Beruflich Dokumente
Kultur Dokumente
June 2016 QP - Unit 1 WJEC Chemistry A-Level
Hochgeladen von
mark sjsieuOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
June 2016 QP - Unit 1 WJEC Chemistry A-Level
Hochgeladen von
mark sjsieuCopyright:
Verfügbare Formate
PMT
Centre Candidate
Surname
Number Number
Other Names 2
GCE AS/A level
1091/01 – LEGACY S16-1091-01
CHEMISTRY – CH1
A.M. FRIDAY, 27 May 2016
1 hour 30 minutes
For Examiner’s use only
Maximum Mark
Question
Mark Awarded
Section A 1. to 6. 10
Section B 7. 14
010 0 01
ADDITIONAL MATERIALS
10 91
8. 16
In addition to this examination paper, you will need a:
• calculator; 9. 13
• copy of the Periodic Table supplied by WJEC.
Refer to it for any relative atomic masses you require. 10. 15
11. 12
INSTRUCTIONS TO CANDIDATES Total 80
Use black ink or black ball-point pen.
Write your name, centre number and candidate number in the spaces at the top of this page.
Section A Answer all questions in the spaces provided.
Section B Answer all questions in the spaces provided.
Candidates are advised to allocate their time appropriately between Section A (10 marks) and
Section B (70 marks).
INFORMATION FOR CANDIDATES
The number of marks is given in brackets at the end of each question or part-question.
The maximum mark for this paper is 80.
Your answers must be relevant and must make full use of the information given to be awarded full
marks for a question.
The QWC label alongside particular part-questions indicates those where the Quality of Written
Communication is assessed.
If you run out of space, use the additional page(s) at the back of the booklet, taking care to number
the question(s) correctly.
© WJEC CBAC Ltd. SM*(S16-1091-01)
PMT
2
Examiner
only
SECTION A
Answer all questions in the spaces provided.
1. Using the convention of arrows to represent electrons, complete the electronic structure of an
atom of chromium. [1]
1s 2s 2p 3s 3p 3d 4s
a
a
a
a
2. State which one of the following statements is true. [1]
A The first ionisation energy of neon is greater than the first ionisation energy of helium.
B The first ionisation energy of sodium is less than the first ionisation energy of neon.
C The first ionisation energy values increase down Group 1.
D The second ionisation energy of sodium is less than the first ionisation energy of sodium.
........................................
24
3. The half-life of a radioactive isotope xZ is 15.0 hours. It decays by beta emission to a stable
isotope of magnesium, 12Mg.
(a) Give the atomic number and symbol of this radioactive isotope. [1]
........................................
24
(b) After a period of 45.0 hours from the start of the decay, only 0.15 g of xZ remains.
24
Calculate the starting mass of the radioactive isotope xZ. [2]
Starting mass = . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . g
© WJEC CBAC Ltd. (1091-01)
PMT
3
Examiner
only
4. The mass spectrum of bromine trifluoride, Br19F3, shows two molecular ion peaks of equal
intensity at m/z 136 and 138.
State what can be deduced about the relative isotopic masses of the bromine atoms present and
their percentage abundances. [2]
5. The graph shows the distribution of energies in a sample of gas at a certain temperature, T1.
T1
Fraction of molecules
with energy, E
010 0 03
10 91
energy E
Sketch on the graph the curve obtained at a higher temperature, T2. [1]
© WJEC CBAC Ltd. (1091-01) Turn over.
PMT
4
Examiner
θ only
6. The standard enthalpy change of formation, ΔHf , of phosphorus(V) chloride is –463 kJ mol−1.
(a) State the standard conditions of temperature and pressure used, showing the units. [1]
Temperature .......................................................................................
Pressure .......................................................................................
(b) Calculate the heat evolved when 45.2 g of phosphorus(V) chloride (Mr 209) is produced
from phosphorus and chlorine under standard conditions. [1]
Heat evolved = . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . kJ
Total Section A [10]
© WJEC CBAC Ltd. (1091-01)
PMT
5
Examiner
only
SECTION B
Answer all questions in the spaces provided.
7. (a) The explosive HMX has the following structural formula. State its empirical formula. [1]
NO2
CH2 CH2
O 2N N N NO2
H 2C CH2
NO2 Empirical formula . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b) Another explosive, RDX, has the formula C3H6N6O6.
010 0 0 5
10 91
(i) Use the data table and the Hess cycle below to calculate the enthalpy of detonation,
ΔH, of RDX. [3]
ΔH
C3H6N6O6(s) 3CO(g) + 3H2O(g) + 3N2(g)
ΔH1 ΔH2
3C(s) + 3H2(g) + 3N2(g) + 3O2(g)
Enthalpy of formation
Compound
ΔHf / kJ mol−1
RDX(s) +62
CO(g) −111
H2O(g) −242
ΔH = . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . kJ mol−1
© WJEC CBAC Ltd. (1091-01) Turn over.
PMT
6
Examiner
only
(ii) The result from (b)(i) can be used to help you sketch the reaction profile for the
explosive detonation of RDX.
Draw this profile using the axes given below. Label your profile with reactants,
products and the activation energy. [2]
Energy
Progress of reaction
(iii) The activation energy for the explosive detonation of RDX is 199 kJ mol−1
whereas the activation energy for the explosive detonation of mercury fulminate is
105 kJ mol−1.
Define the term activation energy and hence comment on the relative stability of
these two explosives. [2]
© WJEC CBAC Ltd. (1091-01)
PMT
7
Examiner
only
(c) The explosive Tetryl is made by adding concentrated nitric acid to N,N-dimethylphenylamine
under suitable conditions. An equation for this is shown below.
C6H5N(CH3)2 + 4HNO3 C6H2(NO2)3N(CH3)(NO2) + CH3OH + 3H2O
Tetryl
(i) State why the atom economy for this reaction is not 100 %. [1]
(ii) Tetryl produced in this reaction needs further treatment. It can be purified by
dissolving it in propanone and then adding water, or by recrystallisation using
benzene as the solvent.
State any factor in the purification of Tetryl that does not fit with the principles of
Green Chemistry. [1]
010 0 07
10 91
© WJEC CBAC Ltd. (1091-01) Turn over.
PMT
8
Examiner
only
(d) Many fireworks contain metal compounds that emit visible light. The colours given by
barium and calcium compounds and their wavelengths are given in the table.
Metal Colour Wavelength / nm
barium green 554
calcium orange-red 616
(i) State which of these two colours has the higher energy, giving a reason for your
answer. [1]
(ii) The colours seen are as a result of the emission of visible light. State how these
colours are produced. [3]
Total [14]
© WJEC CBAC Ltd. (1091-01)
PMT
010 0 0 9
10 91
BLANK PAGE
© WJEC CBAC Ltd. (1091-01) Turn over.
PMT
10
Examiner
only
8. (a) A student was given an aqueous solution of iodic(V) acid, HIO3, and was asked to find its
concentration by titration with sodium hydroxide solution.
NaOH + HIO3 NaIO3 + H2O
He rinsed the burette with water and then filled it with the iodic(V) acid solution. 25.0 cm3
of sodium hydroxide solution of concentration 0.125 mol dm−3 were used for each titration
against the aqueous iodic(V) acid. The following results were obtained.
Titration 1 2 3 4 5
Volume of iodic(V) acid
19.20 18.60 18.70 18.55 18.55
solution used / cm3
(i) Sodium hydroxide is described as a base. State what is meant by the term base.
[1]
(ii) The teacher said that the result of titration 1 was too high. State one reason why a
fault in the practical method could explain this result. [1]
(iii) Use the results from titrations 2 to 5 to calculate the mean volume of iodic(V) acid
solution and hence the concentration of the acid in mol dm−3. [3]
Concentration of iodic(V) acid = . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . mol dm−3
© WJEC CBAC Ltd. (1091-01)
PMT
11
Examiner
only
(iv) Iodic(V) acid is an expensive material to use and a student suggested that it would
be more economical if only 10.00 cm3 of sodium hydroxide solution were used for
each titration.
Suggest one reason why this was not done in this experiment. [1]
0 1 0 0 11
10 91
© WJEC CBAC Ltd. (1091-01) Turn over.
PMT
12
Examiner
only
(b) The percentage by volume of carbon monoxide in a gas mixture can be found by reacting
it with an iodine compound, and titrating with sodium thiosulfate solution, from which the
number of moles of carbon monoxide present can be found.
Two results obtained by this method are shown below.
Volume of sodium
23.5 30.2
thiosulfate solution / cm3
Carbon monoxide / mol × 10−4 1.05 1.30
(i) Plot these two points on the grid provided and then join them with a straight line.
[1]
32.0
30.0
Volume of sodium thiosulfate solution / cm3
28.0
26.0
24.0
22.0
20.0
1.00 1.05 1.10 1.15 1.20 1.25 1.30 1.35
Carbon monoxide / mol × 10−4
© WJEC CBAC Ltd. (1091-01)
PMT
13
Examiner
only
(ii) In an experiment the carbon monoxide in a gas mixture of volume 300 cm3 gave a
reading of 28.40 cm3 of sodium thiosulfate solution.
Use your graph to find the number of moles of carbon monoxide present in the gas
mixture and hence calculate the percentage by volume of carbon monoxide in the
gas mixture. Give your answer to three significant figures. [3]
[1 mol of any gas has a volume of 24 000 cm3 at the conditions used]
Percentage of carbon monoxide by volume = . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . %
(c) Harmful gases from vehicle exhausts include carbon monoxide and nitrogen(II)
oxide, NO. In a catalytic converter these two gases are converted to nitrogen and carbon
dioxide by passing them over a mixture of platinum and rhodium metals.
Give the equation for this reaction. [1]
0 1 0 0 13
10 91
© WJEC CBAC Ltd. (1091-01) Turn over.
PMT
14
Examiner
only
(d) Catalysts are very important in many industrial processes.
Discuss how catalysts
• increase the rate of a reaction
• affect equilibrium reactions [4]
QWC [1]
Total [16]
© WJEC CBAC Ltd. (1091-01)
PMT
15
BLANK PAGE
© WJEC CBAC Ltd. (1091-01) Turn over.
PMT
16
Examiner
only
9. (a) Methanol, CH3OH, is made from a mixture that contains carbon monoxide and hydrogen.
H
CO(g) + 2H2(g) H C O H (g)
(i) Use the table of average bond enthalpies to calculate the enthalpy change for this
reaction. [2]
Bond Bond enthalpy / kJ mol−1
C O 336
C H 413
H H 436
O H 464
C O
1077
in carbon monoxide
Enthalpy change = . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . kJ mol−1
(ii) State why the calculated value for the enthalpy change of reaction may not be the
same as the literature value. [1]
© WJEC CBAC Ltd. (1091-01)
PMT
17
Examiner
only
(iii) The literature value of the enthalpy change for the reaction
CO(g) + 2H2(g) H C O H (I)
is more exothermic than the literature value for the reaction shown opposite.
State why these two values are different, explaining your answer. [1]
(iv) The reaction to make methanol is in dynamic equilibrium.
CO(g) + 2H2(g) a CH3OH(g)
I. State what is meant by the term dynamic equilibrium. [1]
II. Use the equation above and your answer to (i) to suggest and explain the
conditions of temperature and pressure that will give the greatest yield of
methanol. [2]
© WJEC CBAC Ltd. (1091-01) Turn over.
PMT
18
Examiner
only
(b) The equation for the reaction that represents the enthalpy change of combustion of
methanol, ΔHc , is shown below.
CH3OH(l) + 1 12 O2(g) CO2(g) + 2H2O(l)
(i) Estimate the enthalpy change of combustion of methanol by using the following
table, explaining how you obtained your answer. [2]
Number of carbon atoms Enthalpy change of
Name of alcohol
in the alcohol combustion / kJ mol−1
butan-1-ol 4 −2678
pentan-1-ol 5 −3331
hexan-1-ol 6 −3984
Enthalpy change of combustion = . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . kJ mol−1
© WJEC CBAC Ltd. (1091-01)
PMT
19
Examiner
only
(ii) Enthalpy changes of combustion can be measured directly. A student used the
apparatus below to obtain the value for methanol.
calorimeter water
wick
methanol
The result obtained by this method is often lower than the accepted value.
Suggest one way in which the apparatus could be modified in order to obtain a
result closer to the expected value, giving a reason for your answer. [2]
(iii) In another experiment the enthalpy change of combustion of methanol was
measured and found to be −680 kJ mol−1.
Calculate the mass of methanol burned in this experiment if the energy released by
burning the methanol was 18.7 kJ. [2]
Mass of methanol = . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . g
Total [13]
© WJEC CBAC Ltd. (1091-01) Turn over.
PMT
20
Examiner
only
10. (a) The graph below shows the log of the successive ionisation energies for magnesium.
5.5
5.0
4.5
log10 (Ionisation energy)
4.0
3.5
3.0
2.5
0 2 4 6 8 10 12
Number of electrons removed
© WJEC CBAC Ltd. (1091-01)
PMT
21
Examiner
only
Using the electron configuration for magnesium discuss how and why the values for the
ionisation energies change according to the number of electrons removed. [4]
QWC [1]
© WJEC CBAC Ltd. (1091-01) Turn over.
PMT
22
Examiner
only
(b) Strontium, Sr, is another metal found in Group 2 of the Periodic Table. It reacts rapidly
with cold water to produce a solution of the strong base strontium hydroxide, Sr(OH) 2, and
hydrogen.
Sr + 2H2O Sr(OH)2 + H2
In an experiment 1.260 g of strontium gave 0.0140 mol of hydrogen gas.
(i) Use this information to calculate the relative atomic mass of strontium in this sample.
[2]
Relative atomic mass of strontium = . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(ii) A solution of strontium hydroxide can be used to obtain crystals of hydrated strontium
hydroxide, Sr(OH)2.xH2O. On heating to 100 °C the water is lost giving anhydrous
strontium hydroxide.
Sr(OH)2.xH2O(s) Sr(OH)2(s) + xH2O(g)
A sample of hydrated strontium hydroxide of mass 11.95 g was heated and produced
5.47 g of anhydrous strontium hydroxide (Mr 121.62).
Calculate the value of x in Sr(OH)2.xH2O. [3]
x = ......................................
© WJEC CBAC Ltd. (1091-01)
PMT
23
Examiner
only
(iii) Use the information in the question to suggest another method that could be used to
find the value of x in Sr(OH)2.xH2O and how this method could be used to produce
the value of x. [3]
(c) A solution that cannot dissolve any more solute is called a saturated solution. A saturated
solution of strontium chloride can be represented as an equilibrium mixture between the
solid and its solution.
SrCl2(s) a Sr2+(aq) + 2Cl−(aq)
When hydrochloric acid is added, the clear solution of strontium chloride becomes cloudy.
Use Le Chatelier’s Principle to explain the appearance of this cloudiness. [2]
Total [15]
© WJEC CBAC Ltd. (1091-01) Turn over.
PMT
24
Examiner
only
11. Magnesite is a mineral that consists largely of magnesium carbonate. A sample of crushed
magnesite of mass 6.72 g and an excess of dilute hydrochloric acid of concentration 2 mol dm−3
were placed in a conical flask on a balance.
cotton wool
hydrochloric acid
crushed magnesite
Carbon dioxide was given off during the reaction and the loss in mass was recorded at set
intervals.
(a) Suggest why cotton wool was placed in the neck of the flask. [1]
© WJEC CBAC Ltd. (1091-01)
PMT
25
Examiner
only
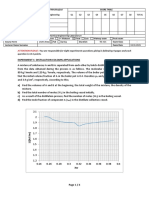
(b) The results from the experiment were plotted in a graph.
4.0
3.0
Loss in mass / g 2.0
1.0
0
0 4 8 12 16 20
Time / min
(i) Use the graph to find the time taken for half of the original mass of magnesite to
react. [1]
Time = . . . . . . . . . . . . . . . . . . . . . . . . . . . . . min
(ii) Use the graph to calculate the initial rate of the reaction, giving its unit. [2]
Rate = . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Unit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
© WJEC CBAC Ltd. (1091-01) Turn over.
PMT
26
Examiner
only
(iii) Use collision theory to explain how the rate of the reaction changes during the
reaction. You should consider both reactants in your answer. [3]
QWC [1]
(iv) The experiment was repeated using the same mass of magnesite but in a lump
form.
Sketch on the graph (previous page) the resulting line for this experiment. [2]
(c) In a further experiment an excess of magnesite was added to some hydrochloric acid.
At the end of the reaction a neutral solution of magnesium chloride and some unreacted
magnesite remained.
Outline how the pH of the mixture would change during this reaction, suggesting pH
values where appropriate. [2]
Total [12]
Section B Total [70]
END OF PAPER
© WJEC CBAC Ltd. (1091-01)
PMT
27
Examiner
only
For continuation only.
© WJEC CBAC Ltd. (1091-01) Turn over.
PMT
28
Examiner
only
© WJEC CBAC Ltd. (1091-01)
PMT
GCE AS/A level
1091/01-A – LEGACY S16-1091-01A
CHEMISTRY – PERIODIC TABLE
FOR USE WITH CH1
A.M. FRIDAY, 27 May 2016
© WJEC CBAC Ltd. SM*(S16-1091-01-A)
THE PERIODIC TABLE
1 2 Group 3 4 5 6 7 0
Period s Block
1.01 4.00
1 H Key He
Hydrogen Helium
1 relative
p Block 2
atomic
6.94 9.01 Ar mass 10.8 12.0 14.0 16.0 19.0 20.2
Li Be Symbol B C N O F Ne
2 Lithium Beryllium Name Boron Carbon Nitrogen Oxygen Fluorine Neon
atomic
3 4 Z 5 6 7 8 9 10
number
23.0 24.3 27.0 28.1 31.0 32.1 35.5 40.0
© WJEC CBAC Ltd.
Na Mg Al Si P S Cl Ar
3 Sodium Magnesium d Block Aluminium Silicon Phosphorus Sulfur Chlorine Argon
11 12 13 14 15 16 17 18
39.1 40.1 45.0 47.9 50.9 52.0 54.9 55.8 58.9 58.7 63.5 65.4 69.7 72.6 74.9 79.0 79.9 83.8
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
4 Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton
23 29 33
2
19 20 21 22 24 25 26 27 28 30 31 32 34 35 36
(1091-01-A)
85.5 87.6 88.9 91.2 92.9 95.9 98.9 101 103 106 108 112 115 119 122 128 127 131
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
5 Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon
37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54
133 137 139 179 181 184 186 190 192 195 197 201 204 207 209 (210) (210) (222)
Cs Ba La ‣ Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn
6 Caesium Barium Lanthanum Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon
55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86
(223) (226) (227)
Fr Ra Ac ‣‣
7 Francium Radium Actinium
87 88 89 f Block
140 141 144 (147) 150 (153) 157 159 163 165 167 169 173 175
‣ Lanthanoid Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
elements Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium
58 59 60 61 62 63 64 65 66 67 68 69 70 71
232 (231) 238 (237) (242) (243) (247) (245) (251) (254) (253) (256) (254) (257)
‣‣ Actinoid Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
elements
Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium
90 91 92 93 94 95 96 97 98 99 100 101 102 103
PMT
Das könnte Ihnen auch gefallen
- Reviewer For Bookkeeping NCIIIDokument18 SeitenReviewer For Bookkeeping NCIIIAngelica Faye95% (20)
- Calculate Breakeven Point in Units and Revenue Dollars: Intermediate Cost Analysis and ManagementDokument52 SeitenCalculate Breakeven Point in Units and Revenue Dollars: Intermediate Cost Analysis and ManagementNavice Kie100% (1)
- Drill Bit Classifier 2004 PDFDokument15 SeitenDrill Bit Classifier 2004 PDFgustavoemir0% (2)
- HBS - Zara Fast Fashion Case Write UpDokument4 SeitenHBS - Zara Fast Fashion Case Write Upaaronhwalton100% (1)
- Welding and Heat Treatment Requirements For Equipment and PipingDokument34 SeitenWelding and Heat Treatment Requirements For Equipment and Pipingonur gunesNoch keine Bewertungen
- June 2015 QP - Unit 1 WJEC Chemistry A-LevelDokument26 SeitenJune 2015 QP - Unit 1 WJEC Chemistry A-LevelYT ChongNoch keine Bewertungen
- A410U10-1-050618 Component 1 PDFDokument28 SeitenA410U10-1-050618 Component 1 PDFZhen Rong YapNoch keine Bewertungen
- Gce As: Friday, 27 May 2022 - Afternoon Energy, Rate and Chemistry of Carbon CompoundsDokument24 SeitenGce As: Friday, 27 May 2022 - Afternoon Energy, Rate and Chemistry of Carbon CompoundsPirate HunterNoch keine Bewertungen
- Gce A Level - New: Physical and Inorganic ChemistryDokument23 SeitenGce A Level - New: Physical and Inorganic ChemistryPirate HunterNoch keine Bewertungen
- Gce As: Tuesday, 13 October 2020 - Morning Energy, Rate and Chemistry of Carbon CompoundsDokument20 SeitenGce As: Tuesday, 13 October 2020 - Morning Energy, Rate and Chemistry of Carbon CompoundsPirate HunterNoch keine Bewertungen
- Paper 1 2018Dokument20 SeitenPaper 1 2018harrywbfraserNoch keine Bewertungen
- Paper 1 2017Dokument24 SeitenPaper 1 2017harrywbfraserNoch keine Bewertungen
- Wjec Paper 3Dokument32 SeitenWjec Paper 3JenNoch keine Bewertungen
- Eduqas PaperDokument36 SeitenEduqas PaperJenNoch keine Bewertungen
- June 2022 QP - Component 3 Eduqas Biology A-LevelDokument44 SeitenJune 2022 QP - Component 3 Eduqas Biology A-LeveltariffgillNoch keine Bewertungen
- Eduqas Paper 2Dokument44 SeitenEduqas Paper 2JenNoch keine Bewertungen
- June 2000 - Paper 2Dokument12 SeitenJune 2000 - Paper 2theyaasir67% (3)
- 8701 w01 qp2Dokument12 Seiten8701 w01 qp2sohailsultanNoch keine Bewertungen
- June 2018 QP - Unit 1 (H) WJEC Biology GCSEDokument28 SeitenJune 2018 QP - Unit 1 (H) WJEC Biology GCSEAsni Tunjung ArantikiNoch keine Bewertungen
- June 2016 (v2) QP - Paper 2 CIE Chemistry A-LevelDokument12 SeitenJune 2016 (v2) QP - Paper 2 CIE Chemistry A-LevelNokutenda KundionaNoch keine Bewertungen
- AS Comp 2Dokument15 SeitenAS Comp 2Ellson LinNoch keine Bewertungen
- Friday, 17 June 2022 - Afternoon Chemical Substances, Reactions and Essential Resources Foundation TierDokument28 SeitenFriday, 17 June 2022 - Afternoon Chemical Substances, Reactions and Essential Resources Foundation TieransssaleemNoch keine Bewertungen
- Ejc h1 Chem p2 AnswerDokument22 SeitenEjc h1 Chem p2 AnswerLim EnningNoch keine Bewertungen
- Wjec Paper 2Dokument16 SeitenWjec Paper 2JenNoch keine Bewertungen
- June 2018 QP - Component 2 WJEC Physics AS-levelDokument15 SeitenJune 2018 QP - Component 2 WJEC Physics AS-levelfadi baqainNoch keine Bewertungen
- June 2017 QP - Unit 1 (F) WJEC Biology GCSEDokument23 SeitenJune 2017 QP - Unit 1 (F) WJEC Biology GCSEAsni Tunjung ArantikiNoch keine Bewertungen
- Paper 3 2018Dokument44 SeitenPaper 3 2018harrywbfraserNoch keine Bewertungen
- Paper 3 2019Dokument44 SeitenPaper 3 2019harrywbfraserNoch keine Bewertungen
- FinalDokument4 SeitenFinalSimge DemirNoch keine Bewertungen
- 0620 m16 QP 42Dokument16 Seiten0620 m16 QP 42reemNoch keine Bewertungen
- Cambridge International General Certificate of Secondary EducationDokument484 SeitenCambridge International General Certificate of Secondary EducationolaNoch keine Bewertungen
- June 2022 (v1) QP - Paper 4 CAIE Chemistry IGCSEDokument16 SeitenJune 2022 (v1) QP - Paper 4 CAIE Chemistry IGCSERimNoch keine Bewertungen
- June 2016 QP - Unit 1 OCR Chemistry A-LevelDokument16 SeitenJune 2016 QP - Unit 1 OCR Chemistry A-Levelmark sjsieuNoch keine Bewertungen
- Paper 2 Nov 2002 PhysicsDokument12 SeitenPaper 2 Nov 2002 PhysicssolarixeNoch keine Bewertungen
- Aqa Phya2 W QP Jun10Dokument20 SeitenAqa Phya2 W QP Jun10Avinash BoodhooNoch keine Bewertungen
- 10 1016@j Micromeso 2017 08 034Dokument15 Seiten10 1016@j Micromeso 2017 08 034Yousef SailiniNoch keine Bewertungen
- Gce As/A Level: Friday, 20 May 2022 - Afternoon Basic Biochemistry and Cell OrganisationDokument32 SeitenGce As/A Level: Friday, 20 May 2022 - Afternoon Basic Biochemistry and Cell OrganisationJ JNoch keine Bewertungen
- 9700 w01 QP 2 PDFDokument12 Seiten9700 w01 QP 2 PDFkarimabdelsamadNoch keine Bewertungen
- wch11 01 Que 20231011Dokument28 Seitenwch11 01 Que 20231011Thoon Nadi Nai0% (1)
- 1 7 - Chemistry 8873/02: 13 September 2018 2 HoursDokument22 Seiten1 7 - Chemistry 8873/02: 13 September 2018 2 HoursLim EnningNoch keine Bewertungen
- Paper 2 June 2007 PhysicsDokument16 SeitenPaper 2 June 2007 PhysicssolarixeNoch keine Bewertungen
- Paper 2 May 2001 PhysicsDokument20 SeitenPaper 2 May 2001 PhysicssolarixeNoch keine Bewertungen
- Cambridge Ordinary Level: Cambridge Assessment International EducationDokument20 SeitenCambridge Ordinary Level: Cambridge Assessment International EducationMohammad faheem AnwarNoch keine Bewertungen
- Paper 3 SL Yr13 PDFDokument29 SeitenPaper 3 SL Yr13 PDFJun Hwan ChangNoch keine Bewertungen
- Sample Paper 2Dokument28 SeitenSample Paper 2omeshwarchaudhari2006Noch keine Bewertungen
- 0625 - w16 - QP - 43 Igcse Paper 4Dokument16 Seiten0625 - w16 - QP - 43 Igcse Paper 4Sultan ShahNoch keine Bewertungen
- Edexcel GCE: ChemistryDokument12 SeitenEdexcel GCE: ChemistrymazlinnairinNoch keine Bewertungen
- November 2015 (v2) QP - Paper 2 CIE Chemistry A-LevelDokument12 SeitenNovember 2015 (v2) QP - Paper 2 CIE Chemistry A-LevelFrengky WijayaNoch keine Bewertungen
- Paper 2 May 2003 PhysicsDokument16 SeitenPaper 2 May 2003 Physicssolarixe100% (1)
- 9701 w09 QP 21Dokument12 Seiten9701 w09 QP 21Hubbak KhanNoch keine Bewertungen
- Chemistry Atoms First 2nd Edition Burdge Solutions ManualDokument20 SeitenChemistry Atoms First 2nd Edition Burdge Solutions Manualpatronaltruncaterxa3100% (27)
- Dwnload Full Chemistry Atoms First 2nd Edition Burdge Solutions Manual PDFDokument35 SeitenDwnload Full Chemistry Atoms First 2nd Edition Burdge Solutions Manual PDFnoahkim2jgp100% (10)
- Paper 2 2017Dokument23 SeitenPaper 2 2017harrywbfraserNoch keine Bewertungen
- Cambridge International General Certificate of Secondary EducationDokument16 SeitenCambridge International General Certificate of Secondary EducationjoshkpatelNoch keine Bewertungen
- Jan 07 U1Dokument16 SeitenJan 07 U1Divyani PatelNoch keine Bewertungen
- Paper 2 May 2006 PhysicsDokument12 SeitenPaper 2 May 2006 PhysicssolarixeNoch keine Bewertungen
- 9700 w02 QP 5Dokument8 Seiten9700 w02 QP 5hasnainf11280Noch keine Bewertungen
- 9701 s10 QP 22Dokument12 Seiten9701 s10 QP 22Hubbak KhanNoch keine Bewertungen
- June 2010 CH5Dokument15 SeitenJune 2010 CH5s5ff22mrtcNoch keine Bewertungen
- Additional Combined Science: Paper 2Dokument16 SeitenAdditional Combined Science: Paper 2rodel.verzosaNoch keine Bewertungen
- Electronics – From Theory Into Practice: Applied Electricity and Electronics DivisionVon EverandElectronics – From Theory Into Practice: Applied Electricity and Electronics DivisionBewertung: 5 von 5 Sternen5/5 (1)
- Electronics—From Theory Into Practice: Pergamon International Library of Science, Technology, Engineering and Social StudiesVon EverandElectronics—From Theory Into Practice: Pergamon International Library of Science, Technology, Engineering and Social StudiesBewertung: 5 von 5 Sternen5/5 (2)
- International Thermodynamic Tables of the Fluid State Helium-4Von EverandInternational Thermodynamic Tables of the Fluid State Helium-4Noch keine Bewertungen
- Non-Destructive Evaluation of Corrosion and Corrosion-assisted CrackingVon EverandNon-Destructive Evaluation of Corrosion and Corrosion-assisted CrackingRaman SinghNoch keine Bewertungen
- Surface Plasmon Enhanced, Coupled and Controlled FluorescenceVon EverandSurface Plasmon Enhanced, Coupled and Controlled FluorescenceNoch keine Bewertungen
- H I Ôn Thi Aptis & Vstep - Tài Liệu - Anna MaiDokument4 SeitenH I Ôn Thi Aptis & Vstep - Tài Liệu - Anna Maihanh.mt2022Noch keine Bewertungen
- Full Download Human Biology 11th Edition Starr Solutions ManualDokument35 SeitenFull Download Human Biology 11th Edition Starr Solutions Manualsheathe.zebrinny.53vubg100% (41)
- Talent-Olympiad 9 Science SampleDokument12 SeitenTalent-Olympiad 9 Science SampleFire GamingNoch keine Bewertungen
- Loch ChildrenDokument4 SeitenLoch ChildrenLauro De Jesus FernandesNoch keine Bewertungen
- Bubble Deck SlabDokument29 SeitenBubble Deck SlabJhimy Rusbel Gutierrez YanapaNoch keine Bewertungen
- ATA212001Dokument3 SeitenATA212001Tarek DeghedyNoch keine Bewertungen
- BF254 BF255Dokument3 SeitenBF254 BF255rrr2013Noch keine Bewertungen
- Exam C - HANATEC142: SAP Certified Technology Associate - SAP HANA (Edition 2014)Dokument10 SeitenExam C - HANATEC142: SAP Certified Technology Associate - SAP HANA (Edition 2014)SadishNoch keine Bewertungen
- Pointers in CDokument25 SeitenPointers in CSainiNishrithNoch keine Bewertungen
- Asme Bladder Accumulator DatasheetDokument3 SeitenAsme Bladder Accumulator DatasheetSamad A BakarNoch keine Bewertungen
- Market Challengers StrategiesDokument19 SeitenMarket Challengers Strategiestobbob007100% (20)
- Probecom 11.3M Antenna System Datasheet 2Dokument2 SeitenProbecom 11.3M Antenna System Datasheet 2Hugo MateoNoch keine Bewertungen
- José Guadalupe PosadaDokument19 SeitenJosé Guadalupe PosadaJudy Baca100% (1)
- Norma Geral Astm Bronze Alumínio - b150b150m.19198Dokument7 SeitenNorma Geral Astm Bronze Alumínio - b150b150m.19198EduardoNoch keine Bewertungen
- Management Glossary - Musa KamawiDokument50 SeitenManagement Glossary - Musa KamawiKazi Nazrul IslamNoch keine Bewertungen
- Buy Wholesale China Popular Outdoor Football Boot For Teenagers Casual High Quality Soccer Shoes FG Ag Graffiti Style & FootballDokument1 SeiteBuy Wholesale China Popular Outdoor Football Boot For Teenagers Casual High Quality Soccer Shoes FG Ag Graffiti Style & Footballjcdc9chh8dNoch keine Bewertungen
- UniFi Quick GuideDokument2 SeitenUniFi Quick GuideAndhika TharunaNoch keine Bewertungen
- Esp Kelompok 2Dokument19 SeitenEsp Kelompok 2Taufiq DiNoch keine Bewertungen
- G2A Glitch DONT LEAK 2Dokument7 SeitenG2A Glitch DONT LEAK 2qDeficiencyNoch keine Bewertungen
- SM-G900F Esquematico Completo Anibal Garcia IrepairDokument2 SeitenSM-G900F Esquematico Completo Anibal Garcia Irepairfix marketNoch keine Bewertungen
- RCD ManagementDokument6 SeitenRCD ManagementPindoterONoch keine Bewertungen
- JAMB Syllabus For BiologyDokument27 SeitenJAMB Syllabus For BiologyOluebube UchennaNoch keine Bewertungen
- 234567890Dokument37 Seiten234567890erppibuNoch keine Bewertungen
- 30xa 100t PDFDokument162 Seiten30xa 100t PDFleung ka kitNoch keine Bewertungen
- Epidemiological Triad of HIV/AIDS: AgentDokument8 SeitenEpidemiological Triad of HIV/AIDS: AgentRakib HossainNoch keine Bewertungen