:A Difference and Definition of (Free Radical and Ions (Cation & Anion) )MUHAMMAD AKRAMNoch keine Bewertungen
- 1Dokument3 Seiten1MUHAMMAD AKRAMNoch keine Bewertungen
- Duhok Polytechnic University: Unit Operation Laboratory of Unit OperationDokument7 SeitenDuhok Polytechnic University: Unit Operation Laboratory of Unit OperationMUHAMMAD AKRAMNoch keine Bewertungen
- 1Dokument3 Seiten1MUHAMMAD AKRAMNoch keine Bewertungen
- 1Dokument3 Seiten1MUHAMMAD AKRAMNoch keine Bewertungen
- 1Dokument3 Seiten1MUHAMMAD AKRAMNoch keine Bewertungen
- Cationic PolmerizationDokument10 SeitenCationic PolmerizationMUHAMMAD AKRAMNoch keine Bewertungen
- PetroleumDokument1 SeitePetroleumMUHAMMAD AKRAMNoch keine Bewertungen
- Pew 209 .02Dokument59 SeitenPew 209 .02Raj BindasNoch keine Bewertungen
- DPP No. 11: Physical ChemistryDokument14 SeitenDPP No. 11: Physical ChemistryViraj ShahNoch keine Bewertungen
- Motor Size SelectionDokument4 SeitenMotor Size SelectionArnoah RamirezNoch keine Bewertungen
- 27.viscoelastic Responses of Polyhedral Oli PDFDokument5 Seiten27.viscoelastic Responses of Polyhedral Oli PDFVansala GanesanNoch keine Bewertungen
- 4 Tension MemberDokument15 Seiten4 Tension Memberhari1008108Noch keine Bewertungen
- The Impact of Preheating and Postcooling On Hot Dip GalvanizingDokument6 SeitenThe Impact of Preheating and Postcooling On Hot Dip GalvanizingkhurshedlakhoNoch keine Bewertungen
- Model Exam - 1Dokument6 SeitenModel Exam - 1Bipul Poudel100% (2)
- I-Puls EN V2Dokument17 SeitenI-Puls EN V2Diego MondragonNoch keine Bewertungen
- ElectroplatingDokument16 SeitenElectroplatingheemadave50% (2)
- Electric Log PDFDokument1 SeiteElectric Log PDFAyu FadhilahNoch keine Bewertungen
- Process Engineers Checklist HAZOPDokument4 SeitenProcess Engineers Checklist HAZOPashumishra007Noch keine Bewertungen
- EuleDokument9 SeitenEuleAnand Utsav KapoorNoch keine Bewertungen
- Tablas SI - Moran & Shapiro - Fundamentals of Engineering Thermodynamics 5th Edition (Con R12)Dokument53 SeitenTablas SI - Moran & Shapiro - Fundamentals of Engineering Thermodynamics 5th Edition (Con R12)Yasir MumtazNoch keine Bewertungen
- The Specific Static Rotor Work YpDokument36 SeitenThe Specific Static Rotor Work YpFiraol DinaolNoch keine Bewertungen
- NatureDokument18 SeitenNatureAs PNoch keine Bewertungen
- Che 3G04 Winter 2004 Mcmaster UniversityDokument33 SeitenChe 3G04 Winter 2004 Mcmaster UniversityAris Koreya100% (2)
- Solar Air Conditioning ThesisDokument7 SeitenSolar Air Conditioning Thesisjjvveqvcf100% (2)
- ChE 100 HW7Dokument5 SeitenChE 100 HW7kelly_wu_5Noch keine Bewertungen
- Laboratory Measurement of Capillary PressureDokument17 SeitenLaboratory Measurement of Capillary Pressureel hadiNoch keine Bewertungen
- Low Power-Consumption CO2 Sensor: (Model NO:MG-812)Dokument6 SeitenLow Power-Consumption CO2 Sensor: (Model NO:MG-812)Nguyen Vu Hoang ThachNoch keine Bewertungen
- Balancing of Reciprocating MassesDokument24 SeitenBalancing of Reciprocating MassesSomnath SomadderNoch keine Bewertungen
- Advantages & Disadvantages of Glass As A Building MaterialDokument9 SeitenAdvantages & Disadvantages of Glass As A Building MaterialRohithNoch keine Bewertungen
- Workshop Exercise - CogenerationDokument3 SeitenWorkshop Exercise - CogenerationJurij BlaslovNoch keine Bewertungen
- A286 Tech DataDokument5 SeitenA286 Tech Datagowtham raju buttiNoch keine Bewertungen
- The Thermoset Difference: Thermoset vs. Thermoplastic: © 2014 Davies Molding LLCDokument4 SeitenThe Thermoset Difference: Thermoset vs. Thermoplastic: © 2014 Davies Molding LLCshafiraNoch keine Bewertungen
- Wall Footing DesignDokument4 SeitenWall Footing DesignAddrien DanielNoch keine Bewertungen
- Duplex and Superduplex Stainless Steel Fittings (Amendments/Supplements To Astm A 815)Dokument11 SeitenDuplex and Superduplex Stainless Steel Fittings (Amendments/Supplements To Astm A 815)Mathew CherianNoch keine Bewertungen
- PSPDokument56 SeitenPSParavind kumarNoch keine Bewertungen
- CH 8 Slides 10th Ed SI Aula 1Dokument26 SeitenCH 8 Slides 10th Ed SI Aula 1M Usama SiddiquiNoch keine Bewertungen
- Xapp1301 Mechanical Thermal Design GuidelinesDokument88 SeitenXapp1301 Mechanical Thermal Design GuidelinesJogirek VekturNoch keine Bewertungen