Beruflich Dokumente
Kultur Dokumente
Physical Chemistry For Engineers II Laboratory
Hochgeladen von
Christina Joana GuzmanOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Physical Chemistry For Engineers II Laboratory
Hochgeladen von
Christina Joana GuzmanCopyright:
Verfügbare Formate
Physical Chemistry for Engineers II Laboratory
TABLE OF CONTENTS
I. Introduction
II. Objectives
III. Review of Related Literatures and Applications
IV. Methodology
A. Materials
B. Experimental Procedure
V. Results and Discussion
VI. Conclusion
VII. Appendix
A. Tables/ Data
B. References
VIII. Documentation
Cagayan State University, Carig Campus Page 1
Physical Chemistry for Engineers II Laboratory
I. Introduction
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to
a surface. This process creates a film of the adsorbate on the surface of the adsorbent. Adsorption is a
surface-based process, while absorption involves the whole volume of the material. The
term sorption encompasses both processes, while desorption is the reverse of it.
Adsorption can either be physical or chemical process in nature. In this experiment, physical
adsorption is involved where charcoal as the adsorbent and the food color solution as the adsorbate. Physical
adsorption resembles the condensation of gases to liquids and depends on the physical, on van der Waals,
force of attraction between the solid adsorbers and the adsorbate molecules.
The aim of this experiment is to determine the equilibrium time under some changed parameters like
ratio of adsorbent to adsorbate and the particle size of the adsorbent.
II. Objectives
The objectives of this experiment are as follows:
1. Determine the equilibrium time as a function of adsorbent to adsorbate ratio and adsorbent particle
size.
2. Determine the relationship of the amount of adsobent to the time of equilibrium.
3. Determine the relationship of the adsorbent’s particle size to the time of equilibrium.
III. Review of Related Literature
Factors affecting the adsorption capacity
Size of adsorbate: Smaller size of particles is preferable .This is because equilibrium is more likely
to achieved by smaller ,reduced and fine particles of adsorbate.If the adsorbate is of smaller size it
can better be hold by the adsorbent.
Contact time and surface area: Adsorption needs time for completion more time given better will be
the adsorption. Also the surface area should be large for complete adsorption and it will lead
adsorption to equilibrium position.
Neutral particles and sparingly soluble adsorbate show better adsorption: Neutral molecules are
easily adsorbed than ionizable molecules because when ionization occurs charges dispersed in
.Sparingly soluble particles are easily adsorbed on to the surface then soluble substances.
Size of Pores to adsorbate size: As we know very well adsorbent contains pores through which
adsorption occurs .If size of adsorbate is larger than size of pores then there is will definitely be a
problem so size of pores should be in accordance with the adsorbate size.
Cagayan State University, Carig Campus Page 2
Physical Chemistry for Engineers II Laboratory
Importance or Applications of Adsorption:
Adsorption finds extensive applications both in research laboratory and in industry. A few
applications are discussed below:
In preserving vacuum: In Dewar flasks activated charcoal is placed between the walls of the flask
so that any gas which enters into the annular space either due to glass imperfection or diffusion
though glass is adsorbed.
In glass masks: All gas masks are devices containing suitable adsorbent so that the poisonous
gases present in the atmosphere are preferentially adsorbed and the air for breathing is purified.
In clarification of sugar: Sugar is decolorized by treating sugar solution with charcoal powder. The
latter adsorbs the undesirable colors present.
In paint industry: The paint should not contain dissolved gases as otherwise the paint does not
adhere well to the surface to be painted and thus will have a poor covering power. The dissolved
gases are therefore, removed by suitable adsorbents during manufacture. Further, all surfaces are
covered with layers of gaseous, liquid or solid films. These have to be removed before the paint is
applied. This is done by suitable liquids which adsorbs these films. Such liquids are called wetting
agents. The use of spirit as wetting agent in furniture painting is well known.
In chromatographic analysis: The selective adsorbent of certain substances from a solution by a
particular solid adsorbent has helped to develop technique for the separation of the components of
the mixture. This technique is called chromatographic analysis. For example: in column
chromatography a long and wide vertical tube is filled with a suitable adsorbent and the solution of
the mixture poured from the top and then collected one by one from the bottom.
In catalysis: The action of certain solids as catalysts is best explained in terms of adsorption. The
theory is called adsorption theory. According to this theory, the gaseous reactants are adsorbed on
the surface of the solid catalyst. As a result, the concentration of the reactants increases on the
surface and hence the rate of reaction increases. The theory is also able to explain the greater
efficiency of the catalyst in the finely divided state, the action of catalyst promoters and poisons.
In adsorption indicators: Various dyes which owe their use to adsorption have been introduced as
indicators particularly in precipitation titrations. For example: KBr is easily titrated with AgNO 3 using
eosin as an indicator.
In softening of hard water: The use of ion exchangers for softening of hard water is based upon
the principle of competing adsorption just as in chromatography.
In removing moisture from air in the storage of delicate instruments:
Such instruments which may be harmed by contact with the moist air are kept out of contact with
moisture using silica gel.
Cagayan State University, Carig Campus Page 3
Physical Chemistry for Engineers II Laboratory
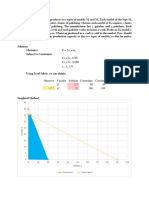
IV. Methodology
A. Materials
1. Charcoal 6. Digital weighing scales
2. Food color 7. Syringe
3. Distilled water 8. Stirring rod
4. Beakers 9. Stopwatch/timer
5. Graduated cylinders
B. Experimental Procedure
i.Density-concentration correlation
1. Prepare 0, 0.5%, 1.0%, 1.5%, 2%, 2.5% and 3% (weight basis) food color solution.
2. Determine the density of each solution.
3. Plot concentration as a function of density using Microsoft Excel.
ii. Adsorbent preparation
1. Crush 1 kg of charcoal.
2. Stack the Tyler screens starting with Number 4 as the top layer followed with 3, 2 and 1.
3. Load the crushed charcoal into the Tyler Number 4. Shake rigorously.
4. Collect the particles at the Tyler Mesh 3, 2 and 1. Particle size found at Tyler Mesh 3 has a particle
size between the Tyler Mesh size 3 and 4 and so on and so forth.
5. Label the particle sizes of the samples.
iii. Adsorption
1. Prepare a 3% w/w food color solution in a beaker.
2. Add the prescribe amount of charcoal to the solution.
3. Stir the solution continuously.
4. Withdraw 0.5 ml of solution every 3 minutes.
5. Determine the density of the solution.
6. Return the withdrawn solution into the beaker.
7. Repeat the process until the density of the solution remains constant over time.
Cagayan State University, Carig Campus Page 4
Physical Chemistry for Engineers II Laboratory
V. Results and Discussion
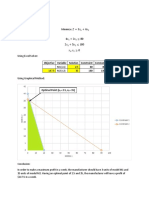
GRAPH 1: CONCENTRATION AS A FUNCTION OF DENSITY
1.2
1
DENSITY (g/mL)
0.8
0.6 Trial 1
Trial 2
0.4
Trial 3
0.2
0
0 0.5 1 1.5 2 2.5 3
CONCENTRATION (%w/w)
Increasing the concentration of the solution results to higher density.
GRAPH 2: EQUILIBRIUM TIME AS A FUNCTION OF ADSORBENT'S AMOUNT
14
12
10
TIME (MINUTES)
8 TRIAL 1
6 TRIAL 2
4 TRIAL 3
2
0
10:01 5:01 1:01
ADSORBENT-ADSORBATE RATIO (GRAMS)
Decreasing the amount of adsorbent leads to slower equilibrium time.
Cagayan State University, Carig Campus Page 5
Physical Chemistry for Engineers II Laboratory
GRAPH 3: DENSITY AS A FUNCTION OF ADSORBENT'S AMOUNT
1.25
1.2
1.15
DENSITY (g/mL)
1.1
Trial 1
1.05
Trial 2
1
Trial 3
0.95
0.9
10:01 5:01 1:01
ADSORBENT-ADSORBATE RATIO (GRAMS)
Densities at equilibrium didn’t change much with decreasing adsorbent’s amount.(This is due to
some experimental errors, the weighing scale used only gives mass with one decimal place. Using
high presicion weighing scale would be better.) Looking at the graph, the density increased at the
volumes with lesser adsorbent.
GRAPH 4: EQUILIBRIUM TIME AS A FUNCTION OF ADSORBENT'S SIZE
20
18
16
TIME IN MINUTES
14
12
10 Trial 1
8 Trial 2
6
Trial 3
4
2
0
1-2(fine) 2-3(finer) 3-4(finest)
MESH
As the absorbent’s size gets finer, the equilibrium time gets faster
Cagayan State University, Carig Campus Page 6
Physical Chemistry for Engineers II Laboratory
GRAPH 5: DENSITY AS A FUNCTION OF ADSORBENT'S SIZE
1.25
1.2
1.15
DENSITY (g/mL)
1.1
Trial 1
1.05
Trial 2
1 Trial 3
0.95
0.9
1-2(fine) 2-3(finer) 3-4(finest)
MESH
As the absorbent’s size gets finer, the density decreases.
VI. Conclusion
Adding more solute to a solvent changes the composition of particles in a given volume of solution,
this resulted in the increase of mass per unit area or what we call the density.
Increasing the amount of adsorbent especially with constant stirring leads to the increase of active
surface area resulting to more dispersion intensity of adsorbent thus attracting more adsorbate in a shorter
period of time. Therefore, the equilibrium will be achieved sooner
Smaller adsorbent particles achieves equilibrium time sooner than that of the adsorbent with larger
particle size because in finer absorbent, more surface area of the adsorbent is in contact with the solution,
thus, greater tendency for adsorption to occur.
Cagayan State University, Carig Campus Page 7
Physical Chemistry for Engineers II Laboratory
VII. Appendix
A. Tables and Data
Table 1: Concentration-Density Data
Concentration Density (g/ml)
(%w/w) Trial 1 Trial 2(g/mL) Trial 3(g/mL)
0 0 0 0
0.5 0.9805 0.9805 0.9805
1.0 0.9854 0.9854 0.9854
1.5 0.9902 0.9902 0.9902
2 0.9714 0.9903 0.9714
2.5 1 0.9952 0.9952
3 1.0049 1 1
Table 2: Equilibrium Time as a Function of Adsorbent to Adsorbate Ratio
Adsorbent Adsorbate Equilibrium time
Ratio min Trial 1 min Trial 2 min Trial 3
10:1 6 1.2 6 1.2 6 1
5:1 6 1.2 6 1.2 9 1.2
1:1 12 1.2 6 1.2 9 1.2
Table 3: Equilibrium time as a function of Particle size
Equilibrium Time
Particle Size
min Trial 1 min Trial 2 Min Trial 3
Tyler Mesh 1-2 9 1.2 9 1.2 18 1.2
Tyler Mesh 2-3 6 1 6 1 9 1.2
Tyler Mesh 3-4 6 1 6 1 6 1
Cagayan State University, Carig Campus Page 8
Physical Chemistry for Engineers II Laboratory
B. References
http://flamesofchemistry.blogspot.com/2013/09/adsorption-capacity-and-
factors.html
https://www.thebigger.com/chemistry/surface-chemistry/what-are-the-applications-
of-adsorption/
https://www.britannica.com/science/adsorption
https://www.researchgate.net/figure/Effect-of-adsorbent-dose-on-
adsorption_fig4_272771088
VIII. Documentation
Charcoal in Tyler mesh Separated charcoals in different particle size
Weighing of charcoal Weighing of food color powder
Cagayan State University, Carig Campus Page 9
Physical Chemistry for Engineers II Laboratory
Food color solution in a beaker
Food color solution with
charcoal in a beaker
Stirring of charcoal solution Weighing of withdrawn charcoal
solution using syringe
Cagayan State University, Carig Campus Page 10
Das könnte Ihnen auch gefallen
- Gas Absorption PDFDokument42 SeitenGas Absorption PDFKim GojoCruz90% (30)
- Activated Carbon AdsorptionDokument16 SeitenActivated Carbon AdsorptionKabilashini Mana Mohan100% (2)
- FINAL LAB Report (Experiment 2)Dokument40 SeitenFINAL LAB Report (Experiment 2)Jonelou CusipagNoch keine Bewertungen
- EXPERIMENT 6 Aeration TestDokument8 SeitenEXPERIMENT 6 Aeration TestMuhammad Faiz Zafuan Idrus100% (2)
- Activated Carbon Adsorption, Isotherms, Kinetics Continuous-Flow OperationDokument7 SeitenActivated Carbon Adsorption, Isotherms, Kinetics Continuous-Flow OperationMuhd Hafetz100% (1)
- Osmotic DehydrationDokument13 SeitenOsmotic DehydrationChristina Joana GuzmanNoch keine Bewertungen
- Global 6000 SystemsDokument157 SeitenGlobal 6000 SystemsJosé Rezende100% (1)
- Notes On The Life and Works of Jose Rizal - IncompleteDokument15 SeitenNotes On The Life and Works of Jose Rizal - Incompleteblock_me_please50% (2)
- MKT-case StudyDokument7 SeitenMKT-case StudyJoe Thampi KuruppumadhomNoch keine Bewertungen
- G4 Exp5Dokument9 SeitenG4 Exp5Naresh GanisonNoch keine Bewertungen
- Chemistry-Theory MergedDokument38 SeitenChemistry-Theory Mergedkemiah IsaacsNoch keine Bewertungen
- Z Notes Chemistry ATP 2023-25Dokument11 SeitenZ Notes Chemistry ATP 2023-25Zoya100% (1)
- Important Terms: Quantitative AnalysisDokument6 SeitenImportant Terms: Quantitative Analysisqasim khokhar100% (1)
- Carbon From Coffee GroundsDokument7 SeitenCarbon From Coffee GroundsSolisNoch keine Bewertungen
- Print Activity 3Dokument10 SeitenPrint Activity 3JaneNoch keine Bewertungen
- Effect of Varying Concentration On AbsorbanceDokument5 SeitenEffect of Varying Concentration On AbsorbanceAriel ChenNoch keine Bewertungen
- Report Oel Task ADokument47 SeitenReport Oel Task APoh Ching HongNoch keine Bewertungen
- Olumide DryingDokument34 SeitenOlumide DryingAnthony Oyindamola AdekoleoyeNoch keine Bewertungen
- Validation of Analytical Methods for Laboratory Calculations and TestingDokument9 SeitenValidation of Analytical Methods for Laboratory Calculations and TestingYana NanaNoch keine Bewertungen
- Fluid Saturation Measurement of Core Sample Using Solvent Extraction MethodDokument12 SeitenFluid Saturation Measurement of Core Sample Using Solvent Extraction MethodRizvanNoch keine Bewertungen
- Lab Report 4Dokument7 SeitenLab Report 4Carol Wei100% (1)
- Caie Igcse Chemistry 0620 Alternative To Practical v2Dokument11 SeitenCaie Igcse Chemistry 0620 Alternative To Practical v2Sanjith KatakamNoch keine Bewertungen
- Water Jar Test DoneDokument8 SeitenWater Jar Test DoneNasEvan'sNoch keine Bewertungen
- Module3 - Gravimetric Method of AnalysisDokument22 SeitenModule3 - Gravimetric Method of AnalysisJoyce Mariele RomeroNoch keine Bewertungen
- Analyzing Metal Ion Mixtures Using SpectrophotometryDokument9 SeitenAnalyzing Metal Ion Mixtures Using SpectrophotometryBano KhanNoch keine Bewertungen
- Chemistry Investigatory Project SEQUENCE To Arrange The Papers in The File-1Dokument19 SeitenChemistry Investigatory Project SEQUENCE To Arrange The Papers in The File-1sky80243Noch keine Bewertungen
- Coagulation and Flocculation Lab ReportDokument15 SeitenCoagulation and Flocculation Lab ReportPamela Mabena100% (1)
- CHEM AISSEDokument28 SeitenCHEM AISSEsusrudhansNoch keine Bewertungen
- ME Laboratory 1: Precious Arlene Villaroza-MelendrezDokument38 SeitenME Laboratory 1: Precious Arlene Villaroza-MelendrezJhonny EnglishNoch keine Bewertungen
- Project Lab: Purpose of The ExperimentDokument10 SeitenProject Lab: Purpose of The ExperimentRobel KahsuNoch keine Bewertungen
- StoichiometryDokument34 SeitenStoichiometryvanditNoch keine Bewertungen
- H2 PsaDokument5 SeitenH2 PsaRathikanti JanardhanNoch keine Bewertungen
- Chemistry Unit 8Dokument3 SeitenChemistry Unit 8cindyNoch keine Bewertungen
- Lab Manual of BiochemistryDokument19 SeitenLab Manual of BiochemistryUsama Javed0% (1)
- Lab Volatile Suspended Solids (EC2206C4A)Dokument9 SeitenLab Volatile Suspended Solids (EC2206C4A)zahir asaharNoch keine Bewertungen
- Group 15 - Lab Report Exp 9Dokument14 SeitenGroup 15 - Lab Report Exp 9lanaNoch keine Bewertungen
- Lab ManualDokument24 SeitenLab ManualUsama JavedNoch keine Bewertungen
- Gravimetric AnalysisDokument5 SeitenGravimetric AnalysisInduNoch keine Bewertungen
- Determination of The Effective Diffusion CoefficientDokument8 SeitenDetermination of The Effective Diffusion Coefficientsexabo7448Noch keine Bewertungen
- Experiment 6Dokument20 SeitenExperiment 6Saniha Aysha AjithNoch keine Bewertungen
- Covenant University, Canaanland, Ota.: Date Submitted: Oct-8-2020Dokument11 SeitenCovenant University, Canaanland, Ota.: Date Submitted: Oct-8-2020Akande AyodejiNoch keine Bewertungen
- Lecture-7 (Gravimetric Analysis)Dokument6 SeitenLecture-7 (Gravimetric Analysis)Mohamed AbdelaalNoch keine Bewertungen
- Porosity and DensityDokument9 SeitenPorosity and DensityNawal HaiderNoch keine Bewertungen
- METU Ch.E. 410 Lab Experiment 32 Batch AdsorptionDokument2 SeitenMETU Ch.E. 410 Lab Experiment 32 Batch AdsorptionResky Ervaldi SaputraNoch keine Bewertungen
- 2019 Experimental Methods in Chemical EngineeringDokument11 Seiten2019 Experimental Methods in Chemical EngineeringTrung NguyenNoch keine Bewertungen
- Diffusion Rate of Different SolventsDokument4 SeitenDiffusion Rate of Different SolventsHope GrantNoch keine Bewertungen
- Experiment 4: The Determination of Partial Molar VolumeDokument5 SeitenExperiment 4: The Determination of Partial Molar VolumeLucile BronzalNoch keine Bewertungen
- UpdatedDokument3 SeitenUpdatedLoeyNoch keine Bewertungen
- Beer'S Law Simulation Lab: 2. Grade LevelDokument14 SeitenBeer'S Law Simulation Lab: 2. Grade LevelShiv JhattuNoch keine Bewertungen
- Gurdaspur Public School Gurdaspur: Project Report TOPIC-determination of The Rates of Evaporation of Different LiquidsDokument15 SeitenGurdaspur Public School Gurdaspur: Project Report TOPIC-determination of The Rates of Evaporation of Different LiquidsDeepak HansNoch keine Bewertungen
- FE 3 Practical 6 - 120057Dokument4 SeitenFE 3 Practical 6 - 120057diksha singhNoch keine Bewertungen
- An Introduction to Adsorption: Characterization, Applications and Krypton SeparationDokument13 SeitenAn Introduction to Adsorption: Characterization, Applications and Krypton SeparationSupriya ChumberNoch keine Bewertungen
- Redox GravimetricDokument6 SeitenRedox GravimetricadillezbaboolalNoch keine Bewertungen
- Organic Chemistry Lab Report on Recrystallization of Benzoic AcidDokument6 SeitenOrganic Chemistry Lab Report on Recrystallization of Benzoic AcidNelvianaNoch keine Bewertungen
- Report 8Dokument7 SeitenReport 8enieynazNoch keine Bewertungen
- Gas AbsorptionDokument12 SeitenGas Absorptionaya mahmoudNoch keine Bewertungen
- Chemistry InvestigatoryDokument16 SeitenChemistry Investigatorypriyanshuroy2006Noch keine Bewertungen
- 2018310334박성제 4조 exp4 prereportDokument7 Seiten2018310334박성제 4조 exp4 prereport성제박Noch keine Bewertungen
- CHEMISTRYDokument15 SeitenCHEMISTRYMAYIL SHANKARI PNoch keine Bewertungen
- GRAVIMETRICDokument11 SeitenGRAVIMETRIC;'SiLeNt';Noch keine Bewertungen
- Laboratory Manuan Lab 2Dokument4 SeitenLaboratory Manuan Lab 2Oka DeeNoch keine Bewertungen
- International Journal of Green and Herbal Chemistry: Decolorization of Textile Waste Water Using Low Cost AdsorbentDokument6 SeitenInternational Journal of Green and Herbal Chemistry: Decolorization of Textile Waste Water Using Low Cost AdsorbentFarrukh JamilNoch keine Bewertungen
- Mapua University: Experiment No. 1Dokument8 SeitenMapua University: Experiment No. 1Denver John TejadaNoch keine Bewertungen
- Silica gel removes methylene blue dyeDokument5 SeitenSilica gel removes methylene blue dyemtanaydinNoch keine Bewertungen
- Confined Fluid Phase Behavior and CO2 Sequestration in Shale ReservoirsVon EverandConfined Fluid Phase Behavior and CO2 Sequestration in Shale ReservoirsNoch keine Bewertungen
- Guzman Christina JoanaDokument23 SeitenGuzman Christina JoanaChristina Joana GuzmanNoch keine Bewertungen
- ExercisewowDokument6 SeitenExercisewowChristina Joana GuzmanNoch keine Bewertungen
- Martinez, Cherryl ADokument23 SeitenMartinez, Cherryl AChristina Joana GuzmanNoch keine Bewertungen
- Air Pollution PaperDokument10 SeitenAir Pollution PaperChristina Joana GuzmanNoch keine Bewertungen
- QMDokument10 SeitenQMChristina Joana GuzmanNoch keine Bewertungen
- Reviewer PDFDokument29 SeitenReviewer PDFChristina Joana GuzmanNoch keine Bewertungen
- Solid Waste ManagementDokument18 SeitenSolid Waste ManagementChristina Joana GuzmanNoch keine Bewertungen
- QMDokument10 SeitenQMChristina Joana GuzmanNoch keine Bewertungen
- Solid Waste PaperDokument11 SeitenSolid Waste PaperChristina Joana GuzmanNoch keine Bewertungen
- Reviewer PDFDokument29 SeitenReviewer PDFChristina Joana GuzmanNoch keine Bewertungen
- MR Particle Size AnalysisDokument37 SeitenMR Particle Size AnalysisChristina Joana GuzmanNoch keine Bewertungen
- Chapter 5: Thermal Effects and Energy Balances 5.1 Temperature Dependence 0f Reaction RatesDokument13 SeitenChapter 5: Thermal Effects and Energy Balances 5.1 Temperature Dependence 0f Reaction RatesChristina Joana GuzmanNoch keine Bewertungen
- Chemical reactor design and operation for maximum efficiencyDokument2 SeitenChemical reactor design and operation for maximum efficiencyChristina Joana GuzmanNoch keine Bewertungen
- NomenclatureDokument2 SeitenNomenclatureChristina Joana GuzmanNoch keine Bewertungen
- Rule of Thumb: Distillation and Gas AdsorptionDokument2 SeitenRule of Thumb: Distillation and Gas AdsorptionChristina Joana GuzmanNoch keine Bewertungen
- Transport PhenomenaDokument21 SeitenTransport PhenomenaChristina Joana GuzmanNoch keine Bewertungen
- Hardware Purchase and Sales System Project ProfileDokument43 SeitenHardware Purchase and Sales System Project Profilesanjaykumarguptaa100% (2)
- Body Scan AnalysisDokument9 SeitenBody Scan AnalysisAmaury CosmeNoch keine Bewertungen
- Presentation On Ich Topics & Guidelines With A Special Reference ToDokument79 SeitenPresentation On Ich Topics & Guidelines With A Special Reference ToVidyaNoch keine Bewertungen
- Call SANROCCO 11 HappybirthdayBramanteDokument8 SeitenCall SANROCCO 11 HappybirthdayBramanterod57Noch keine Bewertungen
- C11 RacloprideDokument5 SeitenC11 RacloprideAvina 123Noch keine Bewertungen
- DMS-2017A Engine Room Simulator Part 1Dokument22 SeitenDMS-2017A Engine Room Simulator Part 1ammarNoch keine Bewertungen
- Basic Calculus: Performance TaskDokument6 SeitenBasic Calculus: Performance TasksammyNoch keine Bewertungen
- AJK Newslet-1Dokument28 SeitenAJK Newslet-1Syed Raza Ali RazaNoch keine Bewertungen
- Kastanakis 2014Dokument8 SeitenKastanakis 2014Andreea Georgiana MocanuNoch keine Bewertungen
- Nature and Scope of Marketing Marketing ManagementDokument51 SeitenNature and Scope of Marketing Marketing ManagementFeker H. MariamNoch keine Bewertungen
- Brooks Instrument FlowmeterDokument8 SeitenBrooks Instrument FlowmeterRicardo VillalongaNoch keine Bewertungen
- Obsolescence 2. Book Value 3. Depreciation 4. Depletion EtcDokument9 SeitenObsolescence 2. Book Value 3. Depreciation 4. Depletion EtcKHAN AQSANoch keine Bewertungen
- COT EnglishDokument4 SeitenCOT EnglishTypie ZapNoch keine Bewertungen
- Pfrs 16 LeasesDokument4 SeitenPfrs 16 LeasesR.A.Noch keine Bewertungen
- Addition and Subtraction of PolynomialsDokument8 SeitenAddition and Subtraction of PolynomialsPearl AdamosNoch keine Bewertungen
- E Learning: A Student Guide To MoodleDokument16 SeitenE Learning: A Student Guide To MoodleHaytham Abdulla SalmanNoch keine Bewertungen
- National Products Classification Code For Services in IndiaDokument92 SeitenNational Products Classification Code For Services in Indiakalanemi0% (2)
- FINAL - Plastic Small Grants NOFO DocumentDokument23 SeitenFINAL - Plastic Small Grants NOFO DocumentCarlos Del CastilloNoch keine Bewertungen
- LLoyd's Register Marine - Global Marine Safety TrendsDokument23 SeitenLLoyd's Register Marine - Global Marine Safety Trendssuvabrata_das01100% (1)
- SuffrageDokument21 SeitenSuffragejecelyn mae BaluroNoch keine Bewertungen
- The Impact of Information Technology and Innovation To Improve Business Performance Through Marketing Capabilities in Online Businesses by Young GenerationsDokument10 SeitenThe Impact of Information Technology and Innovation To Improve Business Performance Through Marketing Capabilities in Online Businesses by Young GenerationsLanta KhairunisaNoch keine Bewertungen
- All MeterialsDokument236 SeitenAll MeterialsTamzid AhmedNoch keine Bewertungen
- BMXNRPDokument60 SeitenBMXNRPSivaprasad KcNoch keine Bewertungen
- 40 Multiple Choice Questions in Basic StatisticsDokument8 Seiten40 Multiple Choice Questions in Basic StatisticsLevi CorralNoch keine Bewertungen
- Color Codes and Irregular Marking-SampleDokument23 SeitenColor Codes and Irregular Marking-Samplemahrez laabidiNoch keine Bewertungen
- Chapter 9-10 (PPE) Reinzo GallegoDokument48 SeitenChapter 9-10 (PPE) Reinzo GallegoReinzo GallegoNoch keine Bewertungen
- Scholars of Hadith Methodology in Dealing With The Two Sahihs: The Criticized Ahadith As A Model. Ammar Ahmad Al-HaririDokument37 SeitenScholars of Hadith Methodology in Dealing With The Two Sahihs: The Criticized Ahadith As A Model. Ammar Ahmad Al-HaririSalah KhanNoch keine Bewertungen