Beruflich Dokumente
Kultur Dokumente
Optic Atrophy
Hochgeladen von
Annisa Badriyyah HakimahOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Optic Atrophy
Hochgeladen von
Annisa Badriyyah HakimahCopyright:
Verfügbare Formate
Optic atrophy
Author: Doctor Christophe Orssaud1
Creation Date: August 2002
Update: November 2003
Scientific Editor: Professor Jean-Louis Dufier
1
Service d'ophtalmologie, Hôpital Européen Georges Pompidou, 20 rue Leblanc, 75908 PARIS Cedex 15,
France. Christophe.Orssaud@hop.egp.ap-hop-paris.fr
Abstract
Keywords
Included diseases
Excluded diseases
Definition/Diagnosis criteria
Differential diagnosis
Frequency
Clinical description
Diagnosis
Etiology
Treatment
References
Abstract
Optic atrophies (OA) refer to a specific group of hereditary optic neuropathies in which the cause of the
optic nerve dysfunction is inherited either in an autosomal dominant or autosomal recessive pattern.
Autosomal dominant optic atrophy (ADOA), type Kjer, is the most common OA, whereas autosomal
recessive optic atrophy (AROA) is a rare form. Prevalence of ADOA ranges from 1:50,000 to 1:10,000.
The frequency of AROA is unknown but the disease seems rare. MRI of patients with ADOA reflects the
important loss of tissue of the optic nerve due to a dramatic reduction of the retinal ganglion cells. The
age at onset of ADOA is usually between 4 and 6 years, although visual symptoms are usually
imperceptible until late in life due to the slowly progressive decrease in visual acuity. There is no
neurological deficit. However, a mild hearing loss may be encountered. In the pure congenital AROA,
optic atrophy is never associated with neurological disorder and visual impairment is severe. Since the
visual symptoms are important, AROA can be discovered very early, usually before the age of 4 years.
ADOA has been associated with More than 60 mutations in the OPA1 gene. OPA1 maps to 3q28 and
encodes an homologue of yeast dynamin-related GTPase, which is ubiquitously expressed in retinal
ganglion cells and optic nerve. To date, no causative gene has been identified in the recessive form of
OA.
Keywords
Optic atrophy, hereditary optic neuropathy, ADOA, AROA, OPA1 gene
Included diseases -Wolfram syndrome
-autosomal dominant optic atrophy, type Kjer; -Normal tension glaucoma
-autosomal recessive optic atrophy. -Acquired optic atrophies
Excluded diseases Definition/Diagnosis criteria
-Leber's hereditary optic neuropathy Optic atrophies (OA) refer to a specific group of
-Leigh syndrome hereditary optic neuropathies in which the cause
Orssaud C. Optic Atrophy. Orphanet Encyclopedia. November 2003.
http://www.orpha.net/data/patho/GB/uk.OA.pdf 1
of the optic nerve dysfunction is inherited either are usually mild until late in life due to the slowly
in an autosomal dominant or autosomal progressive decrease in visual acuity. In most
recessive pattern. Autosomal dominant optic cases, visual acuity is equally reduced in both
atrophy (ADOA), type Kjer, is the most common eyes. Intra or interfamilial phenotypic variations
OA, whereas autosomal recessive optic atrophy are important. Although the entire optic disc may
(AROA) is a rare form. be affected, it sometimes only displays temporal
pallor with an absence of vessels or capillaries.
Differential diagnosis Visual field exhibits central or paracentral
Leber's hereditary optic neuropathy, a maternally scotomas. A blue-yellow dyschromatopsia is
inherited optic atrophy due to mutations of the characteristically observed in color vision testing
mitochondrial DNA, and many other inherited in ADOA patients. MRI of patients with ADOA
optic atrophies such as Leigh syndrome, reveals a significant thinning of the optic nerve
Wolfram syndrome, Costeff optic atrophy along its length, confirming the important loss of
syndrome, can be differentiated from OA by the tissue of the optic nerve due to a dramatic
clinical findings and family history. reduction of the ganglion cells. There is no
Normal tension glaucoma (NTG) is particularly neurological deficit. Hearing loss may be
important to differentiate from OA since it leads encountered during the course of ADOA. But this
also to an optic atrophy and may have a genetic deficit is usually mild.
basis. The characteristic enlargement of the
optic is not specific of NTG and it can be Recessive form
observed in the course of OA. Thus, it is not In the pure congenital AROA, visual impairment
possible to rule out the diagnosis of AO with appears very early and is present at birth or
slow visual impairment when such optic disc appears in the first year of life. In addition, visual
enlargement is observed. High value must be impairment is severe, leading to visually disabled
attributed to the visual field defects or to vascular or to blind children. Since the visual symptoms
manifestations. In addition, it has been stated are important, AROA is discovered very early,
that polymorphisms of the OPA1 gene could be usually before the age of 4 years. Sensory
associated with NTG. nystagmus is the rule. At fundus examination,
normal retina contrasts with the diffuse pallor of
Frequency the optic disc. A cupping may develop with age.
The frequency of each OA varies depending on However, in very young children, it might be
their pattern of inheritance. Prevalence of ADOA difficult to differentiate AROA from Leber’s
ranges from 1:50,000 to 1:10,000. The congenital amaurosis or other retinal
frequency of AROA is unknown but the disease degenerations, since narrowing of retinal vessels
seems rare. and abnormalities of retinal pigmentation, which
are characteristic of these congenital retinal
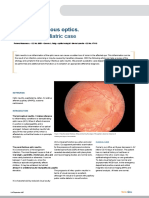
Clinical description disorders, are delayed in juvenile forms.
OA are characterized by a primary degeneration Electrophysiological testing enables the
of retinal ganglion cells, the axons of which join diagnosis to be confirmed. Electroretinogram
to form the optic nerve. This degeneration leads (ERG) is normal in pure congenital AROA,
to ascending atrophy of the optic nerve and is whereas no responses are obtained in
responsible for the clinical manifestations of the congenital retinal degenerations. Visual evoked
OA, which consist mainly in a decreased visual potentials are absent in AROA, confirming the
acuity, abnormalities of the central visual field pathology of the optic tract. Visual field testing,
and a typical color vision defect. when possible, shows a central scotoma. This
form of optic atrophy is never associated with
Dominant form neurological disorder.
ADOA is characterized by a selective
degeneration of the retinal ganglion cells and Diagnosis
fibers of the papillo-macular bundle. This Diagnosis of OA forms is established on the
involvement of the smallest fibers of the optic basis of clinical findings and family history.
nerve is not specific to ADAO since it may be
also encountered in all genetic and acquired Etiology
optic neuropathies resulting from mitochondrial Linkage analysis revealed genetic heterogeneity
dysfunction. The age of onset is usually between in ADOA. Two loci have been identified, one
4 and 6 years, but ADAO rarely causes severe located on chromosome 3 (3q28-qter) and the
impairment of vision in childhood. Acuity ranges other one on chromosome 18 (18 q12.2-q12.3).
from 20/20 to 20/200 in 85% of young adult OPA1, the gene mapping to 3q28, is responsible
patients and is lower than 20/200 in the for most ADOA cases. This OPA1 gene
remaining 15%. But, visual impairment can comprises 28 coding exons and 8 mRNA
major quickly in adults. Thus visual symptoms isoforms have been reported, due to the
Orssaud C. Optic Atrophy. Orphanet Encyclopedia. November 2003.
http://www.orpha.net/data/patho/GB/uk.OA.pdf 2
alternative splicing of 3 different exons. More neuropathies. Neurochemistery. 2002. 40:573-
than 60 OPA1 mutations have been described. 84.
OPA1 encodes a protein which shares a high Eiberg H, Kjer B, Kjer P, Rosenberg T.
homology with a yeast mitochondrial dynamin- Dominant optic atrophy (OPA1) mapped to
related GTPase, Msp1. This Msp1 protein is chromosome 3q region. I. Linkage analysis. Hum
essential for the maintenance of mitochondrial Mol Genet 1994. 3:977-80.
DNA. The OPA1 protein is ubiquitously Hoyt CS. Autosomal dominant atrophy: a
expressed, especially in retinal ganglion cells spectrum of disability. Ophthalmology 1980.
and optic nerve. It is imported into mitochondria 87:245-251.
and is responsible for the maintenance of Kerrisson JB, Arnould V, Barmada MM.
mitochondrial inner membranes and cristae. It Genetic heterogeneity of dominant optic atrophy,
has been suggested that OPA1 mutations lead Kjer type: identification of a second locus on
to a loss of protein function and haplotype chromosome 18q. Arch Ophthalmol 1999. 117:
insufficiency, or are responsible for a dominant 805-10.
negative effect. This leads to the hypothesis that Kjer P, Jensen OA, Klinken L. Histopathology of
OPA1 mutations could induce the changes in eye optic nerve and brain in case of dominant
mitochondrial structure or distribution that are optic atrophy. Acta ophthalmol 1983. 61:300-12.
thought to be implicated in loss of retinal Votruba M, Moore AT, Bhattacharrya SS.
ganglion cells. Clinical features, molecular genetics, and
To date, no causative gene has been identified pathophysiology of dominant atrophy. J Med
in the recessive form of OA. Genet 1998. 35:793-800.
Votruba M, Leary S, Losseff N, Bhattacharrya
Treatment SS, Moore AT, Miller DH, Moseley IF. MRI of the
Treatment is not currently available. intra-orbital optic nerve in patients with
autosomal dominant optic atrophy.
References Neuroradiology 2000. 42:180-3.
Alexander C, Votruba M, Pesch UEA, Thiselton
DL, Mayer S, Moore A, Rodriguez M, Kellner U,
Leo-Kottler B, Auburger G, Bhattacharya SS,
Wissinger B. OPA1, encoding a dynamin-related
GTPase, is mutated in autosomal dominant optic
atrophy linked to chromosome 3q28. Nat Genet
2000. 26:211-15.
Biousse V, Newman NJ. Hereditary optic
neuropathies. Ophthalmol Clin North Am 2001.
14:547-68.
Brown J, Fingert JH, Taylor CM. Clinical and
genetic analysis of a family affected with
dominant optic atrophy (OPA1). Arch
Ophthalmol 1997. 115:95-9.
Delettre C, Lenaers G, Griffoin J-M, Gigarel N,
Lorenzo C, Belenguer P, Pelloquin L,
Grosgeorge J, Turc-Carel C, Perret E, Astarie-
Dequeker C, Lasquellec L, Arnaud B,
Ducommun B, Kaplan J, Hamel CP. Nuclear
gene OPA1, encoding a mitochondrial dynamin-
related protein, is mutated in dominant optic
atrophy. Nal Genet 2000. 26, 207-210.
Delettre C, Griffoin JM, Kaplan J, Dollfus H,
Lorenz B, Faivre L, Lenaers G, Belenguer P,
Hamel, CP. Mutation spectrum and splicing
variants in the OPA1 gene. Hum Genet
2001.109:584-91.
Delettre C, Lenaers G, Pelloquin L, Belenguer
P, Hamel, CP. OPA1 (Kjer type) dominant optic
atrophy: a novel mitochondrial disease. Mol
Genet Metab 2002. 75: 97-107.
Carelli V, Ross-Cisneros FN, Sadun AA. Optic
nerve degeneration and mitochondrial
dysfunction: genetic and acquired optic
Orssaud C. Optic Atrophy. Orphanet Encyclopedia. November 2003.
http://www.orpha.net/data/patho/GB/uk.OA.pdf 3
Das könnte Ihnen auch gefallen
- II. Upper Respiratory Tract DisordersDokument98 SeitenII. Upper Respiratory Tract DisordersarielleortuosteNoch keine Bewertungen
- The Diabetic FootDokument553 SeitenThe Diabetic Footjustme_adry100% (1)
- Case Study On GlaucomaDokument6 SeitenCase Study On GlaucomaKing GamerNoch keine Bewertungen
- 1000 MCQ Book Pain MedicineDokument215 Seiten1000 MCQ Book Pain Medicineihtisham1100% (16)
- MCQ OphthalmologyDokument206 SeitenMCQ OphthalmologyMohammed Mousa Imran100% (4)
- Optic NeuritisDokument25 SeitenOptic NeuritisChikita Artia SariNoch keine Bewertungen
- Retinitis PigmentosaDokument59 SeitenRetinitis PigmentosaAndreaRebeca50% (2)
- 100 Million Years of Food by Stephen LeDokument9 Seiten100 Million Years of Food by Stephen Lesimas0% (1)
- Review of Cellular Division: Cell Division Is The Process by Which A Parent Cell Divides Into Two or MoreDokument7 SeitenReview of Cellular Division: Cell Division Is The Process by Which A Parent Cell Divides Into Two or MoreThirumuraiNoch keine Bewertungen
- 2013 ASA Guidelines Difficult AirwayDokument20 Seiten2013 ASA Guidelines Difficult AirwayStacey WoodsNoch keine Bewertungen
- Retinitis Pigmentosa: Causes, Tests, and Treatment OptionsVon EverandRetinitis Pigmentosa: Causes, Tests, and Treatment OptionsBewertung: 4 von 5 Sternen4/5 (1)
- Ectopia LentisDokument38 SeitenEctopia LentisSaransh Sharma100% (1)
- Optic AtrophyDokument6 SeitenOptic Atrophyrutuparna383Noch keine Bewertungen
- Clinical Approach To Optic Neuropathies: DiagnosisDokument14 SeitenClinical Approach To Optic Neuropathies: Diagnosiskmathewjames100% (1)
- Distrofias RetinialesDokument7 SeitenDistrofias Retinialesmartin.ruda93Noch keine Bewertungen
- Hereditary Retinal and Choroidal Dystrophies: Tamer Moussa Ibrahim MDDokument57 SeitenHereditary Retinal and Choroidal Dystrophies: Tamer Moussa Ibrahim MDSalma EissaNoch keine Bewertungen
- Optic AtrophyDokument34 SeitenOptic AtrophySaif BokhariNoch keine Bewertungen
- Congenital Optic Nerve Anomalies: Nandini Singh S.NO: 63 Roll No:68Dokument54 SeitenCongenital Optic Nerve Anomalies: Nandini Singh S.NO: 63 Roll No:68nandini singhNoch keine Bewertungen
- Neuro-Ophthalmology: Simon J HickmanDokument10 SeitenNeuro-Ophthalmology: Simon J Hickmanhalvi89Noch keine Bewertungen
- Alzheimer and The EyeDokument9 SeitenAlzheimer and The EyeRafiqy Sa'adiy FaizunNoch keine Bewertungen
- Congenital Corneal DisordersDokument101 SeitenCongenital Corneal Disorderseyemd_in_training100% (1)
- Background: Ectopia Lentis. Dislocated Traumatic Lens (Cataract)Dokument7 SeitenBackground: Ectopia Lentis. Dislocated Traumatic Lens (Cataract)Muhammad Khairul AfifNoch keine Bewertungen
- Congenital Nystagmus: Author: Doctor Christophe Orssaud Scientific Editor: Professor Jean-Louis DufierDokument0 SeitenCongenital Nystagmus: Author: Doctor Christophe Orssaud Scientific Editor: Professor Jean-Louis DufierPratiara Syamir FasaNoch keine Bewertungen
- Fundus Fluorescein Angiography and Optical Coherence Tomographic Characteristics in Acute Multiple Evanescent White Dot SyndromeDokument5 SeitenFundus Fluorescein Angiography and Optical Coherence Tomographic Characteristics in Acute Multiple Evanescent White Dot SyndromePriya ChandrasekaranNoch keine Bewertungen
- 6 - Chronic Visual Loss (Dr. Essam)Dokument34 Seiten6 - Chronic Visual Loss (Dr. Essam)REON CEREJONoch keine Bewertungen
- Retinitis Pigmentosa and Allied Disorders: Review ArticleDokument6 SeitenRetinitis Pigmentosa and Allied Disorders: Review Articletipu42Noch keine Bewertungen
- Orphanet Journal of Rare Diseases: Oculocutaneous AlbinismDokument8 SeitenOrphanet Journal of Rare Diseases: Oculocutaneous AlbinismFrancialNoch keine Bewertungen
- Optic Atrophy :major Review March 2010, Kerala Journal of Ophthalmology, Devendra V. Venkatramani Et AlDokument6 SeitenOptic Atrophy :major Review March 2010, Kerala Journal of Ophthalmology, Devendra V. Venkatramani Et AlNavojit ChowdhuryNoch keine Bewertungen
- Core Tip: Leber Congenital Amaurosis (LCA) Is The Most Severe Retinal Dystrophy CausingDokument1 SeiteCore Tip: Leber Congenital Amaurosis (LCA) Is The Most Severe Retinal Dystrophy CausingIdhae LoveSujuNoch keine Bewertungen
- Eye Disorders in Patients With Multiple Sclerosis Natural History and ManagementDokument15 SeitenEye Disorders in Patients With Multiple Sclerosis Natural History and ManagementAndreea PleșaNoch keine Bewertungen
- Inflammatory Optic NeuropathyDokument49 SeitenInflammatory Optic NeuropathyVishal KulkarniNoch keine Bewertungen
- Pérdida Aguda VisualDokument7 SeitenPérdida Aguda VisualYanina Pérez de VillarrealNoch keine Bewertungen
- Stargardt DiseaseDokument9 SeitenStargardt DiseaseEstefhany SCNoch keine Bewertungen
- Artigo Management of Ectopia - Neely - 2001Dokument7 SeitenArtigo Management of Ectopia - Neely - 2001Giovanna SoaresNoch keine Bewertungen
- Congenital Disorders of The CorneaDokument100 SeitenCongenital Disorders of The Corneaeyemd_in_trainingNoch keine Bewertungen
- Report of A Pediatric Case: Neuritis Infectious OpticsDokument5 SeitenReport of A Pediatric Case: Neuritis Infectious OpticsErika SanchezNoch keine Bewertungen
- Congenital Disorders of The Optic Nerve: Excavations and HypoplasiaDokument11 SeitenCongenital Disorders of The Optic Nerve: Excavations and HypoplasiaNurul An NisaNoch keine Bewertungen
- Visual Field ScienceDokument8 SeitenVisual Field ScienceMuhammad Zarak NiaziNoch keine Bewertungen
- Retinitis PigmentosaDokument35 SeitenRetinitis Pigmentosawessam284Noch keine Bewertungen
- Munim 2022Dokument4 SeitenMunim 2022febyolaNoch keine Bewertungen
- 3.visual Sign and Symptomps of Multiple System AtrophyDokument9 Seiten3.visual Sign and Symptomps of Multiple System AtrophyNia AnestyaNoch keine Bewertungen
- AizzzzzzzzzzzzzzzzzzaaaaaDokument2 SeitenAizzzzzzzzzzzzzzzzzzaaaaaAldrin AvelinoNoch keine Bewertungen
- Optic Neuritis: A Review: PN Shams, GT PlantDokument8 SeitenOptic Neuritis: A Review: PN Shams, GT PlantKade SilabanNoch keine Bewertungen
- Approach To Optic Neuropathies: Clinical UpdateDokument12 SeitenApproach To Optic Neuropathies: Clinical UpdateHerdy VeristianNoch keine Bewertungen
- Secondary GlaucomaDokument19 SeitenSecondary GlaucomaRakhmad TriharsadiNoch keine Bewertungen
- Problem 3 "Emergency Medicine Block": Frudensia Kristiana 405110031 Group 10Dokument16 SeitenProblem 3 "Emergency Medicine Block": Frudensia Kristiana 405110031 Group 10Frudensia KristianaNoch keine Bewertungen
- A Review of Neuro-Ophthalmologic EmergenciesDokument5 SeitenA Review of Neuro-Ophthalmologic EmergenciesZakiyul FuadNoch keine Bewertungen
- Congenital Malformation of Eye, Ear, Face and NeckDokument11 SeitenCongenital Malformation of Eye, Ear, Face and Neckمحمد نعيمNoch keine Bewertungen
- By Julie K. Hutchinson, O.D., Andrew S. Gurwood, O.D., and Marc D. Myers, O.DDokument5 SeitenBy Julie K. Hutchinson, O.D., Andrew S. Gurwood, O.D., and Marc D. Myers, O.DChrisNoch keine Bewertungen
- Tjo 51 398Dokument5 SeitenTjo 51 398Richard Chuzón GuevaraNoch keine Bewertungen
- Albinism Classification, Clinical.23Dokument4 SeitenAlbinism Classification, Clinical.23Danny WongNoch keine Bewertungen
- Ophthalmology PosterDokument12 SeitenOphthalmology PosteraneeshafonsecaNoch keine Bewertungen
- 4 - Jonas1994Dokument6 Seiten4 - Jonas1994Eduardo HernandezNoch keine Bewertungen
- Ataxias HereditariasDokument11 SeitenAtaxias HereditariasEder ReyesNoch keine Bewertungen
- Pediatric Optic Neuritis 2016 PDFDokument7 SeitenPediatric Optic Neuritis 2016 PDFangelia beanddaNoch keine Bewertungen
- Ahmed 2010Dokument11 SeitenAhmed 2010Karamjot SinghNoch keine Bewertungen
- Ophthalmology Neuro OphthalmologyDokument7 SeitenOphthalmology Neuro OphthalmologyjbtcmdtjjvNoch keine Bewertungen
- Paediatric NeurophthalmologyDokument49 SeitenPaediatric NeurophthalmologyNavami KrishnaNoch keine Bewertungen
- Graves - Optic.neuropathy-Before OCTDokument4 SeitenGraves - Optic.neuropathy-Before OCTMihnea VulpeNoch keine Bewertungen
- 710-Article Text-2049-1-10-20221220Dokument6 Seiten710-Article Text-2049-1-10-20221220Vanessa StepaniaNoch keine Bewertungen
- Electro-Retinography (ERG) & Electro-Oculography (EOG) : BY Dr. Asif Manzoor (PGR) Eye-Ii Lgh. LHRDokument25 SeitenElectro-Retinography (ERG) & Electro-Oculography (EOG) : BY Dr. Asif Manzoor (PGR) Eye-Ii Lgh. LHRUsman ImtiazNoch keine Bewertungen
- Eye Docs PediatricsDokument133 SeitenEye Docs PediatricsMuneeb ShahzadNoch keine Bewertungen
- Management of DiplopiaDokument5 SeitenManagement of Diplopiacahyati syhrilNoch keine Bewertungen
- Lca EosrdDokument15 SeitenLca EosrdYosua NadyaNoch keine Bewertungen
- 2 - Quigley1982Dokument12 Seiten2 - Quigley1982Eduardo HernandezNoch keine Bewertungen
- Transfusion Guidelines For Neonates and Older Children 2004 PDFDokument21 SeitenTransfusion Guidelines For Neonates and Older Children 2004 PDFAnnisa Badriyyah HakimahNoch keine Bewertungen
- Northrop 2015Dokument4 SeitenNorthrop 2015Annisa Badriyyah HakimahNoch keine Bewertungen
- WJG 15 4627 PDFDokument11 SeitenWJG 15 4627 PDFRynaldi RahmanNoch keine Bewertungen
- Download-fullpapers-JOI Vol 7 No 4 Des 2010 (Putu Yuliawati)Dokument4 SeitenDownload-fullpapers-JOI Vol 7 No 4 Des 2010 (Putu Yuliawati)Annisa Badriyyah HakimahNoch keine Bewertungen
- Wms Main Pocket Guide 2017Dokument29 SeitenWms Main Pocket Guide 2017justrudinNoch keine Bewertungen
- #MediastinumDokument4 Seiten#Mediastinumameerabest100% (1)
- Practical Research 2: Learning CompetenciesDokument32 SeitenPractical Research 2: Learning CompetenciesPrances PelobelloNoch keine Bewertungen
- Monday, October 06, 2014 EditionDokument12 SeitenMonday, October 06, 2014 EditionFrontPageAfricaNoch keine Bewertungen
- Hematologic EffectsDokument8 SeitenHematologic EffectsGiralph NikkoNoch keine Bewertungen
- Critical Illness BrochureDokument2 SeitenCritical Illness BrochureBrijesh RaiNoch keine Bewertungen
- 2nd PU Practical Viva QuestionsDokument10 Seiten2nd PU Practical Viva QuestionsSadhvi CNoch keine Bewertungen
- Adenomiosis CX ConservadoraDokument12 SeitenAdenomiosis CX Conservadorajuan carlos pradaNoch keine Bewertungen
- Test CaeDokument3 SeitenTest CaegabiNoch keine Bewertungen
- 02chapters1-4 Consumo HumanoDokument61 Seiten02chapters1-4 Consumo HumanoLucy BuitronNoch keine Bewertungen
- D021201078 - Muhammad Fadhil Raihan - English Exercise Week 3Dokument3 SeitenD021201078 - Muhammad Fadhil Raihan - English Exercise Week 3Faren KiriNoch keine Bewertungen
- Faktor Resiko Neuropati Perifer Diabetik 09d66707Dokument9 SeitenFaktor Resiko Neuropati Perifer Diabetik 09d66707Lastri AsmuniatiNoch keine Bewertungen
- Congestive Heart FailureDokument2 SeitenCongestive Heart FailureAngYap Lyn Oloan Saipen100% (1)
- Pre-Lab PM10 Sampling in FoodCourtDokument8 SeitenPre-Lab PM10 Sampling in FoodCourtAjlaa RahimNoch keine Bewertungen
- Psoriasis & Psoriatic Arthritis: DR Prathibha J PDokument48 SeitenPsoriasis & Psoriatic Arthritis: DR Prathibha J PjefferyNoch keine Bewertungen
- Final SURGERY PDF 4TH YR 7TH SEM SOLVED ON THE BASIS OF PREVIOUS YR PAPER AND BOOKS ANKIT AKELADokument25 SeitenFinal SURGERY PDF 4TH YR 7TH SEM SOLVED ON THE BASIS OF PREVIOUS YR PAPER AND BOOKS ANKIT AKELAchai rinNoch keine Bewertungen
- Criminal Sociol-WPS OfficeDokument50 SeitenCriminal Sociol-WPS OfficeFelix GatuslaoNoch keine Bewertungen
- 1, 14 PDFDokument164 Seiten1, 14 PDFRudy Nuryadi100% (1)
- Autosomal Recessive Primary Microcephaly (MCPH) : A Review of Clinical, Molecular, and Evolutionary FindingsDokument12 SeitenAutosomal Recessive Primary Microcephaly (MCPH) : A Review of Clinical, Molecular, and Evolutionary FindingsAli HaiderNoch keine Bewertungen
- Chapter 3 - Microorganism in FoodDokument20 SeitenChapter 3 - Microorganism in Foodnaa znlNoch keine Bewertungen
- Asthma: A. DefinitionDokument6 SeitenAsthma: A. DefinitionElvando SimatupangNoch keine Bewertungen
- Asbestos Awareness Quiz #1: AnswersDokument2 SeitenAsbestos Awareness Quiz #1: AnswersMichael NcubeNoch keine Bewertungen
- Chapter 1 Serology DZ 2010Dokument77 SeitenChapter 1 Serology DZ 2010Anduamlak TeferaNoch keine Bewertungen
- Steroid AbuseDokument7 SeitenSteroid Abuseapi-400575655Noch keine Bewertungen
- A Comprehensive Survey of International Soybean Research - Genetics, Physiology, Agronomy and Nitrogen Relationships - J.E.board - 2012 - (InTech)Dokument624 SeitenA Comprehensive Survey of International Soybean Research - Genetics, Physiology, Agronomy and Nitrogen Relationships - J.E.board - 2012 - (InTech)José Pedro Casagrande TrentínNoch keine Bewertungen