Beruflich Dokumente
Kultur Dokumente
Insert.C.f.a.s. PUC.03121330001.V4.en
Hochgeladen von
Guneyden GuneydenCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Insert.C.f.a.s. PUC.03121330001.V4.en
Hochgeladen von
Guneyden GuneydenCopyright:
Verfügbare Formate
03121330001V4.
C.f.a.s. PUC
03121305 122 5 x 1 mL Calibrator
English of care as a patient specimen. In the event of exposure, the directives of the
responsible health authorities should be followed.1,2
System information
For use on Roche/Hitachi analyzers, Roche/Hitachi MODULAR and Handling
cobas c analyzers the calibrator code is 489. The product is ready for use. Mix carefully before use. Avoid the formation
For use on COBAS INTEGRA analyzers the system ID is 07 6755 7. of foam.
The enclosed barcoded labels are intended exclusively for
Intended use Roche/Hitachi MODULAR automated analyzers and cobas c systems to
C.f.a.s. (Calibrator for automated systems) PUC (Proteins in Urine/CSF) is identify the calibrator. Attach the barcoded labels to the tubes carrying the
for use in the calibration of quantitative Roche methods on Roche clinical sample cups containing the calibrator material.
chemistry analyzers as specified in the value sheets.
Storage and stability
Summary Store at 2‑8 °C.
C.f.a.s. PUC is a liquid ready-for-use calibrator based on a buffered
aqueous solution. Criterion for the stability data stated by Roche:
The concentrations of the calibrator components have been adjusted to Recovery within ± 10 % of initial value.
ensure optimal calibration of the appropriate Roche methods on clinical Stability:
chemistry analyzers.
Some methods specified in the relevant value sheet may not be available in Unopened: Up to the stated expiration date at 2‑8 °C.
all countries. After opening: 4 weeks at 2‑8 °C, provided that dispensing of
Reagents – working solutions the calibrator occurs without microbial
Reactive components: contamination, e.g. by pouring out.
HEPES buffer: 20 mmol/L, pH 7.5, and chemical additives and material of Store calibrator tightly capped when not in use.
biological origin as specified.
Materials provided
The origin of the biological additives is as follows:
▪ See “Reagents – working solutions” section
Analyte Origin ▪ Barcoded labels
Albumin human serum Materials required (but not provided)
α1‑Microglobulin human urine ▪ Roche system reagents and clinical chemistry analyzers
Immunoglobulin G human serum ▪ General laboratory equipment
Total protein human serum/sheep serum Assay
Non-reactive components: Use C.f.a.s. PUC as specified in the relevant Method Sheet for the system
reagents.
Preservatives and stabilizers
References
The concentrations of the calibrator components are lot‑specific. The exact
calibrator values are given in the electronically available or enclosed value 1 Occupational Safety and Health Standards: Bloodborne pathogens.
sheets. (29 CFR Part 1910.1030). Fed. Register.
The values are also encoded in the enclosed calibrator barcode sheets for 2 Directive 2000/54/EC of the European Parliament and Council of
Roche/Hitachi MODULAR, COBAS INTEGRA and cobas c 111 analyzers. 18 September 2000 on the protection of workers from risks related to
exposure to biological agents at work.
For the cobas c analyzers (except for the cobas c 111 analyzer) the values
are encoded in electronic files sent via the cobas link to the analyzers. A point (period/stop) is always used in this Method Sheet as the decimal
separator to mark the border between the integral and the fractional parts of
Calibrator values a decimal numeral. Separators for thousands are not used.
The calibrator values were determined using the method stated in the
electronically available or enclosed value sheets. Determinations were Symbols
performed under strictly standardized conditions on Roche analyzers using Roche Diagnostics uses the following symbols and signs in addition to
Roche system reagents and the Roche master calibrator. those listed in the ISO 15223‑1 standard (for USA: see
The calibrator values were obtained via single determinations performed in https://usdiagnostics.roche.com for definition of symbols used):
different laboratories, in several separate runs. The calibrator value Contents of kit
specified is the median of all values obtained.
Traceability information is given in the relevant Method Sheets for the Calibrator
system reagents. Volume after reconstitution or mixing
Precautions and warnings GTIN Global Trade Item Number
For in vitro diagnostic use.
Exercise the normal precautions required for handling all laboratory FOR US CUSTOMERS ONLY: LIMITED WARRANTY
reagents.
Disposal of all waste material should be in accordance with local guidelines. Roche Diagnostics warrants that this product will meet the specifications
Safety data sheet available for professional user on request. stated in the labeling when used in accordance with such labeling and will
For USA: Caution: Federal law restricts this device to sale by or on the be free from defects in material and workmanship until the expiration date
order of a physician. printed on the label. THIS LIMITED WARRANTY IS IN LIEU OF ANY
All human material should be considered potentially infectious. All products OTHER WARRANTY, EXPRESS OR IMPLIED, INCLUDING ANY IMPLIED
derived from human blood are prepared exclusively from the blood of WARRANTY OF MERCHANTABILITY OR FITNESS FOR PARTICULAR
donors tested individually and shown to be free from HBsAg and antibodies PURPOSE. IN NO EVENT SHALL ROCHE DIAGNOSTICS BE LIABLE
to HCV and HIV. The testing methods used assays approved by the FDA or FOR INCIDENTAL, INDIRECT, SPECIAL OR CONSEQUENTIAL
cleared in compliance with the European Directive 98/79/EC, Annex II,
List A. DAMAGES.
However, as no testing method can rule out the potential risk of infection COBAS, COBAS C and COBAS INTEGRA are trademarks of Roche.
with absolute certainty, the material should be handled with the same level All other product names and trademarks are the property of their respective owners.
2018-11, V 4.0 English 1/2
03121330001V4.0
C.f.a.s. PUC
Additions, deletions or changes are indicated by a change bar in the margin.
© 2017, Roche Diagnostics
Roche Diagnostics GmbH, Sandhofer Strasse 116, D-68305 Mannheim
www.roche.com
Distribution in USA by:
Roche Diagnostics, Indianapolis, IN
US Customer Technical Support 1-800-428-2336
2/2 2018-11, V 4.0 English
Das könnte Ihnen auch gefallen
- Lyphochek Immunoassay Plus Control Levels 1, 2 and 3Dokument11 SeitenLyphochek Immunoassay Plus Control Levels 1, 2 and 3Enrique DuarteNoch keine Bewertungen
- Integra 400s Operartion Sop PDFDokument11 SeitenIntegra 400s Operartion Sop PDFBasheer AlmetwakelNoch keine Bewertungen
- Instructions For Use CRP: VITROS Chemistry Products CRP SlidesDokument12 SeitenInstructions For Use CRP: VITROS Chemistry Products CRP SlidesKemal MuratspahicNoch keine Bewertungen
- Therapeutic Nutrition: Khien S. Sasi, RN, ManDokument33 SeitenTherapeutic Nutrition: Khien S. Sasi, RN, ManKhien Sasi100% (4)
- Lab Policies Hemoglobin A1C - Cobas c501 Lab 4004Dokument6 SeitenLab Policies Hemoglobin A1C - Cobas c501 Lab 4004yosefin100% (1)
- UserManual PDFDokument546 SeitenUserManual PDFKader SmailiNoch keine Bewertungen
- 04 FS Package InsertDokument6 Seiten04 FS Package InsertImas NurhayatiNoch keine Bewertungen
- BIOLABO Applications BIOSYSTEMS A15 A25!18!11 2014Dokument31 SeitenBIOLABO Applications BIOSYSTEMS A15 A25!18!11 2014TaThach100% (1)
- Operator Manual BT4500-00ING PDFDokument153 SeitenOperator Manual BT4500-00ING PDFquanvh0% (1)
- CYANSmart App Sheets ENGDokument60 SeitenCYANSmart App Sheets ENGMaria Alejandra Noguera Delgadillo100% (1)
- Epidemiological Cutoff Values For Antifungal Susceptibility TestingDokument12 SeitenEpidemiological Cutoff Values For Antifungal Susceptibility TestingGuneyden GuneydenNoch keine Bewertungen
- Runyankore-Rukiga Dictionary Launch: President Yoweri Museveni's SpeechDokument28 SeitenRunyankore-Rukiga Dictionary Launch: President Yoweri Museveni's SpeechThe New Vision50% (2)
- EXP4 The Diels Alder ReactionsDokument3 SeitenEXP4 The Diels Alder ReactionsLaura GuidoNoch keine Bewertungen
- Insert.C.f.a.s. Lipids.03018415001.V7.enDokument2 SeitenInsert.C.f.a.s. Lipids.03018415001.V7.enGuneyden Guneyden100% (1)
- Calsetfer PDFDokument2 SeitenCalsetfer PDFMadalina Cioroiu-AndronescuNoch keine Bewertungen
- Precinorm U Plus.12173581001.v10.en PDFDokument2 SeitenPrecinorm U Plus.12173581001.v10.en PDFARIF AHAMMED P0% (1)
- Precipath HDL - LDL-C.11818171001.V10.en PDFDokument2 SeitenPrecipath HDL - LDL-C.11818171001.V10.en PDFARIF AHAMMED PNoch keine Bewertungen
- PreciControl Cardiac II - Ms - 04917049190.V9.EnDokument2 SeitenPreciControl Cardiac II - Ms - 04917049190.V9.EnARIF AHAMMED PNoch keine Bewertungen
- PreciControl Varia - Ms 05618860190.V5.EnDokument2 SeitenPreciControl Varia - Ms 05618860190.V5.EnARIF AHAMMED P100% (1)
- CREJ2Dokument4 SeitenCREJ2ARIF AHAMMED PNoch keine Bewertungen
- Urea Ingles PDFDokument1 SeiteUrea Ingles PDFcesiahdezNoch keine Bewertungen
- Value Sheet - PreciControl ClinChem Multi 1.05117208922.Lot-410119.Exp-2023-01-31.V288.enDokument13 SeitenValue Sheet - PreciControl ClinChem Multi 1.05117208922.Lot-410119.Exp-2023-01-31.V288.enLAB CITO RSUDZANoch keine Bewertungen
- PreciControl ClinChem Multi 2.05117224001.V4.EnDokument2 SeitenPreciControl ClinChem Multi 2.05117224001.V4.EnARIF AHAMMED P29% (7)
- Randox ControlDokument17 SeitenRandox ControlLuis Ferdinand Dacera-Gabronino Gamponia-Nonan100% (1)
- PreciControl ISD - Ms - 05889081190.v3.en PDFDokument2 SeitenPreciControl ISD - Ms - 05889081190.v3.en PDFARIF AHAMMED PNoch keine Bewertungen
- ALP Single ReagentDokument2 SeitenALP Single ReagentJames 'jps' SimanjuntakNoch keine Bewertungen
- CK-MB Liquiuv: Liquid Nac Activated Uv Test Creatine Kinase (Ec 2.7.3.2)Dokument1 SeiteCK-MB Liquiuv: Liquid Nac Activated Uv Test Creatine Kinase (Ec 2.7.3.2)Maher100% (1)
- Totalt3 ArcDokument6 SeitenTotalt3 ArcTanveerNoch keine Bewertungen
- ETOHDokument4 SeitenETOHARIF AHAMMED PNoch keine Bewertungen
- HbA1c Control MaterialDokument2 SeitenHbA1c Control MaterialMichael TanglaoNoch keine Bewertungen
- A1C-2 Whole Blood enDokument6 SeitenA1C-2 Whole Blood enSyahdie FahledieNoch keine Bewertungen
- Fibrinogeno Multifibren UDokument6 SeitenFibrinogeno Multifibren UFiore M.Noch keine Bewertungen
- Prolactin - IMMULITE and IMMULITE 1000 - Rev 13 DXDCM 09017fe9802977c5-1538194815088Dokument32 SeitenProlactin - IMMULITE and IMMULITE 1000 - Rev 13 DXDCM 09017fe9802977c5-1538194815088Guneyden GuneydenNoch keine Bewertungen
- BS-200 Brochura ENDokument3 SeitenBS-200 Brochura ENmdkNoch keine Bewertungen
- Diagnostic Reagents: Manual MethodsDokument49 SeitenDiagnostic Reagents: Manual Methodsgerman.martinezd3528Noch keine Bewertungen
- GLLUDokument4 SeitenGLLUARIF AHAMMED PNoch keine Bewertungen
- Urea Liquiuv: GLDH Method Fully Enzymatic Method For Kinetic Determi-Nation of UreaDokument1 SeiteUrea Liquiuv: GLDH Method Fully Enzymatic Method For Kinetic Determi-Nation of UreaMaher100% (2)
- Multi Control Beckman CoulterDokument4 SeitenMulti Control Beckman CoulterDani DAshing100% (2)
- CYSC2Dokument4 SeitenCYSC2ARIF AHAMMED PNoch keine Bewertungen
- Totalt4 ArcDokument6 SeitenTotalt4 Arctesteste testeNoch keine Bewertungen
- En C#k#prest 20180228Dokument1 SeiteEn C#k#prest 20180228dian fantriNoch keine Bewertungen
- Cobas E411 Sell Sheet PDFDokument2 SeitenCobas E411 Sell Sheet PDFrajkumarNoch keine Bewertungen
- ACL TOP Family SOP Manual 2016-10-19 enDokument238 SeitenACL TOP Family SOP Manual 2016-10-19 enAndra Radulescu100% (1)
- Lyphochek Assayed Chemistry Control Levels 1 and 2: Revision Date 2020-10-26 Indicates Revised InformationDokument1 SeiteLyphochek Assayed Chemistry Control Levels 1 and 2: Revision Date 2020-10-26 Indicates Revised InformationcassNoch keine Bewertungen
- Furuno Ca-180 / Ilab 350 / RX Daytona InstructionsDokument75 SeitenFuruno Ca-180 / Ilab 350 / RX Daytona InstructionsDharmesh Patel100% (1)
- Urea DialabDokument2 SeitenUrea DialabDian Ayu UtamiNoch keine Bewertungen
- HM 12 38078v1 WW Ruby Brochure 8.5x11 100112Dokument8 SeitenHM 12 38078v1 WW Ruby Brochure 8.5x11 100112vijayramaswamyNoch keine Bewertungen
- Brochure BS-240PRO MINDRAYDokument4 SeitenBrochure BS-240PRO MINDRAYmiguelNoch keine Bewertungen
- Randox ControlDokument103 SeitenRandox ControlSunlifecare CardNoch keine Bewertungen
- Value Sheet - PreciControl ClinChem Multi 2.05117216190.Lot-535719.Exp-2024-08-31.V317.enDokument7 SeitenValue Sheet - PreciControl ClinChem Multi 2.05117216190.Lot-535719.Exp-2024-08-31.V317.enmirandatapia23Noch keine Bewertungen
- Mindray BS200 User ManualDokument6 SeitenMindray BS200 User ManualMichael OkekeNoch keine Bewertungen
- G26interno Brochure PDFDokument6 SeitenG26interno Brochure PDFCan ArasNoch keine Bewertungen
- Insert - Elecsys ACTH.07026684500.V2.EnDokument4 SeitenInsert - Elecsys ACTH.07026684500.V2.EnGuneyden GuneydenNoch keine Bewertungen
- File - 20230224 - 153859 - 1247ue 2026-01Dokument66 SeitenFile - 20230224 - 153859 - 1247ue 2026-01Trần Văn Bình100% (1)
- T3 IflashDokument4 SeitenT3 IflashNIGHT tubeNoch keine Bewertungen
- Amy 110 - 440 XL-1000 - Xsys0003 - 91 - GDokument4 SeitenAmy 110 - 440 XL-1000 - Xsys0003 - 91 - GpetertrungNoch keine Bewertungen
- SI Units For Clinical DataDokument6 SeitenSI Units For Clinical DataAhmed AliNoch keine Bewertungen
- Atellica HEMA RET PLTO 0.5 L x4 Reagent - Atellica HEMA - Rev 03 DXDCM 09017fe98070c55b-1658506190586Dokument6 SeitenAtellica HEMA RET PLTO 0.5 L x4 Reagent - Atellica HEMA - Rev 03 DXDCM 09017fe98070c55b-1658506190586yousra zeidanNoch keine Bewertungen
- Sodium Reagent Kit (Mono Test) : Colorimetric MethodDokument1 SeiteSodium Reagent Kit (Mono Test) : Colorimetric MethodFazal RazaNoch keine Bewertungen
- BS-200 KineticsDokument52 SeitenBS-200 KineticsHammam Gaza100% (1)
- Instructions For Use TSHDokument12 SeitenInstructions For Use TSHBenjamin MannNoch keine Bewertungen
- Insert - Precinorm PUC.03121321001.V4.enDokument2 SeitenInsert - Precinorm PUC.03121321001.V4.enVegha NedyaNoch keine Bewertungen
- C.F.A.S. Hba1C: English System InformationDokument2 SeitenC.F.A.S. Hba1C: English System Informationtechlab100% (1)
- Insert - Precinorm PUC.03121321001.V5.enDokument2 SeitenInsert - Precinorm PUC.03121321001.V5.enIfthon Adji PrastyoNoch keine Bewertungen
- File 1434571027Dokument1 SeiteFile 1434571027Guneyden GuneydenNoch keine Bewertungen
- Ion 510 & Ion 520 & Ion 530 Kit - Chef: Quick ReferenceDokument7 SeitenIon 510 & Ion 520 & Ion 530 Kit - Chef: Quick ReferenceGuneyden GuneydenNoch keine Bewertungen
- Clinitest®: Rapid COVID-19 Antigen TestDokument32 SeitenClinitest®: Rapid COVID-19 Antigen TestGuneyden GuneydenNoch keine Bewertungen
- EUCAST E Def 9.3.2 Mould Testing Definitive Revised 2020Dokument23 SeitenEUCAST E Def 9.3.2 Mould Testing Definitive Revised 2020Guneyden GuneydenNoch keine Bewertungen
- EUCAST E Def 7.3.2 Yeast Testing Definitive Revised 2020Dokument21 SeitenEUCAST E Def 7.3.2 Yeast Testing Definitive Revised 2020Guneyden GuneydenNoch keine Bewertungen
- HCV Genotype Plus Real-TM: HandbookDokument16 SeitenHCV Genotype Plus Real-TM: HandbookGuneyden GuneydenNoch keine Bewertungen
- Inv 2022 0058Dokument1 SeiteInv 2022 0058Guneyden GuneydenNoch keine Bewertungen
- Cartridge of 50 Disks: Disks For Antifungal Susceptibility TestingDokument3 SeitenCartridge of 50 Disks: Disks For Antifungal Susceptibility TestingGuneyden GuneydenNoch keine Bewertungen
- LifePad Cartridge IFUDokument21 SeitenLifePad Cartridge IFUGuneyden GuneydenNoch keine Bewertungen
- Pneumocystis Stain Kit: Catalog Number: KT030Dokument1 SeitePneumocystis Stain Kit: Catalog Number: KT030Guneyden GuneydenNoch keine Bewertungen
- Assay Summary: ADVIA Centaur CPDokument16 SeitenAssay Summary: ADVIA Centaur CPGuneyden GuneydenNoch keine Bewertungen
- Eucast Rast Breakpoint Table V 4.0 PDFDokument14 SeitenEucast Rast Breakpoint Table V 4.0 PDFGuneyden GuneydenNoch keine Bewertungen
- European Committee On Antimicrobial Susceptibility TestingDokument9 SeitenEuropean Committee On Antimicrobial Susceptibility TestingGuneyden GuneydenNoch keine Bewertungen
- Anti-Ccp Igg (Accp) : Intended UseDokument20 SeitenAnti-Ccp Igg (Accp) : Intended UseGuneyden GuneydenNoch keine Bewertungen
- Assay Summary: ADVIA Centaur CPDokument14 SeitenAssay Summary: ADVIA Centaur CPGuneyden GuneydenNoch keine Bewertungen
- ADVIA Centaur CP Immunoassay System Operator S Guide, En, 086D0320 DXDCM 09017fe9803185c3-1554435436510Dokument364 SeitenADVIA Centaur CP Immunoassay System Operator S Guide, En, 086D0320 DXDCM 09017fe9803185c3-1554435436510Guneyden GuneydenNoch keine Bewertungen
- CA 15-3 Calibrator: Scheduling The CalibratorsDokument2 SeitenCA 15-3 Calibrator: Scheduling The CalibratorsGuneyden GuneydenNoch keine Bewertungen
- Assay Summary: ADVIA Centaur CPDokument18 SeitenAssay Summary: ADVIA Centaur CPGuneyden GuneydenNoch keine Bewertungen
- anti-TPO (aTPO) : Assay For The Detection of Autoantibodies Against Thyroid PeroxidaseDokument10 Seitenanti-TPO (aTPO) : Assay For The Detection of Autoantibodies Against Thyroid PeroxidaseGuneyden GuneydenNoch keine Bewertungen
- Prolactin - IMMULITE and IMMULITE 1000 - Rev 13 DXDCM 09017fe9802977c5-1538194815088Dokument32 SeitenProlactin - IMMULITE and IMMULITE 1000 - Rev 13 DXDCM 09017fe9802977c5-1538194815088Guneyden GuneydenNoch keine Bewertungen
- Assay Summary: ADVIA Centaur CPDokument14 SeitenAssay Summary: ADVIA Centaur CPGuneyden GuneydenNoch keine Bewertungen
- Bsis39 Cobre 2015Dokument2 SeitenBsis39 Cobre 2015Guneyden GuneydenNoch keine Bewertungen
- BMS Iddt 2020 41Dokument8 SeitenBMS Iddt 2020 41Guneyden GuneydenNoch keine Bewertungen
- HB-0546-002 1101279 - PCard - MA - DNA - Blood - M48 - 0316 - WW - WEBDokument2 SeitenHB-0546-002 1101279 - PCard - MA - DNA - Blood - M48 - 0316 - WW - WEBGuneyden GuneydenNoch keine Bewertungen
- Instruction System: Installation and Startup of The AnalyzerDokument32 SeitenInstruction System: Installation and Startup of The AnalyzerGuneyden GuneydenNoch keine Bewertungen
- Ancillary Probe Wash 1 - ADVIA Centaur DXDCM 09008b808123b69a-1553854661843Dokument1 SeiteAncillary Probe Wash 1 - ADVIA Centaur DXDCM 09008b808123b69a-1553854661843Guneyden GuneydenNoch keine Bewertungen
- Dimension Exl™ Dimension Exl™ Dimension Exl™ Dimension Exl™Dokument2 SeitenDimension Exl™ Dimension Exl™ Dimension Exl™ Dimension Exl™Guneyden GuneydenNoch keine Bewertungen
- Aspartate Aminotransferase (AST-GOT) - ColorimetricDokument2 SeitenAspartate Aminotransferase (AST-GOT) - ColorimetricGuneyden Guneyden0% (1)
- 307210Dokument4 Seiten307210Guneyden GuneydenNoch keine Bewertungen
- Lesson Plan in Science 9: I. ObjectivesDokument8 SeitenLesson Plan in Science 9: I. ObjectivesmarichuNoch keine Bewertungen
- Cobalamin in Companion AnimalsDokument8 SeitenCobalamin in Companion AnimalsFlávia UchôaNoch keine Bewertungen
- Flow Chart - QCDokument2 SeitenFlow Chart - QCKarthikeyan Shanmugavel100% (1)
- NF en Iso 5167-6-2019Dokument22 SeitenNF en Iso 5167-6-2019Rem FgtNoch keine Bewertungen
- Worksheet Series 5: Answer Any 3 Questions From 1 To 6. (2 Score Each)Dokument6 SeitenWorksheet Series 5: Answer Any 3 Questions From 1 To 6. (2 Score Each)AswithNoch keine Bewertungen
- Better - Homes.and - Gardens.usa - TruePDF December.2018Dokument136 SeitenBetter - Homes.and - Gardens.usa - TruePDF December.2018MadaMadutsaNoch keine Bewertungen
- TM-8000 HD Manual PDFDokument37 SeitenTM-8000 HD Manual PDFRoxana BirtumNoch keine Bewertungen
- 300 PSI CTS (MP-1115) Operation Manual Rev1.3Dokument18 Seiten300 PSI CTS (MP-1115) Operation Manual Rev1.3Juan Manuel VizosoNoch keine Bewertungen
- 9 5 - 358 362 PDFDokument5 Seiten9 5 - 358 362 PDFمالك مناصرةNoch keine Bewertungen
- Curriculum Vitae - RadikaDokument3 SeitenCurriculum Vitae - RadikaradikahendryNoch keine Bewertungen
- SDHI18 - Komparativna Analiza Primene Vodostana I Sinhronih Regulatora TurbinaDokument13 SeitenSDHI18 - Komparativna Analiza Primene Vodostana I Sinhronih Regulatora TurbinaAleksandar PetkovicNoch keine Bewertungen
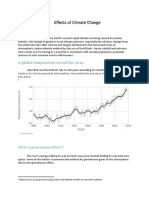
- Effects of Climate ChangeDokument3 SeitenEffects of Climate Changejiofjij100% (1)
- Curing Obesity, WorldwideDokument6 SeitenCuring Obesity, WorldwideHernán SanabriaNoch keine Bewertungen
- Dual Shield 7100 Ultra: Typical Tensile PropertiesDokument3 SeitenDual Shield 7100 Ultra: Typical Tensile PropertiesDino Paul Castro HidalgoNoch keine Bewertungen
- Column, Slab, Footing and Wall Footing Foundations: Class A MixingDokument47 SeitenColumn, Slab, Footing and Wall Footing Foundations: Class A MixingGioharry Nul PanambulanNoch keine Bewertungen
- The Passion For Cacti and Other Succulents: June 2017Dokument140 SeitenThe Passion For Cacti and Other Succulents: June 2017golf2010Noch keine Bewertungen
- LET General Math ReviewerDokument7 SeitenLET General Math ReviewerMarco Rhonel Eusebio100% (1)
- Prestige Institute of Management & Research: Guided By:-Submitted By: - Prof. Arpit Loya Sumeet RattanDokument21 SeitenPrestige Institute of Management & Research: Guided By:-Submitted By: - Prof. Arpit Loya Sumeet RattanSumeet700005Noch keine Bewertungen
- Scott 2001Dokument20 SeitenScott 2001Mariana CatiniNoch keine Bewertungen
- Impact of Retrofitting Existing Combined Heat and Power Plant With Polygeneration of Biomethane PDFDokument16 SeitenImpact of Retrofitting Existing Combined Heat and Power Plant With Polygeneration of Biomethane PDFAwais Salman0% (1)
- Bhil Tribal Mobilisation in AlirajpurDokument14 SeitenBhil Tribal Mobilisation in Alirajpurrahul banerjeeNoch keine Bewertungen
- SCM (Subway Project Report)Dokument13 SeitenSCM (Subway Project Report)Beast aNoch keine Bewertungen
- Assignment 1Dokument3 SeitenAssignment 1farhang_tNoch keine Bewertungen
- Paediatric Intake Form Modern OT 2018Dokument6 SeitenPaediatric Intake Form Modern OT 2018SefNoch keine Bewertungen
- Nissan Copper LTDDokument11 SeitenNissan Copper LTDankit_shahNoch keine Bewertungen
- 123 09-Printable Menu VORDokument2 Seiten123 09-Printable Menu VORArmstrong TowerNoch keine Bewertungen
- Rekomendasi AnimeDokument11 SeitenRekomendasi Animeosvaldo manurungNoch keine Bewertungen