Beruflich Dokumente
Kultur Dokumente
Patente 4.655.879
Hochgeladen von
Yago Boullaude0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
8 Ansichten6 SeitenPATENTE DESTILACION GLYCEROL
Originaltitel
patente 4.655.879
Copyright
© © All Rights Reserved
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenPATENTE DESTILACION GLYCEROL
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
8 Ansichten6 SeitenPatente 4.655.879
Hochgeladen von
Yago BoullaudePATENTE DESTILACION GLYCEROL
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 6
United States Patent [191 [11] Patent Number: 4,655,879
Brockmann et a1. [45] Date of Patent: Apr. 7, 1987
[54] GLYCEROL DISTILLATION PROCESS FOREIGN PATENT DOCUMENTS
[75] Inventors: Rolf Brockmann, Langenfeld; Lutz 0246932 9/ 1963 Australia ........................... .. 568/869
Jeromin, Hilden; Wilhelm 0278651 9/ 1964 Australia .... .. 568/869
Johannisbauer, Erkrath; Helmut 0674001 11/ 1963 Canada . . . . . . . . . . .. 568/869
Meyer, Duesseldorf-Benrath; Otto 1138886 6/1957 France .
0278703 12/1928 United Kingdom .............. .. 568/869
Michel, Langenfeld; Juergen 0737570 9/ 1955 United Kingdom .............. .. 568/869
Plachenka, Mettmann, all of Fed.
Rep. of Germany OTHER PUBLICATIONS
[73] Assignee: Henkel Koinmanditgesellschaft auf D’Souza, “The Importance of Glycerol in the Fatty
Aktien, Duesseldorf, Fed. Rep. of Acid Industry”, Journal of the American Oil Chemists
Germany Society, vol. 56, pp. 812A-819A (1979).
Stromquist et al., “C. P. Glycerol by Ion Exchange”,
[21] App1.No.: 664,902 Industrial and Engineering Chemistry, vol. 53, pp.
1065-1070 (1951).
[22] Filed: Oct. 26, 1984 Ziels, “Recovery and Puri?cation of Glycerol”, The
Journal of the American Oil Chemists Society, vol. 33,
[30] Foreign Application Priority Data pp. 556-565 (1956).
Chemical Abstract 72:1970 42752p “Preparation of
Oct. 28, 1983 [DE] Fed. Rep. of Germany ..... .. 3339051
Alpha-Diamines from Epoxides”.
[51] Int. Cl.‘ ........................ .. B01D 3/34; B01D 1/22 Primary Examiner-—S. Leon Bashore
[52] US. Cl. ...................................... .. 203/37; 203/49; Assistant Examiner-V. Manoharan
203/80; 203/ 87; 203/98; 203/99; 203/DIG. 19; Attorney, Agent, or Firm-—Ernest G. Szoke; Henry E.
203/89; 202/ 186; 202/205; 202/236; 568/854; Millson, Jr.; Mark A. Green?eld
568/869; 159/49; 159/5 [s7] ABSTRACI‘
[58] Field of Search ..................... .. 203/37, 49, 31, 41,
203/36, 98, 80, 87, DIG. 19, 99, 73, 89; An improved process for puri?cation of glycerol ob
435/159; 502/418; 568/854, 869; 210/694; tained from natural sources comprising alkalizing a
202/205, 186, 236; 159/49, 5 glycerol-containing crude mixture in the presence of air
for oxidation, evaporating the mixture in a thin-layer
[56] References Cited evaporator with redistillation of the residue, recti?ca
U.S. PATENT DOCUMENTS tion and reevaporation in a packed column character
ized by low-pressure-loss plates with a falling-?lm evap
1,357,138 10/1920 Bassett .............................. .. 568/869 orator designed for internal and external partial conden
1,474,750 11/ 1923 Willkie .... ... .... .. ... . . . . .. 203/37
sation and to separate off unwanted constituents of the
1,936,497 11/1933 Carothers et a1. .. 568/869
2,578,816 12/1951- Lofdahl et a]. ....... .. 568/869
mixture, bleaching the product with activated carbon
2,960,447 11/ 1960 Anderson et a1. .... .. 203/37
and separating the bleach in known manner.
4,009,188 2/1977 Hcim et a1. ....... .. 203/73
4,225,394 9/1980 Cox et a1. .......................... .1. 203/37 3 Claims, 1 Drawing Figure
4,655,879
1 2
and energy consumption, cannot always be carried out
GLYCEROL DISTILLATION PROCESS continuously and are accompanied by considerable
losses of glycerol. In order to separate glycerol by dis
BACKGROUND OF THE INVENTION tillation from higher boiling impurities as well, the mix
1. Field of the Invention ture has to be additionally subjected to severe thermal
The puri?cation of glycerol obtained from naturally stressing which produces further losses of glycerol and
occurring fats and oils by an improved distillation pro more decomposition products.
cess. SUMMARY OF THE INVENTION
2. Statement of the Relevant Art
Glycerol (propane-1,2,3-triol) is the most important The present invention provides an improved process
of the trihydric alcohols. It is present as its esters in all for the non-degenerative distillation of glycerol, in
animal or vegetable fats and oils and is obtained from which glycerol derived from natural fats and oils is
them as a by-product of the saponi?cation process dur continuously separated from its impurities, particularly
ing soap manufacture. Although glycerol is also synthe salts, water and organic contaminants affecting its
sized from petrochemicals, this invention is concerned chemical and physical properties, without having to be
only with the puri?cation of glycerol obtained from subjected to intense thermal stressing. The yields
naturally occurring fats and oils. amount to 90% and higher and the quality of the glyc
Glycerol is formed during the transesteri?cation, erol is at least of a food and cosmetic standard. Prefera
splitting or saponi?cation of natural oils and fats, by bly, the glycerol is puri?ed to pharmaceutical standards
saponi?cation with alkali or by high pressure hydroly 20 such as that set by the US. Pharmacopeia, Deutsche
sis, and-depending on the saponi?cation proces Industrienorm No. 55,967, or Europharm III.
s-contains relatively large quantities of water, inor The present invention thus relates to a process for the
ganic salts, fats, low molecular weight organic com improved puri?cation by distillation of glycerol ob
pounds and also higher glycerol oligomers and poly tained by the transesteri?cation, splitting or saponi?ca
mers. 25 tion of natural fats and oils. The process comprises
The organic impurities accumulating in the crude alkalizing a glycerol-containing crude mixture in the
glycerol consist primarily of fats and glycerol-like com presence of air for oxidation, evaporating the mixture in
pounds formed by bacterial or chemical decomposition, a thin-layer evaporator with redistillation of the residue,
such as propane-1,2-diol and propane-1,3-diol or even recti?cation and reevaporation in a packed column
other components, such as glycerol methyl ethers having low-pressure-loss plates with a falling ?lm evap
which barely differ from glycerol in their physical orator designed for internal and external partial conden
properties (boiling point, refractive index) and which sation and to separate off unwanted constituents of the
interfere seriously with the puri?cation process because mixture, bleaching the product with activated carbon
they are distilled together with glycerol in conventional and separating the carbon in known manner.
columns having a small number of theoretical plates. The process according to the invention enables high
Some of these impurities are responsible for the undesir
able discoloration and poor color stability of the crude salt, low salt, or salt free crude glycerols to be puri?ed
glycerol; accordingly, their separation is absolutely continuously. It may also be used for the puri?cation of
essential.
crude glycerols from alkaline saponi?cation bottoms
One feature common to all processes carried out on 40
which contain a high percentage of fatty compounds
an industrial scale for extracting puri?ed glycerol ob (mong content 53% by weight), have a high salt con
tained from natural fats and oils is that production of tent (5 6% by weight) and contain up to 19% by weight
high purity glycerol requires a crude mixture of which of water. There is no need for chemical pre-puri?cation,'
the organic impurity content (mong content) amounts even in the case of low quality crude glycerols and
to § 1% by weight and from which fat, soap and other where even the most stringent quality requirements
organic constituents have been removed. To this end, have to be satis?ed. In addition, predrying has only to
the fats are saponi?ed with calcium hydroxide solution be carried out down to 10% by weight of water, which
or by the lime-soda (calcium-sodium hydroxide) pro may still be done at normal pressure without having to
cess in an elaborate chemical prepuri?cation stage and apply vacuum. Through the application of new technol~
are ?ltered off as far as possible in the form of soaps. ogies in the ?eld of distillation and recti?cation, such as
Further separation, particularly to remove inorganic the combination of thin-layer and falling-?lm evapora
impurities, is carried out by distillation. Columns in tors and packed columns with low-pressure-loss plates,
which the plates produce a high pressure loss are used separation ef?ciency is high despite relatively low sump
in the recti?cation stage. In order to obtain adequate temperatures, enabling the glycerol to be very carefully
separation of the various components, the sump temper treated and losses through decomposition reactions to
atures have to be correspondingly high. Limits are im be avoided. Through the integration of various process
posed on this process parameter by the fact that glyc steps, such as the separation of main runnings, ?rst
erol begins to split off water and to decompose and runnings, residue and sump, into a uni?ed process, sepa
polymerize at temperatures of the order of 180° C. ration of the glycerol takes place under optimal condi
Crude glycerol is processed in various ways, depending tions both with regard to apparatus and also to energy
on its salt content. Crude glycerol of low salt content consumption. The yields amount to between 90 and
may be directly puri?ed using ion exchangers whereas 95% of the glycerol processed. The quality of the prod
crude glycerol of high salt content has to be pretreated uct may be regulated according to requirements, the
by distillation to remove the salt. To obtain pure glyc yield even for high purity glycerol still exceeding 90%,
erol of the requisite commercial quality, distillation is 65 depending on the quality of the crude glycerol starting
followed by treatment with ion exchangers, for which material.
purpose the concentrated glycerol has to be rediluted. The packed column used in the process according to
Both processes are very expensive in terms of apparatus the invention is equipped with two evaporators and
4,655,879
3 4
gives a twice distilled end product in one process step. is stirred under nitrogen in a vessel for 15 to 30 minutes
The low-pressure-loss plates provide for a high number at 80° to 90° C. and passed through a frame ?lter press
of separation stages in that process step and lead to to separate the bleaching agent;
nondegenerative separation of the fats and the glycerol (h) bottom runnings are drawn off from the bottom of
like compounds (1,2- and 1,3-propane diol and also the sump and are evaporated in a falling-?lm evaporator
glycerolmethyl ethers), which was not possible in con at temperatures in the range about 150° to 180° C. after
ventional columns because of the inadequate number of which the vapors'are returned to the sump of the col
separation stages responsible for the high pressure loss umn;
per separation stage and the resulting severe thermal (i) a small partial stream of approximately 1% of the
stressing of the glycerol. quantity of crude glycerol used is continuously re
moved from the sump of the column and
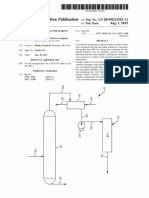
BRIEF DESCRIPTION OF THE DRAWING (i) the vapors deposited in the second condenser,
The FIGURE is a ?ow chart of the process accord optionally together with the sump discharge of the
ing to this invention, for. the non-degenerative puri?ca column, are further processed or partly recycled in
tion of glycerol by distillation. 15 another run.
Variant embodiments of the foregoing process in
DETAILED DESCRIPTION OF THE clude:
INVENTION (1) introducing 2 to 4 kg of superheated steam per 100
Other than in the operating examples, or where oth kg of crude glycerol into the column through the
erwise indicated, all numbers expressing quantities of 20 falling-?lm evaporator to react with and decolor the
ingredients or reaction conditions used herein are to be bottom runnings; and
understood as modi?ed in all instances by the term (2) using salt free and/or salt containing crude glycerols
“about”. as starting materials.
The process of this invention provides an improved It should be understood that in the foregoing and
puri?cation of crude glycerol by distillation. The crude 25 following description, no particular apparatus limita
glycerol may be obtained by the transesteri?cation, tions are intended, other than in the characterization of
hydrolysis and saponi?cation of natural fats and oils. the low-pressure-‘loss plates of the packed column.
Thesuccessive process steps are evaporation, recti?ca Thus, the ?rst, second, third, and re?ux condensers may
tion, bleaching, and separation of the bleaching agent. be any known condensing means. The ?rst and second
The speci?c steps of this process are as follows: 30 stirrer equipped vessels may be any known stirring and
(a) crude glycerol containing up to 10% by weight of container means. The thin-layer evaporator may be any
water-without predrying and chemical prepuri?ca known thin-layer evaporator means and the falling-?lm
tion-is alkalized for 1 hour at 90° to 100° C. with an evaporator may be any known falling-?lm evaporator
aqueous alkali hydroxide solution in a stirrer-equipped means. Any known power or mechanical means may be
vessel, followed by the addition of 10 N/m3 of air/m3 of 35 utilized for introducing or discharging reactants or
crude glycerol; products, where needed. Heat energy, where required,
(b) the resulting mixture is optionally preheated, and may be obtained from any known energy source, al
then is distilled at l65°-180° C./ 10-20 mbar in a thin though it is preferred to conserve heat energy removed
layer evaporator comprising mechanically driven wiper during some process steps by recycling it to energy
blades, the high boiling constituents being discharged 40 consuming steps. Any known ?ltering means may be
through a look into a residue receiver in which they used for separation of the activated carbon bleaching
undergo additional distillation; agent, although frame ?lter presses are preferred. The
(c) the vapors from the evaporator pass through a column itself may be any known column means which is
drop separator into the lower part of a packed column adapted to contain‘ the required low-pressure-loss plates
comprising low-pressure-loss plates (i.e., with a pres 45 and thereby provide at least seven theoretical separa
sure loss of at most 1 mbar/theoretical separation stage tion stages with the indicated minimal pressure loss.
at a comparable air velocity of 2 m/s) and ?ow through Referring more speci?cally to the drawing FIGURE,
that column, at least seven theoretical separation stages the crude glycerol mixture is introduced at 1 into a ?rst
being present; stirrer equipped vessel 2 in which 50% sodium hydrox
(d) the vapors of the column are partially deposited in ide or potassium hydroxide is added at 3 to saponify the
a ?rst condenser at 80° to 90° C. under a head pressure fats and fatty acids, followed by alkalization at 90° C.
of 5 to 10 mbars, the rest being condensed at 20° to 30° for at least one hour, during which air is introduced
C. in a second condenser, the condensate of the second with stirring at 4 to oxidize reducing components of the
condenser corresponding to approximately 1% of the mixture.
quantity of crude glycerol used; 55 The reaction mixture thus treated passes through a
(e) the condensate from the ?rst condenser is cooled preheater 5, in which it is heated to approximately 140°
by 30° .to 50° C. and returned to the head of the column, G, into a thin layer evaporator 6 in which the glycerol
optionally ?rst passing through a re?ux condenser; is carefully concentrated by evaporation in vacuo (max.
(f) the main product runnings is continuously re 15 mbars) with mechanical stirring.
moved from the column as a liquid sidestream at a The residue containing the soaps formed during alka
height of approximately g of the height of the column, lization, salts and polymeric glycerols is discharged
a partial stream being returned to the column immedi through a heated upper ballcock 7 at the lower end of
ately beneath the point of removal either at the same the thin-layer evaporator into a similarly heated re
temperature or cooled by 20° to 40° C.; ceiver 8 in which the residue undergoes additional
(g) the rest of the main product runnings is cooled to 65 “squeezing out” under the effect of higher tempera
80° to 90° C. optionally by passing through a third con tures. The receiver 8 is continuously emptied through
denser and after the addition of activated carbon the similarly heated lower ballcock 70 after the upper
bleaching agent in a quantity of 0.1 to 0.3% by weight, ballcock 7 has been shut off and the receiver vented.
4,655,879 6
5
The receiver is then preevacuated by a ring pump and heat the crude product after alkalization in the pre
the connection between the evaporator 6 and the heated heater 5 preceding the thin layer evaporator 6.
receiver 8 reestablished. Since the stream of condensed ?rst runnings removed
The vapors from the thin-layer evaporator 6 pass at 16 still contains considerable quantities of glycerol,
through a heated vapor tube 9 into the sump 27 of the the entire process may be rerun, although without a
packed column 11 at 10. The vapor tube 9 is ?tted with further alkalization step, optionally together with the
separation aids to prevent droplets of liquid from the sump discharge 21. Any resulting deterioration in color
evaporator 6 from being entrained. in the main product runnings of the column may be
The vapors are recti?ed as they ascend through the corrected by slightly increasing the quantity of acti
packed column 11. The column is ?tted with low-pres vated carbon used in the bleaching stage. Alternatively,
sure-loss plates so that a high number of separation none of the condensed ?rst runnings 16 may be returned
stages is obtained without the glycerol being decom to the alkalization vessel.
posed by severe temperature stressing in the sump 27. In cases where the crude glycerol contains less than
For a comparable air velocity of at least 2 m/s, the 5% by weight of water, an improvement in the color of
low-pressure-loss plates lead to a pressure loss of at the main product runnings removed at 18 may option
most 1 mbar/theoretical separation stage, at least seven ally be obtained by introducing superheated steam at 26
theoretical separation stages being present. into the head of the falling-?lm evaporator 19.
The vapors of the upper separation zone are partly The bleached process products which are run off as
deposited in the ?rst condenser 13 at approximately 85° ?nal runnings and which are obtained at 25 are of excel
C. and under a head pressure of at most 10 mbar (de 20 lent quality. The glycerol content amounts to between
phlegmator). The liquid phase formed is cooled in the 99.8 and 99.9%. After bleaching, the products are col
re?ux condenser 15 and returned to the head 12 of the orless and clear and have I-Iazen numbers of from 5 to
column 11 (i.e., internal and external partial condensa 10. The product does not contain any salt residues and
tion). The lower boiling components of the vapors leav has a water content of less than 0.1%. With product
ing the column at its head 12 condense in a second 25 qualities such as these, a glycerol yield of from 90 to
condenser 14 and are run off as ?rst runnings at 16. In 95% is obtained.
addition to glycerol, the ?rst runnings mainly contain The invention is illustrated by the following Exam
esters, fats and glycerol-like compounds. Vapors which ples, which were carried out with glycerols of various
are not condensible under the preveiling conditions of origin. i
pressure and temperature, mainly water, pass uncon EXAMPLE 1
densed into a vacuum unit at 17.
Higher boiling components, such as dimeric glycerol Crude glycerol mixture of high salt content from the
and colored constituents, may be separated off from the transesteri?cation of coconut oil; water content 2 to
ascending vapors by at least two theoretical separation 10%, salt content 4 to 6%, mong content 2 to 3%, was
stages in the lower third of the column. At the upper used as the starting material.
end of this part of the column, the entire column re?ux EXAMPLE 2
is laterally run off at 18 as the main product runnings.
Part of the main product runnings passes back into the Highly colored, strong-smelling crude glycerol mix
column at about the same point 18 either with the same ture of high salt content from the splitting of a residue
temperature or even slightly cooled. This fraction may 40 accumulating during the splitting of coconut oil; water
be regulated and serves to remove the high boiling and content 2 to 10%, salt content 6%, mong content 1.5%,
colored constituents from the vapor containing good was used as the starting material.
product. Using the crude glycerol mixtures of Examples 1 and
2 in the inventive process, the following values were
A small quantity of the liquid mixture is continuously
removed from the sump 27 at the lower end of the 45 obtained after bleaching with 0.2% and 0.3% of two
column 11 to counteract an excessive concentration of types of active carbon.
colored components. The contents of the sump 27 of the
column 11 are carefully evaporated in the sump falling Tested Value Example 1 Example 2
film evaporator 19 at a maximum temperature of 165° C.
(second evaporation), the vapors being returned to the 50 up (20' C.): 1.4737 1.4737
Glycerin content 99.8-99.9 99.8-99.9
sump 27 at point 20 while the residue is collected as (% by weight):
sump discharge at 21 and may be recycled, optionally l-lazen No.: 5 5-10
together with the ?rst runnings 16, in another run,
S. No.: 0.11 0.11
shown in the FIGURE in dotted lines. The falling-?lm E. No. (ml N-lO HCl): 9.2 9.2
evaporator 19 is preferably designed for internal and 55 A. No.: 0.1 0.1
external partial condensation and to separate off un Red substances
wanted constituents of the crude glycerol. 1st stage: colorless clear colorless clear
The greater part of the main product runnings re 2nd stage: yellowish gray yellow-gray
Miscibility colorless clear colorless clear
moved at 18 is cooled in a main condenser 22 and then water:
continuously bleached by adding activated carbon 28 in 60 Water content 0.07 0.07
a second stirrer equipped vessel 23. Bleaching is carried (% by weight):
out for 30 minutes at 80° C. with from 0.1 to 0.3% by
weight of activated carbon, depending on the quality of Yield (%) 95 90
the crude glycerol, the glycerol/carbon mixture being
placed under a nitrogen blanket. The carbon is removed 65
by means of frame ?lter presses 24. We claim:
The heat removed from the main product runnings in 1. A process for the puri?cation of crude glycerol
the third condenser 22 for cooling to 80° C. is used to derived from the transesteri?cation, hydrolysis, and
4,655,879
7 8
saponi?cation of naturally occurring fats and oils, com- (f) continuously removing a main product from said
prising column as a liquid sidestream at a point approxi
(a) alkalizing crude glycerol containing up to 10% by mately é of the height of said column, and return
weight of water without predrying and chemical ing a partial stream thereof to said column beneath
prepuri?caton by mixing with an aqueous alkali 5 the point of removal;
hydroxide solution for about 1 hour at about (g) cooling the remaining non-returned portion of
90°'—l00° C., followed by the addition of about 10 said main product to about 80°-90° C.; adding
N/m3 of air per cubic meter of said crude glycerol; about 0.1 to 0.3% by weight of activated charcoal
(b) distilling the alkalized mixture at about l65°~l80° to the said non-returned portion as a bleaching
C. and about 10-20 mbar and discharging high 10 agent and stirring under nitrogen for about 15 to 30
boiling constituents for additional distillation; mintures while at about 80°—90° C.;'and then re
(c) passing the vapors generated by said distillation moving said bleaching agent;
into a lower part of a packing column and ?owing (i) drawing off bottom product from the column
said vapors upward through said column, said col- sump, evaporating said bottom product at tempera
umn containing low-pressure-loss plates having a 15 tures of about l50°-l80° C., and returning vapor
pressure loss of at most 1 mbar/theoretical separa- generated thereby to said sump,
tion stage at an air velocity of 2 m/s and having at G) continuously removing a partial stream of about
least seven theoretical separation stages; 1% of the quantity of glycerol being processed
(d) partially ?rst condensing the vapors from said from said bottom product before it is evaporated.
column at about 80°—90° C. under a head pressure 20 2. The process of claim 1 wherein in step (i) said
of about 5-10 mbars, and second condensing the evaporating is by falling-?lm evaporation of said bot
remaining vapors at about 20°-30° C., so that the torn product.
second condensate corresponds to approximately 3. The process of claim 1 or 2 wherein about 2 to 4 kg
l% of the quantity of crude glycerol; of superheated steam per 100 kg of glycerol being pro
(e) cooling the ?rst condensate by about 30°-50° C. 25 cessed is introduced during the evaporation of step (i).
and returning same to the head of said column; * " * * *
35
45
.55
60
65
Das könnte Ihnen auch gefallen
- Us 5162407Dokument9 SeitenUs 5162407Juanan LopezNoch keine Bewertungen
- United States Patent (191: Graves (45) Date of Patent: Oct. 7, 1997Dokument6 SeitenUnited States Patent (191: Graves (45) Date of Patent: Oct. 7, 1997Anonymous vWSYmPNoch keine Bewertungen
- Ipa-Water SeparationDokument8 SeitenIpa-Water SeparationkashifwarsiNoch keine Bewertungen
- Unite Patent (19) : EnglandDokument4 SeitenUnite Patent (19) : EnglandDoinitaNoch keine Bewertungen
- United States Patent (19) 11 Patent Number: 6,107,527: Stec Et Al. (45) Date of Patent: Aug. 22, 2000Dokument4 SeitenUnited States Patent (19) 11 Patent Number: 6,107,527: Stec Et Al. (45) Date of Patent: Aug. 22, 2000cbuhksmkNoch keine Bewertungen
- United States Patent (19) 11 Patent Number: 5,937,665: Kiessel Et Al. (45) Date of Patent: Aug. 17, 1999Dokument9 SeitenUnited States Patent (19) 11 Patent Number: 5,937,665: Kiessel Et Al. (45) Date of Patent: Aug. 17, 1999hussainNoch keine Bewertungen
- United States Patent (19) : Attorney, Agent, or Firm-J. Frederick Thomsen HarryDokument21 SeitenUnited States Patent (19) : Attorney, Agent, or Firm-J. Frederick Thomsen HarryNcTungNoch keine Bewertungen
- United States Patent (19) : (45) Date of Patent: Dec. 8, 1998Dokument6 SeitenUnited States Patent (19) : (45) Date of Patent: Dec. 8, 1998nagy_andor_csongorNoch keine Bewertungen
- United States Patent (19) : Llllllllllllllllllllllllllllllllllllllllllillll - LL - LLLLLLLLLLLLLLLLLLLLLLLDokument6 SeitenUnited States Patent (19) : Llllllllllllllllllllllllllllllllllllllllllillll - LL - LLLLLLLLLLLLLLLLLLLLLLLthomas cookNoch keine Bewertungen
- United States Patent (10) Patent No.: US 6,326,458 B1Dokument26 SeitenUnited States Patent (10) Patent No.: US 6,326,458 B1Citra Adelina SitorusNoch keine Bewertungen
- Apg Sentez HenkelDokument14 SeitenApg Sentez Henkelmaydin74Noch keine Bewertungen
- United States Patent (19) 11 Patent Number: 6,011,134: Marks Et Al. (45) Date of Patent: Jan. 4, 2000Dokument5 SeitenUnited States Patent (19) 11 Patent Number: 6,011,134: Marks Et Al. (45) Date of Patent: Jan. 4, 2000Affinta LorenzaNoch keine Bewertungen
- United States Patent (191: Choi Et A1. (45) Date of Patent: Oct. 7, 1986Dokument15 SeitenUnited States Patent (191: Choi Et A1. (45) Date of Patent: Oct. 7, 1986arbeyvillalbaNoch keine Bewertungen
- United States Patent: 73 Assignee: Acrion Technologies, Inc., Valley View, 3. E. em (I. R35Dokument10 SeitenUnited States Patent: 73 Assignee: Acrion Technologies, Inc., Valley View, 3. E. em (I. R35jeremyhoveNoch keine Bewertungen
- United States Patent (19) 11 Patent Number: 5,997,619Dokument13 SeitenUnited States Patent (19) 11 Patent Number: 5,997,619nooraaNoch keine Bewertungen
- Method for making polysiloxane emulsions using emulsion polymerizationDokument12 SeitenMethod for making polysiloxane emulsions using emulsion polymerizationSinh LeNoch keine Bewertungen
- Us 5356671Dokument5 SeitenUs 5356671wjzabalaNoch keine Bewertungen
- Bolas Ver VerDokument6 SeitenBolas Ver VerpatgabrielNoch keine Bewertungen
- Ulllted States Patent (19) (11) Patent Number: 5,945,409: Crandall (45) Date of Patent: Aug. 31, 1999Dokument9 SeitenUlllted States Patent (19) (11) Patent Number: 5,945,409: Crandall (45) Date of Patent: Aug. 31, 1999عبدالعزيز بدرNoch keine Bewertungen
- Process for isolating tocopherols and sterols from natural sourcesDokument7 SeitenProcess for isolating tocopherols and sterols from natural sourcesAndres GuzmanNoch keine Bewertungen
- Processing of Heavy OilsDokument9 SeitenProcessing of Heavy Oilsmohammed salmanNoch keine Bewertungen
- United States Patent (19) : 11 Patent Number: 45 Date of PatentDokument10 SeitenUnited States Patent (19) : 11 Patent Number: 45 Date of PatentFernando SImonelliNoch keine Bewertungen
- Protective Polymeric CoatingsDokument7 SeitenProtective Polymeric CoatingsAlexander Franco CastrillonNoch keine Bewertungen
- Us 4432328Dokument6 SeitenUs 4432328Kristee Ann KellyNoch keine Bewertungen
- Riedl 1980 US4225520 PDFDokument6 SeitenRiedl 1980 US4225520 PDFManuel GonzalezNoch keine Bewertungen
- US8529731Dokument6 SeitenUS8529731Harshad Vinay SavantNoch keine Bewertungen
- United States Patent (191: Epperson, JRDokument6 SeitenUnited States Patent (191: Epperson, JRAparnaNoch keine Bewertungen
- Us5844037 PDFDokument12 SeitenUs5844037 PDFKelly GuerreroNoch keine Bewertungen
- LL - LLLLLLLLLLLLLL - LLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLDokument16 SeitenLL - LLLLLLLLLLLLLL - LLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLLIrelena RomeroNoch keine Bewertungen
- US5640718Dokument6 SeitenUS5640718Shweta ChauhanNoch keine Bewertungen
- US6953534Dokument13 SeitenUS6953534MaboodNoch keine Bewertungen
- APPARATUS FOR QUENCHING A GAS STREAM IN VINYL CHLORIDE PRODUCTIONDokument9 SeitenAPPARATUS FOR QUENCHING A GAS STREAM IN VINYL CHLORIDE PRODUCTIONAbdulrahman ezzaldeenNoch keine Bewertungen
- Rotational Moulding Process for Improved Plastics ArticlesDokument8 SeitenRotational Moulding Process for Improved Plastics ArticlesG DragonNoch keine Bewertungen
- Adhesive Containing IodineDokument4 SeitenAdhesive Containing IodineVansala GanesanNoch keine Bewertungen
- Patent Number 5194299Dokument11 SeitenPatent Number 5194299Lope Nam-iNoch keine Bewertungen
- Molde PatenteadoDokument12 SeitenMolde PatenteadoFabiano SchincariolNoch keine Bewertungen
- United States Patent (19) 11 Patent Number: 5,935,885Dokument12 SeitenUnited States Patent (19) 11 Patent Number: 5,935,885Yogesh KumarNoch keine Bewertungen
- Us 5733856Dokument11 SeitenUs 5733856omer reisNoch keine Bewertungen
- US Patent Manufacturing of Propylene GlycolDokument5 SeitenUS Patent Manufacturing of Propylene GlycolRegiyanti RNoch keine Bewertungen
- Water-Based Gravure Printing Ink PatentDokument6 SeitenWater-Based Gravure Printing Ink PatentHiba Naser100% (1)
- Iiiiiiiiiiiiiiii: United States Patent 1191Dokument10 SeitenIiiiiiiiiiiiiiii: United States Patent 1191Anonymous vWSYmPNoch keine Bewertungen
- Us 6251262Dokument8 SeitenUs 6251262peymanNoch keine Bewertungen
- Us 6458570Dokument4 SeitenUs 6458570NovrieNoch keine Bewertungen
- Fermentation and recovery process for lactic acid production from potato wasteDokument11 SeitenFermentation and recovery process for lactic acid production from potato wasteALxiitoo SancHez NtsNoch keine Bewertungen
- United States Patent (19) 11 Patent Number: 5,673,939: Bees Et Al. 45 Date of Patent: Oct. 7, 1997Dokument9 SeitenUnited States Patent (19) 11 Patent Number: 5,673,939: Bees Et Al. 45 Date of Patent: Oct. 7, 1997Mohit PadheeNoch keine Bewertungen
- United States Patent (19) 11 Patent Number: 5,928,426: Aitchison (45) Date of Patent: Jul. 27, 1999Dokument17 SeitenUnited States Patent (19) 11 Patent Number: 5,928,426: Aitchison (45) Date of Patent: Jul. 27, 1999hosseinNoch keine Bewertungen
- Us5955039 PDFDokument8 SeitenUs5955039 PDFMayur B NeveNoch keine Bewertungen
- United States Patent: (10) Patent No.: (45) Date of PatentDokument5 SeitenUnited States Patent: (10) Patent No.: (45) Date of PatentLechugaGuerreroHerreraNoch keine Bewertungen
- United States Patent (10) Patent No.: US 7,438,897 B2Dokument6 SeitenUnited States Patent (10) Patent No.: US 7,438,897 B2Amir HamzahNoch keine Bewertungen
- US5461179Dokument11 SeitenUS5461179Muhammad Akbar FahleviNoch keine Bewertungen
- US8749333Dokument19 SeitenUS8749333Anonymous GIIncAfLRNoch keine Bewertungen
- Us 6489527Dokument6 SeitenUs 6489527Gracelia IrmalindaNoch keine Bewertungen
- Microorganismos ExpoDokument8 SeitenMicroorganismos ExpoSofia AmadorNoch keine Bewertungen
- United States Patent: (10) Patent No.: US 6,293,891 B1Dokument8 SeitenUnited States Patent: (10) Patent No.: US 6,293,891 B1Dejan ZdravkovskiNoch keine Bewertungen
- United States Patent (10) Patent No.: US 6,282,863 B1: Christian Et Al. (45) Date of Patent: Sep. 4, 2001Dokument20 SeitenUnited States Patent (10) Patent No.: US 6,282,863 B1: Christian Et Al. (45) Date of Patent: Sep. 4, 2001Munir KadernaniNoch keine Bewertungen
- Method of Treating Lignocellulosic Biomass to Produce CelluloseDokument11 SeitenMethod of Treating Lignocellulosic Biomass to Produce CelluloseahmetNoch keine Bewertungen
- United States Patent (191: YollesDokument8 SeitenUnited States Patent (191: YollesCitra Adelina SitorusNoch keine Bewertungen
- Adsorption 5ADokument9 SeitenAdsorption 5ANguyễn Thị Kim PhượngNoch keine Bewertungen
- United States Patent (10) Patent No.: US 7,207,268 B2: Brunst (45) Date of Patent: Apr. 24, 2007Dokument8 SeitenUnited States Patent (10) Patent No.: US 7,207,268 B2: Brunst (45) Date of Patent: Apr. 24, 2007Quý Đình Mai MaiNoch keine Bewertungen
- Energetic Materials: Particle Processing and CharacterizationVon EverandEnergetic Materials: Particle Processing and CharacterizationUlrich TeipelNoch keine Bewertungen
- Industrial CarbonDokument9 SeitenIndustrial CarbonRonel GarciaNoch keine Bewertungen
- Compressed Air Filters LeafletDokument20 SeitenCompressed Air Filters LeafletAnonymous FZs3yBHh7Noch keine Bewertungen
- 9.5.1 Meat Packing PlantsDokument7 Seiten9.5.1 Meat Packing PlantsdkaringamNoch keine Bewertungen
- CyanideDokument52 SeitenCyanideWilliamEliezerClNoch keine Bewertungen
- Final Year PPT (Satwik)Dokument25 SeitenFinal Year PPT (Satwik)Satwik Singh100% (1)
- Coconut Shell Based Activated Carbon With No Green House Gas EmissionDokument4 SeitenCoconut Shell Based Activated Carbon With No Green House Gas EmissionAbrar AhmedNoch keine Bewertungen
- Application of Wheeler-Jonas Equation and Relative Breakthrough Time (RBT) in Activated Carbon Beds of Respirator Gas FiltersDokument7 SeitenApplication of Wheeler-Jonas Equation and Relative Breakthrough Time (RBT) in Activated Carbon Beds of Respirator Gas FiltersBong SomvixayNoch keine Bewertungen
- Odor Control: Wastewater Treatment/Odor Control Methods Odor Control SystemDokument21 SeitenOdor Control: Wastewater Treatment/Odor Control Methods Odor Control SystemRB MortxNoch keine Bewertungen
- Lecture Notes Clarification, Filtration, Adsorption (Activated Carbon, and Water Stability CEG8103Dokument22 SeitenLecture Notes Clarification, Filtration, Adsorption (Activated Carbon, and Water Stability CEG8103yusufNoch keine Bewertungen
- Removal of Reactive Dyes From Aqueous Solution Using Bagasse Fly AshDokument12 SeitenRemoval of Reactive Dyes From Aqueous Solution Using Bagasse Fly AshSergio Bazan YnostrozaNoch keine Bewertungen
- Exp. (3) Determination of Adsorption Isotherm of Acetic Acid On Activated Charcoal.Dokument21 SeitenExp. (3) Determination of Adsorption Isotherm of Acetic Acid On Activated Charcoal.soran najeb100% (1)
- 2010 - Desulfurization of Diesel Fuels by Selective Adsorption On Activated CarbonsDokument11 Seiten2010 - Desulfurization of Diesel Fuels by Selective Adsorption On Activated CarbonsloremncNoch keine Bewertungen
- Adsorption Review: Key Concepts and ProblemsDokument4 SeitenAdsorption Review: Key Concepts and ProblemsJohn Bryan Aldovino0% (2)
- Voc PDFDokument4 SeitenVoc PDFrkhandelwal9604Noch keine Bewertungen
- Distribution Pattern of Nile Water Algae With Reference To Its Treatability in Drinking Water. J. Appl., Sci. Res. 4, 722-730.Dokument10 SeitenDistribution Pattern of Nile Water Algae With Reference To Its Treatability in Drinking Water. J. Appl., Sci. Res. 4, 722-730.Sabry ZaghloulNoch keine Bewertungen
- Performance of Activated Carbon From Cassava Peel in Reducing Phosphate Concentration in Laundry Liquid WasteDokument10 SeitenPerformance of Activated Carbon From Cassava Peel in Reducing Phosphate Concentration in Laundry Liquid WastesimsonNoch keine Bewertungen
- Activated Carbon Filter Calculation Note - Odor Control Unit - Mall of Qatar - Phase 2Dokument4 SeitenActivated Carbon Filter Calculation Note - Odor Control Unit - Mall of Qatar - Phase 2Ahmed Labib100% (1)
- 1.prog Report 3Dokument4 Seiten1.prog Report 3Subhajit GhoshNoch keine Bewertungen
- Formulasi Sediaan Pasta Gigi Karbon Aktif DENGAN BASIS Virgin Coconut Oil (VCO)Dokument10 SeitenFormulasi Sediaan Pasta Gigi Karbon Aktif DENGAN BASIS Virgin Coconut Oil (VCO)Ghina Mukti LuthfiaNoch keine Bewertungen
- VW Passat b6 Heating Air Conditioning System EngDokument174 SeitenVW Passat b6 Heating Air Conditioning System EngFadel B ChNoch keine Bewertungen
- Adsorption of Butyric Alcohol From Solution On Activated CharcoalDokument4 SeitenAdsorption of Butyric Alcohol From Solution On Activated CharcoalTranggNoch keine Bewertungen
- Adsorption Capacity and Removal Efficiency of Heavy Metal Ions by Moso and Ma Bamboo Activated CarbonsDokument10 SeitenAdsorption Capacity and Removal Efficiency of Heavy Metal Ions by Moso and Ma Bamboo Activated Carbonsanand sagarNoch keine Bewertungen
- Filters and Filtration Unit Model NumbersDokument8 SeitenFilters and Filtration Unit Model NumbersPromagEnviro.comNoch keine Bewertungen
- Project of Parle AgroDokument45 SeitenProject of Parle AgroDhirender Singh100% (5)
- MED12 142plus Medical Air Purifier ISO Sales Leaflet en 2212021829Dokument8 SeitenMED12 142plus Medical Air Purifier ISO Sales Leaflet en 2212021829RUN GONoch keine Bewertungen
- LT-ACB Active Carbon Filter Bag Features & SpecsDokument2 SeitenLT-ACB Active Carbon Filter Bag Features & Specsnabila OktavianiNoch keine Bewertungen
- Paten Etanol Kel3 B IDLDokument18 SeitenPaten Etanol Kel3 B IDLFazaaNoch keine Bewertungen
- Large Scale Recycling Process For Scrap Tires and Rubber Products PDFDokument11 SeitenLarge Scale Recycling Process For Scrap Tires and Rubber Products PDFbachirNoch keine Bewertungen
- Liquid Liquid ExtractionDokument50 SeitenLiquid Liquid ExtractionArrianne Jaye Mata86% (14)
- Life Cycle Assessment of Activated Carbon Production From CoconuDokument10 SeitenLife Cycle Assessment of Activated Carbon Production From CoconuMaría Del Mar LondoñoNoch keine Bewertungen