Beruflich Dokumente
Kultur Dokumente
Electronicstructure Periodic Table
Hochgeladen von
api-445198464Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Electronicstructure Periodic Table
Hochgeladen von
api-445198464Copyright:
Verfügbare Formate
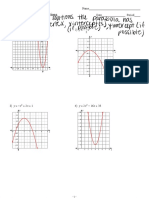
Electronic structure of the first twenty elements in the Periodic Table
Group I Group II Group III Group IV Group V Group VI Group VII Group 0
1 4
1H 2He
1. Draw the electronic structure for each element (this is shown for neon)
2. In the grey area under each structure write out the electronic structure (this is shown for neon – 2,8)
Questions – What do the elements in each Group have in common?
What do the elements in each Period (row) have in common?
Draw and write out the electronic structure for a) a sodium ion b) a chloride ion
7 9 11 12 14 16 19 20
3 Li 4 Be 5B 6 C 7 N 8 O 9F 10 Ne
x x
x x
x
x x
x
xx
2,8
23 24 27 28 31 32 35 40
11 Na 12 Mg 13 Al 14 Si 15 P 16 S 17 Cl 18 Ar
39 40
19 K 20 Ca TRANSITION Ga Ge As Se Br Kr
METALS
Rb Sr In Sn Sb Te I Xe
Das könnte Ihnen auch gefallen
- Solving Equations PacketDokument5 SeitenSolving Equations Packetalooshi Z0% (2)
- Thomas Calculus Early Transcendentals 14th Edition Hass Solutions ManualDokument26 SeitenThomas Calculus Early Transcendentals 14th Edition Hass Solutions ManualRhondaFisherjity98% (47)
- The Radius of A Molecule From Viscosity MeasurementsDokument5 SeitenThe Radius of A Molecule From Viscosity MeasurementsRhett Adrian Seduco50% (2)
- Storage Tank Design For Lactic Acid ProductionDokument30 SeitenStorage Tank Design For Lactic Acid ProductionPrabuddha GopeNoch keine Bewertungen
- Concentration of Solutions DLPDokument3 SeitenConcentration of Solutions DLPLouise Meara Severo70% (10)
- Exponent Worksheet ReviewDokument2 SeitenExponent Worksheet ReviewAgung JatiariskaNoch keine Bewertungen
- Simplifying Expressions Differentiated SheetsDokument3 SeitenSimplifying Expressions Differentiated Sheetsapi-445198464Noch keine Bewertungen
- Emac Gold 2e Year 9 Ots Tests ch7 Indices Test BDokument4 SeitenEmac Gold 2e Year 9 Ots Tests ch7 Indices Test Bapi-445198464Noch keine Bewertungen
- Electronic Structure of The First Twenty Elements in The Periodic TableDokument1 SeiteElectronic Structure of The First Twenty Elements in The Periodic TableFathimath ShiyaraNoch keine Bewertungen
- Electronic Structure of The First Twenty Elements in The Periodic TableDokument1 SeiteElectronic Structure of The First Twenty Elements in The Periodic TableshredderNoch keine Bewertungen
- Electronicstructure Periodic TableDokument1 SeiteElectronicstructure Periodic TableCicy IrnaNoch keine Bewertungen
- CW5-Draw Electronic Structure 1-20 in Periodic TableDokument1 SeiteCW5-Draw Electronic Structure 1-20 in Periodic TableJerry LouNoch keine Bewertungen
- Electronicstructure Periodic TableDokument2 SeitenElectronicstructure Periodic TableZainab FatimaNoch keine Bewertungen
- Electron Structure WorksheetDokument1 SeiteElectron Structure WorksheetKirti KumarNoch keine Bewertungen
- 1-Soal Pas Bindo 8 k13 RevDokument1 Seite1-Soal Pas Bindo 8 k13 RevRizky KurniawatiNoch keine Bewertungen
- 7.5 HW Solve Radical EquationsDokument3 Seiten7.5 HW Solve Radical EquationsNathan HoschouerNoch keine Bewertungen
- Más Material Teoría de Exponentes.Dokument3 SeitenMás Material Teoría de Exponentes.AndréNoch keine Bewertungen
- 04 - Angles and Angle MeasureDokument4 Seiten04 - Angles and Angle MeasureRolando QuintanaNoch keine Bewertungen
- Chapter 6 Practice Test-2Dokument10 SeitenChapter 6 Practice Test-2DRIP CHECK100% (1)
- 1 GCF LCMDokument3 Seiten1 GCF LCMTongtun TuntunNoch keine Bewertungen
- MATH 4073 Numerical Analysis in Test Notes (Cheat Cheat Sheet) v4.0Dokument5 SeitenMATH 4073 Numerical Analysis in Test Notes (Cheat Cheat Sheet) v4.0jfishryanNoch keine Bewertungen
- Multi-Step InequalitiesDokument4 SeitenMulti-Step Inequalitiesioana_adeline23Noch keine Bewertungen
- 03 - 5 - Inverses of LogarithmsDokument4 Seiten03 - 5 - Inverses of LogarithmsSUNGMIN CHOINoch keine Bewertungen
- Exponents ReviewDokument7 SeitenExponents ReviewMa Juwan Xyza MalazaNoch keine Bewertungen
- PH Ysicsguide: Problems and Solutions in 1D Potentials: Part-1Dokument54 SeitenPH Ysicsguide: Problems and Solutions in 1D Potentials: Part-1Subhradip Bhowmik100% (1)
- 313 Chapter 5Dokument1 Seite313 Chapter 5seamesbNoch keine Bewertungen
- @bohring Bot 08-10-23 SR Iit Star Co Scmodel @heyitsyashxdDokument12 Seiten@bohring Bot 08-10-23 SR Iit Star Co Scmodel @heyitsyashxdDinesh BabuNoch keine Bewertungen
- Exponents and Radicals - Worksheet1Dokument4 SeitenExponents and Radicals - Worksheet1Johnmar FortesNoch keine Bewertungen
- XI - IIT - IR - FTM-4 - 07-08-2023 - Key & SolDokument14 SeitenXI - IIT - IR - FTM-4 - 07-08-2023 - Key & Soliitb.akkharcheNoch keine Bewertungen
- 3 The Midpoint FormulaDokument5 Seiten3 The Midpoint FormulaJohn Ramer Lazarte InocencioNoch keine Bewertungen
- 06 - Polar and Rectangular Forms of EquationsDokument2 Seiten06 - Polar and Rectangular Forms of EquationsmelindaNoch keine Bewertungen
- ME2112 - (Part 1) - 2D Stress and Strain-L3 PDFDokument4 SeitenME2112 - (Part 1) - 2D Stress and Strain-L3 PDFShang PingNoch keine Bewertungen
- Parallel Lines and TransversalsDokument3 SeitenParallel Lines and TransversalsArnel ManaleNoch keine Bewertungen
- Assignment PolynomialDokument320 SeitenAssignment Polynomialpittaya_teeNoch keine Bewertungen
- Department of Mathematics National Institute of Technology SrinagarDokument2 SeitenDepartment of Mathematics National Institute of Technology SrinagarSubham KarmakarNoch keine Bewertungen
- Electromagnetics Antennas and Propagation - ProblemsDokument85 SeitenElectromagnetics Antennas and Propagation - ProblemsAnum AhmedNoch keine Bewertungen
- Review Problems Chapter 6Dokument8 SeitenReview Problems Chapter 6Yue FeiNoch keine Bewertungen
- S.S. Bhavikatti - Finite Element Analysis-New Age Publications (Academic) (2004) - 232-253Dokument27 SeitenS.S. Bhavikatti - Finite Element Analysis-New Age Publications (Academic) (2004) - 232-253Gokul KNoch keine Bewertungen
- Mathematics: Daily Practice ProblemsDokument2 SeitenMathematics: Daily Practice Problemsmanoj sharmaNoch keine Bewertungen
- Mathematics: Daily Practice ProblemsDokument2 SeitenMathematics: Daily Practice Problemsmanoj sharmaNoch keine Bewertungen
- 3-The Midpoint FormulaDokument5 Seiten3-The Midpoint FormulaJaniene BathanNoch keine Bewertungen
- Inequalities Worksheet 2Dokument2 SeitenInequalities Worksheet 2amjamil2010Noch keine Bewertungen
- 2D Problems - LST: Higher Order Triangular ElementsDokument3 Seiten2D Problems - LST: Higher Order Triangular ElementsBIRUK FEKADUNoch keine Bewertungen
- 21.04.24 - Osr - Star Co-Sc - Jee-Adv - 2023 - P1 - Gta-7 (P2) - Key & SolDokument12 Seiten21.04.24 - Osr - Star Co-Sc - Jee-Adv - 2023 - P1 - Gta-7 (P2) - Key & Solvenkateswararao.y100% (1)
- Mock Math TU64 เฉลยละเอียด Ver 4 - Copy - removed-1-1Dokument19 SeitenMock Math TU64 เฉลยละเอียด Ver 4 - Copy - removed-1-1sapa123Noch keine Bewertungen
- Graphing Linear Inequalities: y X y XDokument2 SeitenGraphing Linear Inequalities: y X y XAndrew RichardsonNoch keine Bewertungen
- Math HWDokument2 SeitenMath HWAndrew RichardsonNoch keine Bewertungen
- 03 SOL. CHP. CorrelationDokument5 Seiten03 SOL. CHP. CorrelationAWAIS ALTAFNoch keine Bewertungen
- Factoring Worksheet With AnswersDokument2 SeitenFactoring Worksheet With Answersapi-314809600Noch keine Bewertungen
- 17339-28-07 Morning Maths 2022Dokument11 Seiten17339-28-07 Morning Maths 2022princekumarg013Noch keine Bewertungen
- Tutorial - 5 Eigenvalues and Linear Transformation PDFDokument2 SeitenTutorial - 5 Eigenvalues and Linear Transformation PDFbendanNoch keine Bewertungen
- 1 Definate Indafinate Integration Paper 01Dokument7 Seiten1 Definate Indafinate Integration Paper 01ANNENoch keine Bewertungen
- Dividing Rational Expressions PDFDokument4 SeitenDividing Rational Expressions PDFPasia PaulNoch keine Bewertungen
- @bohring Bot × @JEE Tests 17 03 24 SR STAR CO SC JEE ADV 2016 P2Dokument12 Seiten@bohring Bot × @JEE Tests 17 03 24 SR STAR CO SC JEE ADV 2016 P2Amit YadavNoch keine Bewertungen
- Definite Integration EstimationDokument3 SeitenDefinite Integration Estimationakash16260102Noch keine Bewertungen
- Exercise 9, 10, 11: A Ex. 9 Little Drummer BoyDokument1 SeiteExercise 9, 10, 11: A Ex. 9 Little Drummer BoyterzianoNoch keine Bewertungen
- Unit 2 Odd AnswersDokument58 SeitenUnit 2 Odd AnswersGloria TaylorNoch keine Bewertungen
- Semana1 Teoriadeexponentes 4escolar 2015 150313183809 Conversion Gate01 PDFDokument3 SeitenSemana1 Teoriadeexponentes 4escolar 2015 150313183809 Conversion Gate01 PDFLuis HuacchaNoch keine Bewertungen
- Lay 1.2Dokument11 SeitenLay 1.2pri shNoch keine Bewertungen
- Graphing Quadratic FunctionsDokument2 SeitenGraphing Quadratic FunctionsNora HalawiNoch keine Bewertungen
- Fundamentals of Simplex Method 1. Simplex Method in Matrix Notation The Standard Form Max S.TDokument10 SeitenFundamentals of Simplex Method 1. Simplex Method in Matrix Notation The Standard Form Max S.TAhmad AdnanNoch keine Bewertungen
- FunctionDokument9 SeitenFunctionAfifa jamiatun SakillahNoch keine Bewertungen
- 08 Definite Integration - Exercise-2Dokument11 Seiten08 Definite Integration - Exercise-2vishNoch keine Bewertungen
- Frame Analysis Byfinite Element Method: Finite Element Method by G. R. Liu and S. S. QuekDokument29 SeitenFrame Analysis Byfinite Element Method: Finite Element Method by G. R. Liu and S. S. QuekananiaNoch keine Bewertungen
- Year 8 Simplifying Expressions Home LearningDokument1 SeiteYear 8 Simplifying Expressions Home Learningapi-445198464Noch keine Bewertungen
- Simplifying by Multiplying ExpressionsDokument4 SeitenSimplifying by Multiplying Expressionsapi-445198464Noch keine Bewertungen
- Simplifying Expressions by Multiplying Terms LessonDokument9 SeitenSimplifying Expressions by Multiplying Terms Lessonapi-445198464Noch keine Bewertungen
- Simplifying by Multiplying ExpressionsDokument6 SeitenSimplifying by Multiplying Expressionsapi-445198464Noch keine Bewertungen
- Simplifying Algebra Home Learning HarderDokument2 SeitenSimplifying Algebra Home Learning Harderapi-445198464Noch keine Bewertungen
- Simplifying Algebra Home Learning EasierDokument2 SeitenSimplifying Algebra Home Learning Easierapi-445198464Noch keine Bewertungen
- Emac Gold 2e Year 9 Ots Ans ch7 Indices Test ADokument1 SeiteEmac Gold 2e Year 9 Ots Ans ch7 Indices Test Aapi-445198464Noch keine Bewertungen
- Test (40 Marks) :: Polygons, Solids and TransformationsDokument8 SeitenTest (40 Marks) :: Polygons, Solids and Transformationsapi-445198464Noch keine Bewertungen
- Algebra Yr 7rDokument27 SeitenAlgebra Yr 7rapi-445198464Noch keine Bewertungen
- Basic Substitution Matching CardsDokument2 SeitenBasic Substitution Matching Cardsapi-445198464Noch keine Bewertungen
- Algebra Yr 7lDokument30 SeitenAlgebra Yr 7lapi-445198464Noch keine Bewertungen
- Simplifying Expressions Recap Yr 8Dokument10 SeitenSimplifying Expressions Recap Yr 8api-445198464Noch keine Bewertungen
- Emac Gold 2e Year 9 Ots Ans ch7 Indices Test BDokument1 SeiteEmac Gold 2e Year 9 Ots Ans ch7 Indices Test Bapi-445198464Noch keine Bewertungen
- Substitution 8rsDokument10 SeitenSubstitution 8rsapi-445198464Noch keine Bewertungen
- Emac Gold 2e Year 9 Ots Tests ch7 Indices Test ADokument8 SeitenEmac Gold 2e Year 9 Ots Tests ch7 Indices Test Aapi-445198464Noch keine Bewertungen
- Index LawsDokument41 SeitenIndex Lawsapi-445198464Noch keine Bewertungen
- 9 7zero and Negative ExponentsDokument9 Seiten9 7zero and Negative Exponentsapi-445198464Noch keine Bewertungen
- Gold7trp Test ch9 Shapes and TransformationsDokument6 SeitenGold7trp Test ch9 Shapes and Transformationsapi-445198464Noch keine Bewertungen
- Emac7trp Test Ans 09Dokument2 SeitenEmac7trp Test Ans 09api-445198464Noch keine Bewertungen
- 8Dokument12 Seiten8api-445198464Noch keine Bewertungen
- Chapter 9: Shapes and Transformations Test Answers: C B C D E A C D EDokument1 SeiteChapter 9: Shapes and Transformations Test Answers: C B C D E A C D Eapi-445198464Noch keine Bewertungen
- Simplifying Expressions Algebra l2Dokument21 SeitenSimplifying Expressions Algebra l2api-445198464Noch keine Bewertungen
- Algebraic Expressions and TermsDokument13 SeitenAlgebraic Expressions and TermsavgNoch keine Bewertungen
- Gold7trp Test ch8 Statistics and ProbabilityDokument8 SeitenGold7trp Test ch8 Statistics and Probabilityapi-445198464Noch keine Bewertungen
- Chapter 8: Statistics and Probability Test Answers: C C E A B DDokument2 SeitenChapter 8: Statistics and Probability Test Answers: C C E A B Dapi-445198464Noch keine Bewertungen
- Chapter 3: Number Properties and Patterns Test (40 Marks) : Part A - Multiple-Choice QuestionsDokument8 SeitenChapter 3: Number Properties and Patterns Test (40 Marks) : Part A - Multiple-Choice Questionsapi-445198464Noch keine Bewertungen
- Chapter 3: Number Properties and Patterns Test Answers: © Cambridge University Press 2011 1Dokument2 SeitenChapter 3: Number Properties and Patterns Test Answers: © Cambridge University Press 2011 1api-445198464Noch keine Bewertungen
- AlgebraicexpressionsbingoDokument2 SeitenAlgebraicexpressionsbingoapi-445198464Noch keine Bewertungen
- Competition & Luxury Vehicle Club of Darlington SuitDokument31 SeitenCompetition & Luxury Vehicle Club of Darlington SuitBenjamin DuerNoch keine Bewertungen
- MOLECONCEPTREDOXREACTIONCOMPLETEPACAKGEDokument52 SeitenMOLECONCEPTREDOXREACTIONCOMPLETEPACAKGENikhil PalNoch keine Bewertungen
- Surface Chemistry of Solid and Liquid Interfaces PDFDokument365 SeitenSurface Chemistry of Solid and Liquid Interfaces PDFSuchat KotcheapNoch keine Bewertungen
- TB400 Painting and Corrosion ProtectionDokument21 SeitenTB400 Painting and Corrosion ProtectionAliZenatiNoch keine Bewertungen
- Pixl Knowit!: Gcse BiologyDokument66 SeitenPixl Knowit!: Gcse BiologyEsther SparksNoch keine Bewertungen
- Hub. Stereokimia & Aktivitas - 3Dokument85 SeitenHub. Stereokimia & Aktivitas - 3Nia Nurdinia RahmahNoch keine Bewertungen
- Flojetpump HandleidingDokument4 SeitenFlojetpump HandleidingnitroboozterNoch keine Bewertungen
- Lied Mann 2017Dokument8 SeitenLied Mann 2017Chandra SekarNoch keine Bewertungen
- MS DirectPhD Sample PDFDokument4 SeitenMS DirectPhD Sample PDFArunKumarNoch keine Bewertungen
- Effects of Three Different Dietary Binders On Juvenile Sea CucumberDokument8 SeitenEffects of Three Different Dietary Binders On Juvenile Sea CucumberEnrique MartinezNoch keine Bewertungen
- Process UtilityDokument13 SeitenProcess UtilityAnupam Manoj100% (1)
- Lecture Notes Materials and Ecological EngineeringDokument463 SeitenLecture Notes Materials and Ecological EngineeringInhake AutechreNoch keine Bewertungen
- Rociadores - FT - GFS-100B - GL SeriesDokument2 SeitenRociadores - FT - GFS-100B - GL SeriesJimmy FernándezNoch keine Bewertungen
- BM 12 Uc Usa 2011 08Dokument44 SeitenBM 12 Uc Usa 2011 08dangomezvNoch keine Bewertungen
- Jasmuheen - Telepathic CommunicationDokument6 SeitenJasmuheen - Telepathic Communicationmelrakki100% (3)
- Loctite SF 8046: Technical Data SheetDokument2 SeitenLoctite SF 8046: Technical Data Sheeterick daniel veraNoch keine Bewertungen
- Anomalous Doping Effect in Black Phosphorene From First-Principles CalculationsDokument8 SeitenAnomalous Doping Effect in Black Phosphorene From First-Principles Calculationsh shekarlabNoch keine Bewertungen
- Recycling of Li Ion Batteries EUDokument28 SeitenRecycling of Li Ion Batteries EUdcahyasturaNoch keine Bewertungen
- Excipients PDFDokument2 SeitenExcipients PDFMarioNoch keine Bewertungen
- Service Manual CSCU-RE12GKE PDFDokument16 SeitenService Manual CSCU-RE12GKE PDFJose Vicente Ausina100% (1)
- Solution Manual For Chemistry 10th Edition by Kenneth W Whitten Larry Peck Raymond e Davis and George G StanleyDokument14 SeitenSolution Manual For Chemistry 10th Edition by Kenneth W Whitten Larry Peck Raymond e Davis and George G Stanleycuonganh51wNoch keine Bewertungen
- Sika PDS E Intraplast ZDokument2 SeitenSika PDS E Intraplast Zlwin_oo2435100% (1)
- Main PropulsionDokument27 SeitenMain PropulsionalsitorNoch keine Bewertungen
- Calculate Jockey Pump Capacity in Fire Fighting SystemDokument1 SeiteCalculate Jockey Pump Capacity in Fire Fighting SystemkarpakkiNoch keine Bewertungen
- Analytical ChemistryDokument79 SeitenAnalytical ChemistryDipeshBardoliaNoch keine Bewertungen
- High Pressure Boiler Water TreatmentDokument90 SeitenHigh Pressure Boiler Water Treatmentak_thimiri100% (1)
- Carbon Structural Steel: Standardspecification ForDokument4 SeitenCarbon Structural Steel: Standardspecification ForlinaNoch keine Bewertungen