Beruflich Dokumente
Kultur Dokumente
Us Patent
Hochgeladen von
Aditya Yudantoko0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
32 Ansichten8 Seitenus patent from methacrylat acid from isobuthylene and air
Originaltitel
(us patent)
Copyright
© © All Rights Reserved
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenus patent from methacrylat acid from isobuthylene and air
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
32 Ansichten8 SeitenUs Patent
Hochgeladen von
Aditya Yudantokous patent from methacrylat acid from isobuthylene and air
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 8
United States Patent (19) (11) 4,124,634
Gotoh et al. (45) Nov. 7, 1978
(54) PROCESS FOR PRODUCING (56) References Cited
METHACRYLIC ACID FROM U.S. PATENT DOCUMENTS
ISOBUTYLENE BY TWO STEP OXIDATION
3,725,472 3/1973 Kowano et al. ................. 260/.530 N
3,882,047 5/1975 Nuna et al. ...................... 260/.530 N
(75. Inventors: Isao Gotoh; Junji Endoh, both of
Yokohama; Tohru Ueno, Hatano, all FOREIGN PATENT DOCUMENTS
of Japan 939,713 10/1963 United Kingdom ................ 260/.530 N
73) Assignee: Asahi Glass Company, Ltd., Tokyo, Primary Examiner-Vivian Garner
Japan Attorney, Agent, or Firm-Oblon, Fisher, Spivak,
McClelland & Maier
(21) Appl. No.: 775,427 57 ABSTRACT
In a process for producing methacrylic acid by two step
(22 Filed: Mar. 8, 1977 oxidation comprising a first oxidation step of producing
methacrolein by reacting isobutylene with molecular
oxygen in the presence of a diluent gas and a catalyst,
Related U.S. Application Data and a second oxidation step of producing methacrylic
acid by reacting methacrolein with molecular oxygen in
63 Continuation of Ser. No. 624,646, Oct. 22, 1975, the presence of a diluent gas and a catalyst, the im
abandoned. provement comprises using nitrogen as the diluent gas
in the first oxidation step and nitrogen and steam as the
(30) Foreign Application Priority Data diluent gas in the second oxidation step, mixing the
Oct. 23, 1974 JP Japan ................................ 49-121510
reaction products obtained in the first and second oxida
tion steps, separating and recovering methacrolein and
methacrylic acid from the mixture and feeding the re
51) Int. C.? ....................... C07C 45/04; C07C 51/32 covered methacrolein back into the second oxidation
52 U.S. Cl. ................................ 562/532; 260/604 R; step as a starting material.
562/535
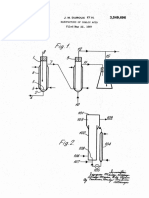
58) Field of Search ........... 260/.530 N, 604 R, 533 N 8 Claims, 1 Drawing Figure
U.S. Patent Nov. 7, 1978 4,124,634
4,124,634
1. 2
characteristics of the catalyst. The consumption of oxy
PROCESS FOR PRODUCING METHACRYLIC gen is correspondingly small and a large amount of
ACIED FROM ISOBUTYLENE BY TWOSTEP oxygen remains in the reaction product. Accordingly,
OXIDATION the overall composition of the methacrolein-containing
This is a continuation of application Ser. No. 624,646, 5 gas formed in the recovery process becomes explosive.
filed Oct. 22, 1975, now abandoned. In order to prevent this formation of an explosive gas,
BACKGROUND OF THE INVENTION decreasing the supply of oxygen while increasing the
1. Field of the Invention supply of nitrogen used as a diluent gas has been sug
gested.
The present invention relates to a process for produc- 10 conditions Unfortunately, in this method, the resultant
ing methacrylic acid from isobutylene. More particu catalyst performance are far from those which are optimum for
larly, it relates to a novel process for producing meth whereby the yield of the in the second oxidation step,
acrylic acid from isobutylene by a two step oxidation oxidation step is disadvantageously object product in the second
consisting of a first step of oxidizing isobutylene to quently, there remains a need for an improvement decreased. Conse
methacrolein and a second step of oxidizing methacro- 15 two step oxidation preparation of methacrylic acid. in the
lein to methacrylic acid.
2. Description of the Prior Art SUMMARY OF THE INVENTION
Heretofore, it has been known to produce meth Accordingly, it is an object of the present invention
acrylic acid by reacting isobutylene with molecular
oxygen in the presence of a diluent gas and steam. How- 20 to provide a process for producing methacrylic acid
ever, a catalyst suitable for industrial production of from isobutylene which maintains optimum conditions
methacrylic acid from isobutylene in high yield, has not for the performance of the catalyst in the second oxida
been found. Accordingly, it is industrially advantageous tion step and prevents the formation of an explosive gas
to conduct the reaction in two steps using a desirable during the process for recovering the unused methacro
catalyst for production of methacrolein from isobutyl- 25 lein and the desired methacrylic acid from the reaction
ene and a desirable catalyst for production of meth product, thereby enabling safe separation of these prod
acrylic acid from methacrolein. lucts,
However, when methacrylic acid is produced in such It is another object of the invention to provide a
a two step oxidation, it is relatively difficult to attain process for producing methacrylic acid from isobutyl
high selectivity of methacylic acid in the second oxida- 30 ene wherein methacrolein and methacrylic acid are
tion step if the conversion of methacrolein is high. That effectively separated and recovered from the reaction
is, the selectivity of methacrylic acid formation de products in the first and second oxidation steps.
creases with increasing conversion of methacrolein. It is still another object of this invention to provide a
Accordingly, it becomes advantgeous to conduct the process for producing methacrylic acid from isobutyl
second oxidation step under conditions such that the 35 ene which includes separating and recovering methac
conversion of methacrolein is relatively low and then to rolein and methacrylic acid from the reaction products
recover the unreacted methacrolein from the reaction in the first and second oxidation steps while preventing
products and recycle them back into the second oxida their polymerization.
tion step. The unreacted methacrolein is separated from It is a further object of this invention to provide a
the reaction products in the second oxidation step while 40 process for producing methacrylic acid from isobutyl
the object product, methacrylic acid is recovered. This
is accomplished by cooling the reaction products in the ene wherein methacrolein and methacrylic acid are
respectively produced in high yield in the first and
second oxidation step to liquefy them, and/or further second oxidation steps.
contacting them with water to separate the gaseous These and other objects of this invention as will here
components of oxygen, nitrogen, carbon dioxide gas 45 inafter become clear from the ensuing discussion have
and the like. As a result, a liquid phase containing meth been attained by providing a process for producing
acrolein and/or methacrylic acid, is obtained and these methacrylic acid from isobutylene by a two step oxida
are separated by distillation. tion consisting of first oxidizing isobutylene to methac
However, the present inventors have determined that
in many cases a dangerous self-explosive gas is formed 50 rolein and then oxidizing methacrolein to methacrylic
in the process of recovering the products of the second acid, wherein nitrogen is used as a diluent gas in the first
oxidation step. The self-explosive gas includes self oxidation step and nitrogen and steam are used as a
flammable gas, usually called a detonating gas. The diluent gas in the second oxidation step, and the reac
present inventors have further determined that the ex tion products of the first and second oxidation steps are
plosive gas is apparently formed by the following mech- 55 mixed and methacrolein and methacrylic acid are sepa
anism. The methacrolein in the reaction product has a rated and recovered from the mixture of their reaction
relatively low boiling point and a low solubility in wa products and the recovered methacrolein is recycled as
ter. As a result, it is relatively difficult to recover a high a starting material into the second oxidation step.
proportion of the methacrolein in the liquid phase dur
ing the recovery process, because it is so insoluble. 60 BRIEF DESCRIPTION OF THE DRAWING
However, the methacrylic acid and steam in the reac A more complete appreciation of the invention and
tion product can be easily recovered in the liquid phase. many of the attendant advantages thereof will be
Accordingly, during the recovery process, methacro readily attained as the same becomes better understood
lein remains present in the gaseous system of oxygen, by reference to the following detailed description when
nitrogen and carbon dioxide gas of the reaction prod- 65 considered in connection with the accompanying draw
uct. ings, wherein:
Furthermore, the conversion of methacrolein in the FIG. 1 is a flow diagram of one embodiment of an
second oxidation step is relatively low because of the apparatus used for the process of this invention.
3
4,124,634 4.
DETAILED DESCRIPTION OF THE
dation step conducted in a reactor 5, it is necessary to
PREFERRED EMBODIMENTS
include steam in the diluent gas 6 containing nitrogen
gas so as to increase the yield of the resultant meth
Referring to FIG. 1, one embodiment of the process acrylic acid. The amount of the diluent gas 6 in the feed
of this invention will be illustrated. In the specification, 5 gas should be in the same range as in the first oxidation
the terms "conversion' and "selectivity' have the fol step. It is preferred to have 5-90 mole %, especially 15
lowing meanings. - 75 mole %, of steam in the feed gas. It is also possible
to add other diluent gases as in the first oxidation step.
Conversion = Converted Fed The amount of molecular oxygen 7 fed in the second
component -- component X100. 10 oxidation step should be in the range of 1.2 - 2.0 moles,
Selectivity
(moles)
= Resulting
(moles)
Converted especially 1.5 - 1.8 moles, for 1 mole of methacrolein in
product -- component X100, order to maintain a good activity of the catalyst. It is
(moles) (moles) preferred to use air as the source of the molecular oxy
gen, for the process of the invention because thereby
The first and second oxidation steps in the process of 15 nitrogen gas is also supplied in a sufficient amount to be
this invention can be operated as follows. The first used as the diluent gas for the oxidation. Suitable cata
oxidation step involves oxidizing isobutylene 3 with lysts for use in the second oxidation step include the
molecular oxygen 4 in the presence of a diluent gas 2 metal oxides used in the first oxidation step, and also
and a catalyst (not shown) in a reactor 1. Nitrogen in air molybdenum-thallium oxides. Oxides with rhenium,
is used as the diluent gas 2. However, if desired, it is 20 copper, cobalt, vanadium or mercury added if desired;
possible to utilize other suitable diluent gases such as molybdenum-phosphorus oxides with an oxide of vana
carbon dioxide, helium, steam, and the like, which do dium, antimony, arsenic, aluminum, or cobalt added, if
not interfere with the effect of this invention. The mo desired; molybdenum-palladium oxides with an oxide of
lecular oxygen 4 can be supplied from any conventional antimony, or arsenic added, if desired; phosphomolyb
source for providing molecular oxygen under the con 25 dic acid; and an ammonium, bismuth, antimony, potas
ditions of the reaction. It is preferred to use air since it sium, cesium, rubidium or thallium salt of phosphomo
is economical and also is the source of the diluent gas. lybdic acid, which has a central phosphorus atom and a
The concentration, of isobutylene to be used as the ligand of molybdenum. Suitable reaction temperatures
starting material is preferred to be less than 4 mole % and pressures for the second oxidation step are substan
from the viewpoint of temperature control. The molar 30 tially in the same range as those of the first oxidation
ratio of molecular oxygen to isobutylene is preferred to step. In the second step, in order to increase the selec
be in the range of 1/3 - 4/1, especially 1/1 - 3/1. The tivity of methacrylic acid, the conversion of methacro
amount of diluent gas in the feed gas is preferred to be lein should be less than 80%, preferably in the range of
in the range of 50-97 mole% especially 65-90 mole 40 - 70%, because of the characteristics of the catalyst.
%. It is necessary that the diluent gas contain more than In this manner, the selectivity of methacrylic acid is
30 mole % preferably more than 50 mole %, of a gas kept as high as 65-85%. The first and second oxidation
which is not condensed in the subsequent recovery steps should be conducted according to the above-men
process. When air is used as the source of molecular tioned parameter limitations.
oxygen, nitrogen, which is a diluent gas and is not con
densed in the subsequent recovery process, can be sup 40 thatIn the the process of this invention, it is characteristic
plied in amounts corresponding to the composition of oxidationreaction steps
products in both the first and second
are mixed 8 as shown in FIG. 1,
air. That is, the amount of nitrogen in air is sufficient for whereby methacrylic acid can be smoothly recovered
use as the diluent gas. Accordingly, it is preferred to use in high yield without formation of an explosive gas in
air as the source of molecular oxygen. the recovery process. According to the comparative
Suitable catalysts for use in the first oxidation step 45 tests described below, the possibility of forming an
include metal oxides and other conventional catalysts explosive gas in the recovery process for the reaction
which can be supported by carriers such as silica, alu product of the second step varies depending upon the
mina, silicon carbide, molecular sieve and the like. Such
suitable catalysts include molybdenum-antimony OX amount of oxygen fed into the second oxidation system.
ides, molybdenum-tellurium oxides, molybdenum-bis 50 the second found
It has been that the conversion of methacrolein in
oxidation step should satisfy the conditions
muth oxides and antimony-iron oxides or mixtures stated in Table 1 in order to prevent formation of the
thereof with copper, phosphorus, cobalt, niobium or explosive gas. In the following, the amount of oxygen is
tantalum. The reaction can be conducted by using the given as the number of moles per 1 mole of methacro
above-mentioned starting gases and catalyst in a contin lein,
uous type, batch type, fixed-bed type or fluidized-bed 55
TABLE
type reactor. The reaction temperature is preferred to
be in the range of 200 - 500 C, especially 250 - 450 COMPARATIVE TESTS
C. The pressure is preferred to be in the range of 0.5- Amount of oxygen
Conversion of methacrolein
required for preventing formation
10 atm, especially 1 - 3 atm. The apparent contact time (moles) of explosive gas (%)
for the reaction is preferred to be in the range of 0.1 - 20 60 0.75 > 40
seconds, especially 1 - 10 seconds. In the first oxidation 1 > 50
step of this invention, it is preferred to achieve a conver 1.25
15
> 65
80.90
sion of isobutylene higher than 80%, especially higher
than 90%. In this use, methacrolein can be produced in
a high selectivity of 80-90%. 65. As can be seen from the data of Table 1, the conversion
In the second oxidation step, the conditions are sub of methacrolein must be higher than 60% when using
stantially the same as those of the first oxidation step 1.2 moles of oxygen, the minimum amount preferred for
except for the following. In the case of the second oxi use with the catalyst in the second oxidation step, in
4,124,634
5 6
order to prevent formation of an explosive gas. It is ably small because of this indirect cooling, and, accord
necessary to have a safety allowance of about 5%. Ac ingly, effective recovery can be attained. The solution
cordingly, the conversion of methacrolein in the second 10 of methacrolein and methacrylic acid diluted with
oxidation step should be more than 65%. Under this water, which controls the polymerization thereof, can
condition, it is difficult to attain a high selectivity of 5 be discharged from the bottom of the heat-exchanger9.
methacrylic acid when using the catalyst in the second It has been found that the polymerizations of methac
oxidation step. When the amount of oxygen is higher rolein and methacrylic acid can be further controlled by
than 1.5 moles, the conversion of methacrolein should cooling the mixture 8 in two or more cooling steps.
be 80-90%, and when the safety allowance is consid According to these studies, methacrolein and meth
ered, the conversion of methacrolein should be 90 - O acrylic acid are relatively difficult to polymerize in a
100%, which cannot be achieved in practice. gaseous condition even at high temperatures. However,
On the other hand, when the reaction products in the they are easily polymerized, with attendant loss, when
first oxidation step are mixed with the reaction products they are kept at a high temperature in a liquefied condi
in the second oxidation step according to this invention, tion. Accordingly, when the mixture 8 of the reaction
the formation of an explosive gas in the recovery pro 15
products is suddenly cooled, part of the methacrolein
cess is easily controlled. Even though the amount of and methacrylic acid is condensed during the cooling
oxygen in the reaction system is high, it is possible to operation, thereby being kept at a high temperature is a
utilize a low conversion of methacrolein while obtain
ing a high selectivity of methacrylic acid. As can be liquefied condition. The tendency is especially high in
seen from the data in Table 2, the conversion of methac 20 the case of methacrylic acid which has a high boiling
rolein required in the second oxidation step in order to point and is easily liquefied. Accordingly, the cooling
prevent the formation of an explosive gas is greatly operation is preferably conducted in multistages, such
decreased with respect to the amount of oxygen, as as in 2-3 steps. In such a case where the cooling opera
compared with the comparative test results of Table 1. tion is conducted in at least two steps, in the first step,
25 the mixture of the reaction products is cooled to a tem
TABLE 2
perature which prevents condensation of methacrolein
and methacrylic acid, preferably 120 - 170° C. In the
THIS INVENTION second step, the mixture is cooled to 1-50' C which is
Conversion of methacrolein
Amount of oxygen required for preventing formation finally required by this invention. The second step of
(moles) of explosive gas (%) 30 the cooling operation can also be conducted in multi
1 > 32 steps, if desired. In accordance with such a multi-step
.25
1.5
>
>
44
56 cooling operation, the amount of polymerization is de
1.75 > 72 creased. The cooling methods to be employed depend
2 > 90 upon the cooling temperatures involved. For example, a
35 cooling method using water can be used in high temper
As Table 2 shows, in accordance with the process of ature systems and a cooling method using a desirable
this invention, when using 1.2 moles - 1.8 moles of coolant, e.g., aqueous solutions of inorganic salts, gly
oxygen for 1 mole of methacrolein, which is the pre cols, alcohols, and the like can be used in low tempera
ferred amount for the catalyst in the second oxidation ture systems.
step, the required conversion of methacrolein is less The gaseous components 11 including those compo
than 80% and generally 40-70%, which is preferred for nents having low boiling points and sparingly soluble in
providing high selectivity of methacrylic acid. water which are separated by the heat-exchanger 9, can
In the process of this invention, the reaction products be discharged without recovery if the content of meth
of the first and second oxidation steps are mixed before acrolein is small. However, it is customary to feed the
cooling the reaction products as shown in FIG. 1. How 45 gaseous components 11 into an absorption tower 12 to
ever, it is possible to mix the reaction products during contact them with water 13, whereby the water soluble
or after cooling depending upon the safety limitations, methacrolein is selectively absorbed and dissolved in
resulting from the amount of oxygen used and the con water. The absorbed solution 14 is distilled by a distilla
version of methacrolein. The mixture of the reaction tion tower 15 to recover methacrolein. The water dis
products 8 contains methacrolein, methacrylic acid, SO charged from the distillation tower 15 can be recycled
isobutylene, acetic acid, oxygen, nitrogen, steam, car to the absorption tower 12. The gaseous components
bon dioxide gas and carbon monoxide in relative which are not absorbed in water are discharged as an
amounts depending upon the conditions used in the first exhaust gas 16. In the conventional process, there is a
and second oxidation steps. The mixture of the reaction possibility of forming an explosive gas in the absorption
products is cooled by any suitable cooling method such 55 tower 12 by decreasing the concentration of methacro
as an indirect heat-exchanger 9 at a temperature for lein in the gaseous components when contacting them
liquefying methacrylic acid, and if desirable methacro with water.
lein, preferably at 1 - 50 C, especially 5 - 20 C. However, according to the process of this invention,
During the cooling, it is characteristic that methacrylic the formation of the explosive gas is prevented.
acid and methacrolein are cooled with steam in the 60 When methacrolein and methacrylic acid are sepa
reaction mixture to form a liquid since a large amount of rated and recovered from the liquefied components 10
steam is included. When the reaction products are indi discharged from the heat-exchanger 9, the liquefied
rectly cooled, i.e., when the reaction products are not components can be directly distilled. However, an
contacted with coolant, methacrolein and methacrylic amount of water 7 - 70 times (molar ratio) that of the
acid are condensed together with steam to form a solu 65 methacrylic acid is included in the liquefied component.
tion diluted with water. Accordingly, easily polymer Accordingly, it is necessary to distill the water. This is
ized methacrolein and methacrylic acid are not poly not advantageous for efficiency, and furthermore, the
merized. The amount of the resultant solution is remark products are azeotropically distilled with the water.
4,124,634 8
7
Accordingly, before the liquefied components are dis such as unreacted isobutylene, carbon monoxide, car
tilled, it is preferred to separate the water from the bon dioxide and the like, can be fed into the second
liquefied components as much as possible by any suit oxidation step. According to studies, in this case, the
able conventional method. Such methods include the catalytic activity in the second oxidation step can be
extraction method of extracting methacrolein and meth maintained at a high level for a long-period of time,
acrylic acid by using a desirable extracting reagent such whereby the yield of methacrylic acid can be kept high.
as aliphatic or aromatic hydrocarbons, halohydrocar The reason for this is not clear. However, the tendency
bons, ketones and the like; and the salting-out method of is very significant and advantageous when catalysts
separating methacrolein and methacrylic acid from having a high catalytic activity such as potassium, ce
water by adding a desirable salting-out agent such as 10 sium, rubidium or thallium salts of phosphomolybdate
water soluble inorganic acids or inorganic salts. In ac having a central phosphorus atom and a ligand of mo
cordance with these methods, methacrolein and meth lybdenum are used in the second oxidation step. In
acrylic acid can be separated in high efficiency by dis accordance with the process of this invention, impuri
tilling a solution having a relatively low content of ties such as isobutylene, carbon monoxide, carbon diox
Water. 15 ide, and the like, are not included in the methacrolein
FIG. 1 shows one preferred embodiment using an used in the second oxidation step. Accordingly, it is
extracting reagent, wherein the solution 10 is fed to a possible to use a more compact apparatus since a need
distillation tower 17 to distill methacrolein 18 having a for an increase in the capacity due to the impurities is
low boiling point whereby methacrolein is separated not present. In accordance with this invention, meth
and recovered. A small amount of water is included in 20 acrylic acid can be produced by a two step oxidation in
the recovered methacrolein. This is preferred for use as high yield and safety using a compact apparatus without
the starting material for second oxidation step because formation of a detonating gas in the recovery process.
the reaction for producing methacrylic acid is con A further understanding can be attained by reference
ducted in the presence of water. However, if necessary, to certain specific examples which are provided for
a purified product can be obtained by removing water 25 purposes of illustration only and are not intended to be
and the other impurities. The components having high limiting. In the examples, the percent of each compo
boiling points 19 discharged from the distillation tower nent refers to mole percent, except where otherwise
17 are fed to the extraction tower 20 wherein the com indicated.
ponents are contacted with an extracting reagent 21 EXAMPLE 1.
such as water insoluble organic solvents e.g., aliphatic 30
or aromatic hydrocarbons, halohydrocarbons, esters, A feed gas containing 4.0% of isobutylene, 8.0% of
and the like, whereby the water phase 23 is separated oxygen, 30.1% of nitrogen and 57.9% of water (oxygen
from the extraction phase 22 containing methacrylic and nitrogen are fed as air) was used in the first oxida
acid. The extraction phase 22 is fed to the distillation tion step. The gas was passed through a reactor made of
tower 24 and the extracting reagent 21 is separated by 35 stainless steel (having a diameter of 1 inch; a length of
distillation to obtain methacrylic acid having a purity of 1.5 m) which was filled with a catalyst of oxides of
higher than 99% which may contain small amounts of metal components: Mo12Nb1Te1Sb2 (atomic ratio) sup
water and organic materials in most cases. On the other ported on silicon carbide (having a diameter of 4 mm) to
hand, the water phase 23 separated by the extraction react it at 430 C with a 5 seconds contact time. The
tower 20 contains acetic acid, and is fed to the distilla reaction products contained 2.86% of methacrolein,
40
tion tower 26 to separate the extracting reagent 21 from 0.4% of isobutylene, 1.3% of oxygen, 29.9% of nitro
the other components such as acetic acid. gen, 63.2% of water and 3.3% of carbon dioxide. The
The embodiment for separating and recovering meth conversion of isobutylene was 90.0% and the selectivity
acrolein and methacrylic acid by mixing the reaction of methacrolein was 79.4%. On the other hand, a feed
products in the first and second oxidation steps and 45 gas containing 4.0% of methacrolein, 5.0% of oxygen,
cooling the mixture to liquefy the component has been 18.8% of nitrogen and 72.1% of water was used in the
illustrated in FIG. 1. However, it is possible to employ second oxidation step. The gas was passed through a
other embodiments wherein the reaction products in the reactor made of stainless steel (having a diameter of 1
first and second oxidation steps are respectively pre inch and a length of 1.5 m) which was filled with a
cooled, and then directly contacted with water to have 50 catalyst of a mixture of antimony phosphomolybdale
the water soluble components of methacrolein and and oxides of iron and zirconium supported on silicon
methacrylic acid absorbed in the water. The water carbide (having a diameter of 4 mm) to react it at 340
phase is then distilled to separate and recover the prod C with a 4 second contact time. The reaction products
ucts. In this case, the contacting of the reaction prod contained 2.0% of methacrolein, 1.6% of methacrylic
ucts with water is preferably conducted at 1 - 60 C, 55 acid, 73.4% of water, 2.7% oxygen, 18.7% of nitrogen,
under a pressure of 1 - 10 atm, at a rate of 1 - 7 kg of 0.9% of carbon dioxide and 0.5% of carbon monoxide.
water per 1 m of the gas. The resulting aqueous solu The conversion of methacrolein was 50.0% and the
tion of methacrolein and methacrylic acid is preferably selectivity of methacrylic acid was 80.0%. The reaction
treated to remove as much of the water as possible by products in the first and second oxidation steps were
the extraction method or the salt-out method. Thereaf 60 mixed to form a gaseous mixture of reaction products
ter, methacrolein and methacrylic acid are recovered . containing 0.2% of isobutylene, 2.4% of methacrolein,
by distillation. 0.9% of methacrylic acid, 69.1% of water, 2.1% of
The resulting methacrolein and methacrylic acid can oxygen, 23.5% of nitrogen, 1.2% of carbon dioxide gas,
be used as products with or without post treatments. A and 0.5% of carbon monoxide. The gaseous mixture of
portion or all of the methacrolein is fed as a starting 65 reaction products was cooled in a shell-tubular, heat
material for producing methacrylic acid back into the exchanger with water and brine in three cooling steps:
second oxidation step. In the process of this invention, 150° C in the first step; 40' C in the second step and 5°
pure methacrolein which does not contain impurities C in the third step. As a result, gaseous components
9
4,124,634 10
containing 0.5% of isobutylene, 6.4% of methacrolein, COMPARATIVE TEST
0.06% of methacrylic acid, 4.1% of water, 6.7% of
oxygen, 76.5% of nitrogen, 3.9% of carbon dioxide gas, 1. In the process of Example 1, the amount of oxygen
and 1.5% of carbon monoxide and liquefied compo in the second oxidation step and the convention of
nents containing 0.6% of methacrolein, 1.3% of meth methacrolein was varied. The respective reaction prod
acrylic acid and 97.9% of water, were obtained. The ucts in the second oxidation step were mixed with the
gaseous components did not form a detonating gas be reaction products in the first oxidation step and the
cause the methacrolein components were highly diluted resulting mixture was cooled by the same apparatus
with the nitrogen diluent. The gaseous components under the same conditions as above to separate it into
were counter-currently contacted with water in an 10 gaseous components and liquefied components. A part
absorption tower(Rasching rings filled; 3 atm of inner of the gaseous components discharged from the cooling
pressure; 5° C) at a ratio of 78 moles of water to 1 mole apparatus was sampled and was fed to an explosion
of methacrolein. From the top of the absorption tower, tester, wherein the gaseous components were tested to
a gas containing 0.6% of isobutylene, 7.5% of oxygen confirm the formation of an explosive gas by fusing a
15 platinum resistance wire to ignite the explosion. The
and 85.7% of nitrogen was obtained and from the bot
tom of the absorption tower, a solution containing 4.0% critical points of conversion of methacrolein (%) for
of methacrolein and 95.8% of water was obtained. The preventing formation of an explosive gas relative to the
solution was distilled to obtain methacrolein containing amount of oxygen (moles) used in the second oxidation
about 29.4% of water. On the other hand, a 22% aque 20 step were measured. The results are shown in above
mentioned Table 2.
ous solution of lithium chloride was added to the lique 2. Also, in the process of Example 1, the amount of
fied components in a mixing vessel and the mixture was
stirred and fed to a separating funnel for separation. The oxygen in the second oxidation step and the conversion
upper phase liquid contained 6.0% of methacrolein, of methacrolein were varied, and only the reaction
60.8% of methacrylic acid and 32.9% of water. The 25 products in the second oxidation step were cooled by
lower phase liquid contained 0.2% of methacrolein, the same apparatus under the same conditions without
0.2% of methacrylic acid, 90.6% of water and 8.8% of oxidationthesteps.
mixing reaction products in the first and second
The gaseous components were tested
lithium chloride. The upper phase liquid was fed to a by the same method to confirm the formation of an
distillation tower, to obtain methacrolein containing explosive gas. The critical points of conversion of meth
29.4% of water from the top and crude methacrylic 30 acrolein (%) for preventing
acid containing 33% of water from the bottom. The gas relative to the amounts formation of oxygen
of an explosive
(moles) in the
lower phase liquid was distilled in a distillation tower to second oxidation step were measured. The results are
obtain a methacrolein fraction (59.7% of methacrolein shown in above-mentioned Table 1.
and 25.8% of water) and a methacrylic acid fraction
(5.0% of methacrylic acid and 95.0% of water) from the 35 in 3.theInfirst
the process of (1), the conversion of isobutylene
top and a residual solution containing 9.2% of lithium relationshipoxidation step was decreased to 80% and the
between the critical points of conversion of
chloride and 90.7% of water from the bottom. The
residual solution was fed to a concentrating vessel to methacrolein (%) for preventing formation of an explo
obtain a concentrated slurry containing 22% of lithium sive gas and the amounts of oxygen (moles) in the sec
chloride and 77.7% of water. The slurry was recycled ond oxidation step were measured by the same method.
and fed to the mixing vessel. The methacrolein fractions The results are shown in Table 3.
obtained from each of the distillation towers were TABLE 3
mixed and were recycled as starting materials in the Conversion of methacrolein
required for preventing for
second oxidation step. The resulting crude methacrylic Amount of oxygen
(moles) mation of an explosive gas (%)
acid was purified by a distillation to obtain methacrylic 45 1. > 42
acid having a purity of 97 - 99% in substantially anhy 1.25 > 54
drous condition. 1.5 > 66
1.75 > 82
EXAMPLE 2
The gaseous mixture of reaction products obtained in 50 Having now fully described the invention, it will be
the first and second oxidation steps of Example 1 was apparent to one of ordinary skill in the art that many
cooled to 150° C by the heat-exchanger of Example 1, changes and modifications can be made thereto without
and was fed to an absorption tower filled with Raschig departing from the spirit or scope of the invention as set
rings to be contacted with water at a ratio of 351 moles forth herein.
of water to 1 mole of methacrolein. The absorbed solu 55 What is claimed a new and intended to be covered by
tion contained 0.28% of methacrolein, 0.10% of meth Letters Patent is:
acrylic acid and 99.62% of water. The solution was 1. A process for producing methacrylic acid by an
remarkably higher than that of Example 1. oxidation procedure, which comprises:
EXAMPLE 3
oxidizing isobutylene in a feed gas at a temperature of
60 200-500' C and a pressure of 0.5 to 10 atm over a
The process of Example 1 was repeated except that metal oxide oxidation catalyst suitable for oxidizing
the gaseous mixture of reaction products obtained in the isobutylene to methacrolein at a contact time of
first and second oxidation steps of Example 1 was indi 0.1-20 seconds in the presence of oxygen as the
rectly cooled by the shell-tubular type heat-exchanger oxidizing agent and nitrogen as a gaseous diluent,
to 20 C to separate the mixture into gaseous compo 65 wherein the amount of diluent gas in the feed gas
nents and liquefied components. As a result, the fraction ranges from 50-97 mole percent and wherein the
of methacrylic acid was decreased by about 20% as molar ratio of molecular oxygen to isobutylene
compared with Example 1. ranges from 1/3-4/1, such that a methacrolein
1.
4,124,634 12
containing reaction effluent is obtained in which phase and a liquid phase, the gas phase is contacted with
the conversion of said isobutylene is greater than water to form a solution, and methacrolein and meth
80%; acrylic acid are separated and recovered by distilling
simultaneously oxidizing methacrolein in a feed gas the solution and the liquid phase.
over an oxidation catalyst selected from the group 5 3. The process for producing methacrylic acid of
consisting of metal oxides, phosphomolybdic acid, claim 1 wherein said mixture is cooled to a temperature
and ammonium, bismuth, antimony, potassium, within the range of 1 - 50° C.
cesium and thallium salts of phosphomolybdic 4. The process for producing methacrylic acid of
acid, which is suitable for oxidizing methacrolein to claim 2 wherein said liquid phase is extracted with an
methacrylic acid in the presence of oxygen as the O agent for removing water therefrom prior to said distill
oxidizing agent and nitrogen and stream as a gase 1ng.
ous diluent under the reaction conditions specified 5. The process for producing methacrylic acid of
above for the oxidation of isobutylene, wherein the claim 2, wherein the mixture of the reaction products
amounts of said diluent gas and said steam in said obtained in the isobutylene oxidation and methacrolein
feed gas range from 50-97 mole percent and 5–90 15 oxidation steps is cooled in several steps.
mole percent respectively and wherein the amount 6. The process for producing methacrylic acid of
of oxygen relative to 1 mole of methacrolein ranges claim 1, wherein the reaction products obtained in the
from 1.2 to 2.0 moles, so that a methacrylic acid isobutylene oxidation and methacrolein oxidation steps
containing reaction effluent is obtained in which
the conversion of methacrolein is 40% to less than 20 are mixed, the mixture is contacted with water and the
80%; resulting solution is distilled to separate methacrolein
combining both of said reaction effluents; and methacrylic acid.
effecting separation and isolation of methacrolein and 7. The process for producing methacrylic acid of
methacrylic acid from the combined effluents, claim 1, wherein the mole ratio of oxygen to isobutylene
wherein the combined effluents are of such a resul 25 in the gas feed in the isobutylene oxidation step is in the
tant composition that the danger of the formation range of 1/1-3/1, and the molar ratio of oxygen to
of an explosive gaseous mixture derived from the methacrolein in the feed gas in the methacrolein oxida
oxidation processes in the isolation and separation tion step is in the range of 1.2/1-2.0/1.
steps is eliminated; and 8. The process for producing methacrylic acid of
returning the isolated methacrolein to the methacro- 30 claim 1, wherein the catalyst used in the isobutylene
lein oxidation step. oxidation step is a metal oxide and the catalyst used in
2. The process for producing methacrylic acid of the methacrolein oxidation step is an ammonium, bis
claim 1, wherein the mixture of the reaction products muth, antimony, potassium, cesium, rubidium or thal
obtained in the isobutylene oxidation and methacrolein lium salt of phosphomolybdic acid.
oxidation steps is indirectly cooled to separate out a gas 35 is
45
SO
55
60
65
Das könnte Ihnen auch gefallen
- Fuels, Chemicals and Materials from the Oceans and Aquatic SourcesVon EverandFuels, Chemicals and Materials from the Oceans and Aquatic SourcesFrancesca M. KertonNoch keine Bewertungen
- Nanoporous Catalysts for Biomass ConversionVon EverandNanoporous Catalysts for Biomass ConversionFeng-Shou XiaoNoch keine Bewertungen
- US4365087Dokument8 SeitenUS4365087ali.a.yahyaNoch keine Bewertungen
- '" Is "S" 165,443 3/1965 U.S.S.R. 260/531: United States Patent 15 3,678,107Dokument5 Seiten'" Is "S" 165,443 3/1965 U.S.S.R. 260/531: United States Patent 15 3,678,107budispartanNoch keine Bewertungen
- United States Patent (19) : 54 Process For Producing AcrylicacidDokument10 SeitenUnited States Patent (19) : 54 Process For Producing AcrylicacidKatia Gutierrez GalaNoch keine Bewertungen
- FGFHGHJHJKDokument9 SeitenFGFHGHJHJKMary Grace VelitarioNoch keine Bewertungen
- US5723679 Paten MEK 1Dokument3 SeitenUS5723679 Paten MEK 1Lathifa Rahma AstutiNoch keine Bewertungen
- United States Patent (10) Patent No.: Us 6,670,504 B1: Borchert Et Al. (45) Date of Patent: Dec. 30, 2003Dokument5 SeitenUnited States Patent (10) Patent No.: Us 6,670,504 B1: Borchert Et Al. (45) Date of Patent: Dec. 30, 2003GI2015Noch keine Bewertungen
- Us 5455369Dokument3 SeitenUs 5455369korope8705Noch keine Bewertungen
- United States Patent (19) : HHHHHHHHHDokument8 SeitenUnited States Patent (19) : HHHHHHHHHtasyiaNoch keine Bewertungen
- United States Patent (19) : Ohyama Et Al. 45) Date of Patent: Jan. 24, 1995Dokument7 SeitenUnited States Patent (19) : Ohyama Et Al. 45) Date of Patent: Jan. 24, 1995DILIP KulkarniNoch keine Bewertungen
- Syngas Composition FIX Haldor Topsoe US6224789B1Dokument4 SeitenSyngas Composition FIX Haldor Topsoe US6224789B1Ilham FajriNoch keine Bewertungen
- United States Patent (19) 11 Patent Number: 6,093,845: Van Acker Et Al. (45) Date of Patent: Jul. 25, 2000Dokument8 SeitenUnited States Patent (19) 11 Patent Number: 6,093,845: Van Acker Et Al. (45) Date of Patent: Jul. 25, 2000falya aronaNoch keine Bewertungen
- United States Patent (10) Patent No.: US 8,158,822 B2Dokument4 SeitenUnited States Patent (10) Patent No.: US 8,158,822 B2JFPacNoch keine Bewertungen
- Us4342699 PDFDokument18 SeitenUs4342699 PDFKhabib FirmansyahNoch keine Bewertungen
- Us 4378342Dokument9 SeitenUs 4378342هیمن مNoch keine Bewertungen
- TCCA - US5039800 - P - Process For Producing Trichloroisocyanuric AcidDokument7 SeitenTCCA - US5039800 - P - Process For Producing Trichloroisocyanuric AcidListya Eka AnggrainiNoch keine Bewertungen
- US8269048Dokument6 SeitenUS8269048Lara MartinezNoch keine Bewertungen
- United States Patent (19) : Kiyuma Et Al. Apr. 3, 1990Dokument7 SeitenUnited States Patent (19) : Kiyuma Et Al. Apr. 3, 1990mario 311Noch keine Bewertungen
- Perpindahan PanasDokument9 SeitenPerpindahan Panasadit dwipawarmanNoch keine Bewertungen
- Process For Purification of HCL From Edc PyrolysisDokument6 SeitenProcess For Purification of HCL From Edc Pyrolysisu2051721Noch keine Bewertungen
- Copper Chromite SizeDokument5 SeitenCopper Chromite SizeJose HernándezNoch keine Bewertungen
- Patent US5218146Dokument11 SeitenPatent US5218146Santiago BorgesNoch keine Bewertungen
- United States Patent (19) : 75 Inventors: Pekka Knuuttila, Porvoo AilaDokument4 SeitenUnited States Patent (19) : 75 Inventors: Pekka Knuuttila, Porvoo AilaMOHAMMED SHAKEIBNoch keine Bewertungen
- US4668495Dokument5 SeitenUS4668495Chemist Ahmed FoudaNoch keine Bewertungen
- Us 5231222Dokument11 SeitenUs 5231222Ratu TiaraNoch keine Bewertungen
- A01 269Dokument11 SeitenA01 269icingrockNoch keine Bewertungen
- Patent US4317926Dokument9 SeitenPatent US4317926Santiago BorgesNoch keine Bewertungen
- United States Patent (19) : 73) Assignee: Huron Tech Corp., Delco, N.CDokument14 SeitenUnited States Patent (19) : 73) Assignee: Huron Tech Corp., Delco, N.CShahid AliNoch keine Bewertungen
- Us6210562 PDFDokument8 SeitenUs6210562 PDFSyuhadah NoordinNoch keine Bewertungen
- Us4371456 PDFDokument3 SeitenUs4371456 PDFGraciaVelitarioNoch keine Bewertungen
- United States Patent (19) : 75) Inventors: Vlastimil Kadlec, Litvinov VojtéchDokument3 SeitenUnited States Patent (19) : 75) Inventors: Vlastimil Kadlec, Litvinov VojtéchGraciaVelitarioNoch keine Bewertungen
- US6545178Dokument6 SeitenUS6545178Santiago BorgesNoch keine Bewertungen
- US5679826Dokument5 SeitenUS5679826J Venkat RamanNoch keine Bewertungen
- Us 7393981Dokument6 SeitenUs 7393981jose_martinez_392Noch keine Bewertungen
- Us 3549696Dokument4 SeitenUs 3549696budispartanNoch keine Bewertungen
- US4472587Dokument15 SeitenUS4472587Gunung PatiselatanNoch keine Bewertungen
- Us 6143920Dokument4 SeitenUs 6143920J Venkat RamanNoch keine Bewertungen
- US6767528Dokument6 SeitenUS6767528Helwinda ApriliaNoch keine Bewertungen
- 2008 March 4 Acetaldehyde From Ethylene OxidationDokument4 Seiten2008 March 4 Acetaldehyde From Ethylene OxidationameymurudkarNoch keine Bewertungen
- United States Patent (19) : Homme, Jr. 11 4,088,742Dokument11 SeitenUnited States Patent (19) : Homme, Jr. 11 4,088,742Hedi Ben MohamedNoch keine Bewertungen
- Us 7393981Dokument6 SeitenUs 7393981Wojciech RedutkoNoch keine Bewertungen
- US4684750Dokument4 SeitenUS4684750Deep PatelNoch keine Bewertungen
- BusaDokument10 SeitenBusadewiNoch keine Bewertungen
- (Patent) US4915930Dokument9 Seiten(Patent) US4915930Pavita SalsabilaNoch keine Bewertungen
- United States Patent (19) (11) Patent Number: 5,770,677: Sridhar Et Al. (45) Date of Patent: Jun. 23, 1998Dokument6 SeitenUnited States Patent (19) (11) Patent Number: 5,770,677: Sridhar Et Al. (45) Date of Patent: Jun. 23, 1998bvritNoch keine Bewertungen
- Diluție! PatentDokument7 SeitenDiluție! PatentMarina ButuceaNoch keine Bewertungen
- Argon Recovery From Silicon Crystal FurnaceDokument7 SeitenArgon Recovery From Silicon Crystal FurnaceasdfqwerNoch keine Bewertungen
- Us 6489527Dokument6 SeitenUs 6489527Gracelia IrmalindaNoch keine Bewertungen
- US3903185Dokument6 SeitenUS3903185Muhammad Akbar FahleviNoch keine Bewertungen
- Paten Pembuatan Magnesium Klorida Dari Magnesium Hidroksida Dan Asam KloridaDokument6 SeitenPaten Pembuatan Magnesium Klorida Dari Magnesium Hidroksida Dan Asam KloridaRaudhah AqilahNoch keine Bewertungen
- United States Patent Office: Ch-Ch-OnDokument3 SeitenUnited States Patent Office: Ch-Ch-OnChanachai PuckNoch keine Bewertungen
- United States Patent (19) : (45) Date of Patent: Mar 23, 1999Dokument9 SeitenUnited States Patent (19) : (45) Date of Patent: Mar 23, 1999Yosef Camposano RodriguezNoch keine Bewertungen
- Acetaldehido A01 031Dokument4 SeitenAcetaldehido A01 031DwiPurwatiNoch keine Bewertungen
- Us 6034269Dokument5 SeitenUs 6034269Rahmat SunaryaNoch keine Bewertungen
- United States Patent (19) : 11 Patent Number: 45 Date of PatentDokument25 SeitenUnited States Patent (19) : 11 Patent Number: 45 Date of Patentfitri rowiyahNoch keine Bewertungen
- US4495107ADokument2 SeitenUS4495107AWojciech RedutkoNoch keine Bewertungen
- United States Patent: (54) Encapsulating and PottingDokument8 SeitenUnited States Patent: (54) Encapsulating and PottingVansala GanesanNoch keine Bewertungen
- Us 4945186Dokument3 SeitenUs 4945186J Venkat RamanNoch keine Bewertungen
- Extracting RicinDokument3 SeitenExtracting Ricinまど ばにNoch keine Bewertungen
- Arsenic Pollution in West Bengal PDFDokument5 SeitenArsenic Pollution in West Bengal PDFKim Hiền100% (1)
- PT3 Speaking TestDokument7 SeitenPT3 Speaking TestJc ApeNoch keine Bewertungen
- 0 PDF Testing and Commissioning Document For Water ScribdDokument90 Seiten0 PDF Testing and Commissioning Document For Water ScribdARNOLD ALINGNoch keine Bewertungen
- Jingen PK MS-100 MSDS UpdatedDokument7 SeitenJingen PK MS-100 MSDS UpdatedMainulHoqueNoch keine Bewertungen
- Emulgante CO-55 - MSDSDokument4 SeitenEmulgante CO-55 - MSDSMohamed HalemNoch keine Bewertungen
- What Is The Water CycleDokument3 SeitenWhat Is The Water CycleArnold Arada PaombongNoch keine Bewertungen
- The Book of DishesDokument56 SeitenThe Book of Dishesabuzai100% (2)
- NGVF 2016 D1.T2.4.1 Gordon Farquharson WFI - New PH Eur Production Specification PDFDokument39 SeitenNGVF 2016 D1.T2.4.1 Gordon Farquharson WFI - New PH Eur Production Specification PDFParth PatelNoch keine Bewertungen
- Verka Complete Traning FilrDokument46 SeitenVerka Complete Traning FilrSaab Rana100% (1)
- Revised National Plumbing Code of The Philippines - 1 PDFDokument112 SeitenRevised National Plumbing Code of The Philippines - 1 PDFKyra Aleson100% (1)
- Management of Family Resources: Code: TLE6HE-0a-1Dokument29 SeitenManagement of Family Resources: Code: TLE6HE-0a-1JUVELYN CANOYNoch keine Bewertungen
- Scrap Tire Recycling, Elt Proposal Project Desing, Wo PricesDokument19 SeitenScrap Tire Recycling, Elt Proposal Project Desing, Wo PricesCemalKaldırımcıNoch keine Bewertungen
- WABAG Image ENG 2016 WebDokument20 SeitenWABAG Image ENG 2016 WebNguyen AnNoch keine Bewertungen
- MIP Method For Hydrology CalculationDokument5 SeitenMIP Method For Hydrology Calculationtrilokbist04Noch keine Bewertungen
- Grade 6 Agriculture T2 2020Dokument6 SeitenGrade 6 Agriculture T2 2020MuramCoNoch keine Bewertungen
- Eco Cycle DesignDokument85 SeitenEco Cycle DesignKiran Sunku100% (1)
- Soft Eng Techniques For Coastal ProtectionDokument49 SeitenSoft Eng Techniques For Coastal ProtectionHaryo ArmonoNoch keine Bewertungen
- WWTPD Lab Assign 3Dokument3 SeitenWWTPD Lab Assign 3Zohaib BaigNoch keine Bewertungen
- Balsamo - Bisnar - Keita - Ramirez - San Jose Marketing Plan Tropicana Coco QuenchDokument46 SeitenBalsamo - Bisnar - Keita - Ramirez - San Jose Marketing Plan Tropicana Coco Quenchapi-292484841Noch keine Bewertungen
- Water Cycle Atomospheric ConditionsDokument26 SeitenWater Cycle Atomospheric ConditionsEUNICE CORREANoch keine Bewertungen
- Drainage-Systems-Installation-Guide - OffsetDokument72 SeitenDrainage-Systems-Installation-Guide - OffsetPaqui MorentNoch keine Bewertungen
- (WWW - Banksoal.web - Id) Soal Ujian Semester I SMA Kelas XI IPA-IPS - Bahasa InggrisDokument4 Seiten(WWW - Banksoal.web - Id) Soal Ujian Semester I SMA Kelas XI IPA-IPS - Bahasa InggrisYuanita Maya DamayantiNoch keine Bewertungen
- Waste Not Want Not (Tests & Exams)Dokument15 SeitenWaste Not Want Not (Tests & Exams)sara wilson100% (3)
- Loctite Frekote 770-NCDokument6 SeitenLoctite Frekote 770-NCreussavelNoch keine Bewertungen
- 8.chain of LifeDokument15 Seiten8.chain of LifeBiraj PoudelNoch keine Bewertungen
- MSDS Aldehid General: Toxicity DataDokument27 SeitenMSDS Aldehid General: Toxicity DataHarliana Rahim100% (1)
- Comparative Study of Codal Provisions For Durability of Concrete StructuresDokument98 SeitenComparative Study of Codal Provisions For Durability of Concrete StructuresPriyam AndhariaNoch keine Bewertungen
- Detailed Lesson Plan. Phase ChangeDokument6 SeitenDetailed Lesson Plan. Phase ChangeChupapi Munyanyo80% (5)
- Unit 8 (HIGHWAY DRAINAGE)Dokument27 SeitenUnit 8 (HIGHWAY DRAINAGE)Zara Nabilah91% (22)
- English 4 q3 PT ValidatedDokument5 SeitenEnglish 4 q3 PT ValidatededelynNoch keine Bewertungen