Beruflich Dokumente
Kultur Dokumente
Atypical MHC
Hochgeladen von
kalichemaxOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Atypical MHC
Hochgeladen von
kalichemaxCopyright:
Verfügbare Formate
REVIEWS
Atypical MHC class II‑expressing
antigen-presenting cells: can anything
replace a dendritic cell?
Taku Kambayashi1 and Terri M. Laufer2
Abstract | Dendritic cells, macrophages and B cells are regarded as the classical
antigen-presenting cells of the immune system. However, in recent years, there has been a rapid
increase in the number of cell types that are suggested to present antigens on MHC class II
molecules to CD4+ T cells. In this Review, we describe the key characteristics that define an
antigen-presenting cell by examining the functions of dendritic cells. We then examine the
functions of the haematopoietic cells and non-haematopoietic cells that can express MHC
class II molecules and that have been suggested to represent ‘atypical’ antigen-presenting cells.
We consider whether any of these cell populations can prime naive CD4+ T cells and, if not,
question the effects that they do have on the development of immune responses.
Dendritic cells (DCs), B cells and macrophages constitu- antigen processing and presentation capabilities, and
tively express MHC class II molecules and are regarded their expression of co-stimulatory molecules, DCs
as the ‘professional’ antigen-presenting cells (APCs) of express pattern recognition receptors (PRRs) that medi-
the immune system (FIG. 1). However, the universe of ate activation in response to pathogens. Following PRR-
APCs, seemingly so well established, has recently been mediated maturation, DCs can migrate to the lymph
changing as more and more cell types are proposed nodes to promote the activation of naive T cells. Indeed,
to express MHC class II molecules and have antigen- DCs have been shown to be both necessary and suffi-
presenting functions (FIG. 1; TABLE 1). A number of cient for the activation of naive T cells1,2. The APC that
haematopoietic cell types have been suggested to pre- initiates an immune response must make multiple deci-
sent antigens on MHC class II molecules to CD4+ T cells, sions — for example, whether to actively respond to or
including mast cells, basophils, eosinophils, neutrophils, tolerate the antigenic challenge, and what type of active
innate lymphoid cells (ILCs) and CD4+ T cells themselves. immune response to induce. DCs clearly predominate in
In addition, MHC class II expression has been detected on the induction of T cell proliferation. Interestingly, how-
non-haematopoietic cell types in the periphery, such as ever, DCs are not required for the development of CD4+
endothelial cells, epithelial cells and lymph node stromal T cell responses under certain conditions3,4, suggesting
1
Department of Pathology cells (LNSCs). MHC class II‑expressing cells also support that other APCs may be able to replace the function of
and Laboratory Medicine and thymocyte development and tolerance induction in the DCs. Thus, we consider whether any non-classical APC
Division of Rheumatology,
Department of Medicine,
thymus. However, for the purposes of this article, we focus can prime naive CD4+ T cells, and discuss the roles that
Perelman School of Medicine, on how atypical MHC class II+ cells interact with mature these cells may have in modulating DC‑dependent and
University of Pennsylvania, CD4+ T cells that have exited the thymus. In this Review, DC‑independent immune responses.
Philadelphia, Pennsylvania we delineate the qualities that define a true APC and then
19104, USA.
examine whether any of the atypical MHC class II+ cell Mast cells as APCs
2
Philadelphia Veterans Affairs
Medical Center, Philadelphia, populations have these functional capabilities. Mast cells are tissue-resident innate immune cells that are
Pennsylvania 19104, USA. strategically localized at mucosal and submucosal sites
Correspondence to T.M.L. Classical APC characteristics in close proximity to the external environment. They
e-mail: A consideration of the properties of DCs that facilitate have established roles in allergic disease. Following the
tlaufer@mail.med.upenn.edu
doi:10.1038/nri3754
the activation of naive CD4+ T cells provides a com- activation of high-affinity Fc receptor for IgE (FcεRI),
Published online prehensive list of characteristics that a bona fide APC mast cells degranulate and release pre-stored immuno
17 October 2014 should have (BOX 1). Specifically, in addition to their modulatory molecules — such as tumour necrosis factor
NATURE REVIEWS | IMMUNOLOGY VOLUME 14 | NOVEMBER 2014 | 719
© 2014 Macmillan Publishers Limited. All rights reserved
REVIEWS
Professional APCs Atypical APCs
DCs and macrophages B cells Mast cells Basophils Eosinophils ILC3s
Key features Key features Key features
• Phagocytic • Internalize antigens via • Inducible expression of MHC class II molecules
• Express receptors for apoptotic BCRs • Antigen-presenting functions limited to
cells, DAMPs and PAMPs • Constitutively express specific immune environments (especially
• Localize to tissues MHC class II molecules type 2 immune settings)
• Localize to T cell zone of lymph and antigen processing • Lack of compelling evidence that they can
nodes following activation (DCs) machinery activate naive CD4+ T cells in an antigen-
• Constitutively express high levels • Express co-stimulatory specific manner
of MHC class II molecules and molecules following
antigen processing machinery activation
• Express co-stimulatory molecules
following activation
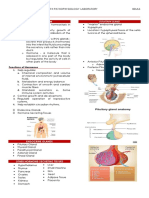
Figure 1 | Key antigen-presenting functions of professional and atypical antigen-presenting cells. The figure compares
and contrasts the features of ‘professional’ and ‘atypical’ antigen-presenting cells (APCs). Professional
Nature APCs
Reviewsinclude dendritic
| Immunology
cells (DCs) and, with a lesser role, macrophages and B cells. These cells constitutively express MHC class II structural proteins
and associated proteins, and antigen-processing machinery. By contrast, atypical APCs — such as mast cells, basophils,
eosinophils and innate lymphoid cells (ILCs) — do not constitutively express MHC class II molecules, but can upregulate
the expression of MHC class II under certain conditions. Basophils, eosinophils and mast cells that express MHC class II may
contribute to T helper 2 cell differentiation, but there is less evidence that they can prime naive CD4+ T cells. B cell receptor;
DAMP, damage-associated molecular pattern; PAMP, pathogen-associated molecular pattern.
(TNF) and histamine — which can promote T cell activa- reflect the different protocols that have been used
tion both directly and indirectly, through stimulation of to generate mast cells. The earlier studies generated
APCs. Furthermore, mast cell-deficient mice have defec- mast cells from short-term bone marrow cultures
tive CD4+ T cell responses in experimental autoimmune (~3 weeks) that were supplemented with IL‑3 alone21.
encephalomyelitis5 and in Leishmania major infection6, By contrast, later studies used long-term bone
suggesting that CD4+ T cell responses could be altered marrow cultures that were supplemented with both IL‑3
in the absence of mast cells. and stem cell factor (SCF; also known as KIT ligand).
Many of the cells derived from the short-term cultures
Expression of MHC class II molecules by mast cells. Mast expressed FcεRI but lacked the mast/stem cell growth
cells can directly present antigens to T cells. Both rodent7,8 factor receptor KIT22, suggesting that these cultures
and human9,10 mast cells have been reported to constitu- also contained non-mast cells, including basophils3,23
tively express MHC class II molecules. The expression of and FcεRI+ DCs24,25, both of which expressed MHC
MHC class II by mast cells correlated with their ability class II but not KIT. By contrast, bone marrow cells
to present antigen in vitro to naive CD4+ T cells and to grown in cultures supplemented with IL‑3 and SCF
T cell hybridomas. Cytokines such as interleukin‑4 (IL‑4), for more than 6 weeks yielded mast cells that express
interferon‑γ (IFNγ) and granulocyte–macrophage high, homogeneous levels of both KIT and FcεRI18,20.
colony-stimulating factor (GM‑CSF; also known as Alternatively, the maturation status of mast cells may
CSF2) further enhanced the APC function of mast have affected their APC function, as only mast cells
cells11,12. However, these reports were contradicted in from bone marrow cultures less than 3 weeks old were
follow‑up studies by the same group showing that the able to present antigen to T cells26.
activation of antigen-specific T cells still occurred when Although resting bone marrow-derived mast
the T cells were cultured with MHC-mismatched mast cells from long-term cultures do not constitutively
cells13. Moreover, co‑culture of mast cells with spleno express MHC class II, its expression can be induced on
cytes resulted in antigen-independent activation of these cells. After activation of bone marrow-derived
Experimental autoimmune T cells14, which was probably mediated by immuno mast cells with Toll-like receptor 4 (TLR4) agonists
encephalomyelitis logically active exosomes released by mast cells15 (BOX 2). and, to a lesser extent, TLR2 agonists in the presence
An experimental model for These results were consistent with subsequent studies of IFNγ, a large proportion of cells expressed MHC
the human disease multiple
showing that MHC class II molecules mainly reside class II and other molecules that are associated with
sclerosis. Autoimmune disease
is induced in experimental in intracellular lysosomal compartments of mast cells, antigen presentation — namely, MHC class II transacti
animals by immunization with rather than at the cell surface16. vator (CIITA), the invariant chain (Ii) and H2‑DM20.
myelin or peptides derived More recently, several groups have reported that Similarly, although freshly isolated mast cells from the
from myelin. The animals resting or FcεRI-activated mast cells do not express peritoneal cavity lacked MHC class II expression, these
develop a paralytic disease
with inflammation and
MHC class II molecules, either on the cell surface mast cells expressed MHC class II after in vitro treat-
demyelination in the brain or intracellularly 17–20. The apparent discrepancies ment with IL‑4 and IFNγ27. MHC class II+ mast cells
and spinal cord. between the earlier and more recent studies may were also found in lymph node sinuses; treatment of
720 | NOVEMBER 2014 | VOLUME 14 www.nature.com/reviews/immunol
© 2014 Macmillan Publishers Limited. All rights reserved
REVIEWS
Table 1 | Features of ‘atypical’ antigen-presenting cells
Atypical APC Features of atypical APC
Ability to promote T cell activation Expression of Lymph node Factors that induce Expression of other
co-stimulatory migration by expression of MHC MHC class II‑related
Naive T cells Activated T cells
molecules APC class II on APC genes
Mast cells +/– + +/– (CD80 and + TLR agonists, IFNγ, Ii and HLA‑DM
CD86) GM‑CSF, IL‑4 and Notch
Basophils +/– +/– + (mouse) + FcεRI, IL‑3 and papain Ii and HLA‑DM
– (human)
Eosinophils + + CD80 and CD86 + IL‑3, IFNγ and GM‑CSF Unknown
Neutrophils +/– +/– CD80 and CD86 + Co‑culture with T cells, Unknown
GM‑CSF and IFNγ
ILC2s + Unknown Unknown Unknown Unknown Unknown
ILC3s – Tolerance induction – + Unknown Ii and HLA‑DM
CD4 T cells
+
– Tolerance induction – + TCR stimulation Unknown
LNSCs Tolerance Unknown ICOSL (subset) NA TLR agonists and IFNγ Ii and HLA‑DM
induction
Endothelial cells – + CD137L, OX40L – IFNγ Unknown
and ICOSL
Epithelial cells Tolerance + – – IFNγ and microbial Ii and HLA‑DM
induction stimuli
APC, antigen-presenting cell; CD137L, CD137 ligand (also known as TNFSF9); FcεRI, high-affinity Fc receptor for IgE; GM‑CSF, granulocyte–macrophage
colony-stimulatory factor; ICOSL, ICOS ligand; IFN, interferon; Ii, invariant chain; IL, interleukin; ILC, innate lymphoid cell; LNSC, lymph node stromal cell;
NA, not applicable; OX40L, OX40 ligand (also known as TNFSF4); TCR, T cell receptor; TLR, Toll-like receptor.
mice with lipopolysaccharide or infection with L. major allografts and in preventing nephrotoxic serum neph
increased the number of mast cells in the draining ritis, which are processes that were proposed to involve
lymph nodes and further upregulated MHC class II IL‑9 production by TReg cells to recruit mast cells to the
expression20. The apparent constitutive expression of graft or injury site29,30. It has been shown that endogenous
MHC class II by lymph node mast cells may be owing proteins are effectively presented on MHC class II mol-
to the fact that these cells have matured and migrated ecules of mast cells20 and as such, many of the peptides
following their activation in the tissue. Alternatively, presented by mast cell MHC class II may be self antigens
endogenous ligands that induce the expression of MHC that stimulate TReg cells.
class II on mast cells may exist at specific anatomical
sites. The latter hypothesis is supported by data identi Does IgE support mast cell antigen-presenting functions?
fying Notch–Delta-like protein 1 interactions as an Antigen-specific IgE molecules enhance APC function
inducer of mast cell MHC class II expression19. The in a subset of human DCs that express FcεRI by shut-
requirement for IFNγ and either TLR-mediated or tling incorporated antigens to the MHC class II path-
Notch-mediated signalling for MHC class II expression way31. FcεRI could also potentially facilitate the uptake
by mast cells may be related to the roles of these path- of antigens into mast cells for more efficient antigen
ways in inducing the transcription factor PU.1, which presentation. Indeed, large particulate antigens were
regulates the CIITA promoter 28. taken up by mast cells through an IgE-dependent
mechanism both in vitro and in vivo32. Moreover, ear-
Functions of MHC class II on mast cells. After MHC lier studies using cultured mast cells demonstrated
class II was expressed by activated bone marrow-derived that the uptake of antigens through antigen-specific
mast cells, they could process and present soluble protein IgE molecules enhances the T cell-stimulating capac-
antigens to previously activated CD4+ T cells in vitro, but ity of mast cells18,22,33. However, in the latter scenario,
they could not prime naive T cells20, perhaps because they the activation of T cells was not mediated through
lacked co‑stimulatory molecule expression. However, direct antigen presentation by mast cells, as the mast
given that lymph node-resident and peritoneal mast cells used in these experiments failed to express MHC
cells that have been activated by IL‑4 and IFNγ express class II 17,18,20. Rather, mast cells transferred FcεRI-
both CD80 and CD86 (REFS 20,27), it is possible that some incorporated antigens to DCs that secondarily pre-
mast cells can present antigens to naive T cells in vivo. In sented the antigens to T cells18. Antigens incorporated
addition to activated T cells, mast cells appear to prefer- by mast cells through FcεRI were found to colocal-
entially expand antigen-specific regulatory T (TReg) cells ize with secretory compartments, and were released
rather than naive T cells20. The activation of TReg cells by by the mast cells upon reactivation32. Thus, although
MHC class II+ mast cells may contribute to the protec- FcεRI may transport antigens to the MHC class II
tive effects of mast cells in mediating tolerance to skin pathway under specific conditions22,33, mast cells that
NATURE REVIEWS | IMMUNOLOGY VOLUME 14 | NOVEMBER 2014 | 721
© 2014 Macmillan Publishers Limited. All rights reserved
REVIEWS
Box 1 | What are the key properties of an antigen-presenting cell?
Basophils modulate T cell responses
Basophils are a rare population of granulocytes that
Burnet’s ‘clonal selection’ theory presupposes that the T cell repertoire contains comprise ~1% of circulating peripheral leukocytes, and
a vast number of T cell clones expressing unique antigen receptors. Thus, an effective they are structurally and phenotypically related to mast
adaptive immune response requires a second cell to select and expand those few T cell cells. Like mast cells, basophils contain abundant gran-
clones that express ‘useful’ antigen receptors. Ralph Steinman termed this quality
ules with pre-formed inflammatory mediators that can
‘immunogenicity’ (REF. 145), and crucial work by McDevitt, Sela and Humphrey146–148
showed that immunogenicity required ‘immune response’ genes, which mapped to
be immediately released upon crosslinking of FcεRI34–37.
the MHC locus. Zinkernagel and Doherty149 first demonstrated that the presentation Important new reagents have recently been used to show
of viral antigens was MHC restricted and multiple laboratories subsequently showed that basophils and mast cells have functionally unique
that MHC class I and class II proteins present peptide fragments that are derived from and non-overlapping roles in IgE-mediated and IgG-
antigens to the T cell receptor (TCR). Thus, immunogenicity requires that proteins are mediated allergic inflammation in mice38–43. In addition,
processed and presented on MHC molecules to be recognized by T cells. it has been suggested that basophils are central to the dif-
However, T cell activation requires more than the simple expression of MHC molecules ferentiation of T helper 2 (TH2) cells. Indeed, a number of
by antigen-presenting cells (APCs). The activation of a naive T cell requires interaction studies have suggested that basophil-derived IL‑4 is cru-
with an APC that provides multiple signals (see the figure): ‘signal 1’ is delivered through cial for the development of TH2 cell responses to cysteine
interaction of the TCR with peptide–MHC complexes; ‘signal 2’ involves co-stimulatory
proteases, allergens and extracellular parasites4,23,42,44–50.
molecules; and ‘signal 3’ is mediated by instructive cytokines. The ability to deliver these
three signals is the defining characteristic of a professional APC150.
The search to identify APCs focused on cells that could induce B cell and T cell Basophils as APCs. Although basophil-derived IL‑4
responses in vitro and in vivo. This antigen-presenting function was initially ascribed to can skew T cells to a TH2 cell phenotype, it had been
macrophages, because the crucial ‘accessory cell’ found in these assays was adherent, presumed that antigen-specific T cell activation by
as are macrophages151. However, in 1973, Steinman and Cohn152 identified a different professional APCs, such as DCs, is still necessary for
population of adherent cells with large pseudopods in the mouse spleen; they TH2 cell induction. To examine the requirement for
subsequently demonstrated that these ‘dendritic cells’ (DCs) were the most potent DCs in antigen presentation during TH2 cell differen-
inducers of T cell proliferation in primary mixed lymphocyte responses153. Additionally, tiation, a number of groups have used mice expressing
DCs are particularly well adapted to process proteins into the peptides that are the human diphtheria toxin receptor (DTR) under the
presented by MHC class II molecules. They express the MHC class II structural α- and
Cd11c promoter (Cd11c–DTR mice); in these mice,
β-chains; however, they also express the associated chaperone invariant chains,
HLA‑DM and HLA-DO, which regulate peptide loading, and the complement of
diphtheria toxin treatment results in the ablation of
lysosomal proteases and cathepsins that can operate in the acidic phagolysosomal CD11c‑expressing cells. When bone marrow from
pathway (recently reviewed in REF. 154). Although many analyses probe for expression Cd11c–DTR mice was used to reconstitute irradiated
of the invariant chain (Ii), HLA-DM and HLA-DO, there have been very few stringent wild-type mice, diphtheria toxin treatment of these chi-
evaluations of the phagolysosomal pathways of atypical APCs. meric mice did not alter TH2 cell responses to papain
or Trichuris muris 3,4. Furthermore, papain injection or
DAMPs T. muris infection of mice in which MHC class II
and PAMPs
expression was restricted to DCs3,4,51 did not result in a
MHC TH2 cell response. Together, these results suggested
PRR
class II CD4 that MHC class II expression by DCs alone was neither
Peptide necessary nor sufficient for TH2 cell induction.
Signal 1: antigen- This suggested that another MHC class II‑expressing
specific interactions cell type might be required for TH2 cell induction in
TCR these models. Indeed, basophils were found to consti-
Signal 2: co-stimulatory tutively express MHC class II, CD80 and CD86, and the
molecules expression of these molecules was further upregulated
CD40L
CD40 by activation with IL‑3 (REF. 23) or papain3. Moreover,
Signal 3: instructive co‑culture of ovalbumin (OVA)-specific naive CD4+
cytokines
T cells with antigen-pulsed basophils alone was suffi-
IL-12 IL-12R cient to induce T cell proliferation and TH2 cell differen-
Activated DC CD4+ T cell
tiation in vitro3,4,23. Basophils could also present antigens
to T cells in vivo, as antigen-specific naive CD4+ T cells
displayed a TH2 cell phenotype in CIITA-deficient mice
CD40L, CD40 ligand; DAMP, damage-associated molecular pattern; IL‑12, interleukin‑12;
Nature
IL‑12R, IL‑12 receptor; PAMP, pathogen-associated molecular pattern; Reviews
PRR, | Immunology (which lack MHC class II expression) injected with
pattern recognition
receptor. peptide-pulsed basophils23. Basophils were found to
effectively incorporate soluble but not particulate anti-
gens by macropinocytosis3, which was further enhanced
have captured antigens may also act as reservoirs by antigen-specific IgE molecules bound to FcεRI
of antigen that can be presented to T cells by other on basophils. IgE-coated basophils efficiently incor-
APCs. Therefore, evidence from in vitro studies sup- porated specific antigens in vivo 52, and the addition
ports both direct and indirect roles for mast cells in of antigen-specific IgE molecules to CD4+ T cell and
antigen presentation. However, although some mast basophil co‑cultures augmented the induction of TH2
cells certainly express MHC class II when analysed cells23. However, it is unclear whether the augmented
directly ex vivo, whether they truly present antigen T H2 cell response mediated by IgE was owing to
in vivo remains to be determined. enhanced antigen presentation or increased IL‑4
722 | NOVEMBER 2014 | VOLUME 14 www.nature.com/reviews/immunol
© 2014 Macmillan Publishers Limited. All rights reserved
REVIEWS
Box 2 | A confounding role for exosomes?
TH2 cell phenotype ex vivo and required the addition of
basophils to the co‑cultures51. Thus, it was concluded that
Naive T cells are usually activated by T cell receptor (TCR)-dependent contact with although DCs present antigen to T cells, basophils were
peptide–MHC complexes on intact antigen-presenting cells (APCs). However, APCs required for TH2 cell polarization.
may also release antigen-presenting vesicles, or exosomes. Exosomes are secreted A similar caveat to these models lies in the basophil
membrane vesicles that form within late multivesicular endosomal compartments and
ablation methods that were used. In a house dust mite
are released into the environment following fusion of the multivesicular bodies with
the plasma membrane.
(HDM) allergen model, which induces a TH2 cell response
Exosomes can be secreted by numerous cell types including dendritic cells (DCs), in the lungs, different results were obtained when baso-
B cells, mast cells and microglial cells. They are also secreted by several different phils were depleted using different antibodies — namely,
tumour cell types. Intestinal epithelial cells (IECs) may also secrete MHC class II‑bearing MAR‑1 and Ba103 (REF. 24). Treatment with MAR‑1,
exosomes; putative exosomes carrying IEC-specific proteins have been identified by which is specific for FcεRI, had a stronger effect than
immunogold analysis of intestinal tissue sections155. Ba103 (which recognizes CD200R3) in attenuating
Immunologically active exosomes were first described by Thery and Amigorena156,157, TH2 cell polarization in HDM-challenged mice, although
who noted that exosomes derived from DCs contain immunologically relevant both antibodies reduced basophil numbers to an
proteins such as MHC class I, MHC class II and CD86, as well as the heat shock protein equivalent extent 24. The authors showed that in addi-
HSC73, which is involved in peptide delivery. Exosomes bearing peptide–MHC
tion to basophils, MAR‑1 also ablated a population of
complexes can stimulate naive T cells in vitro; however, Amigorena and his colleagues
have suggested that exosomes must be recaptured by endogenous DCs for antigen
FcεRI+ DCs, whereas Ba103 only depleted basophils.
presentation to naive T cells. Furthermore, basophils from HDM-challenged mice were
We discuss in the main text how mast cells may contribute to the immune response poor at presenting antigen ex vivo compared to FcεRI+
via the transfer of exosomes to DCs. Similarly, MHC class II‑loaded exosomes may be DCs isolated from the same lymph nodes, suggesting
secreted by IECs and act as sources of antigen for tissue-resident or migratory DCs158,159. that DCs mediated antigen presentation. This argument
Thus, the interpretation of many in vivo studies on the immunological functions of was supported by experiments showing that diphtheria
different cell types — including some types of DCs — is confounded by the possibility toxin treatment of Cd11c–DTR mice abrogated TH2 cell
that peptide–MHC complexes from non-conventional cells may simply be transferred responses upon HDM challenge24. Two other groups also
to DCs via exosomes for presentation to T cells. questioned the role of basophils in TH2 cell responses by
using novel mouse strains that constitutively lack baso-
phils owing to mast cell protease 8‑driven expression of
production by FcεRI-activated basophils. Together, these Cre recombinase58,59. Papain-induced TH2 cell responses
results suggested that basophils are a source of IL‑4 and were intact in these basophil-deficient mice but not in
may act as the primary APC in the activation of T cells DC‑deficient mice, suggesting that DCs but not baso-
in a variety of TH2‑type immune settings. phils had an important role in papain-induced TH2 cell
polarization58,59. These results are in contrast to the afore-
Different findings in different systems. More recently, the mentioned studies demonstrating an important role for
view that basophils might be the primary APC for TH2 cell basophils in papain-induced TH2 cell polarization3,42,53.
responses in vivo has been challenged by studies suggest- However, a more recent study targeted DTR expression
ing that previous results may have been confounded by to basophils under the control of regulatory elements in
the depletion methods that were used. First, the ablation the gene encoding IL‑4 and found that basophils were
of DCs in Cd11c–DTR mice — as opposed to in wild-type necessary for TH2 cell differentiation to peptide antigens,
chimeric mice reconstituted with Cd11c–DTR bone mar- but dispensable for priming to protein antigens60. It is
row — markedly reduced the papain-induced TH2 cell clear that the immunological effects of acute or consti-
response53. As a proportion of skin-resident migratory tutive depletion of basophils are quite dependent on the
DCs are radioresistant 54–57, diphtheria toxin treatment method of depletion and the biological setting.
Mixed lymphocyte of wild-type mice reconstituted with bone marrow from
responses Cd11c–DTR mice could have spared migratory DCs in Do human basophils show APC functions? Given
A tissue-culture technique
the skin that were sufficient to induce TH2 cell responses. the contradictory data generated in mouse systems,
for testing T cell reactivity.
The proliferation of one Similarly, Cd11c–Aβb mice lack MHC class II expression researchers soon began exploring the antigen-presenting
population of T cells — induced on migratory skin Langerhans cells and dermal DCs, and capabilities of human basophils. In humans, basophils
by exposure to inactivated this may confound the findings from studies using these comprise <1% of circulating granulocytes and they
MHC-mismatched stimulator mice51. Indeed, surgical excision of the injection site a few express FcεRI, CD203c (also known as ENPP3) and
cells — is determined by
measuring the incorporation
hours after antigen challenge also attenuated the TH2 cell CD123 (also known as IL‑3RA). Resting blood baso-
of 3H-thymidine into the response, suggesting that radioresistant skin DC popula- phils are HLA‑DR negative and early studies showed that
DNA of dividing cells. tions — either Langerhans cells or dermal DCs — might short-term activation with allergens, FcεRI engagement
be important51. As Langerhans cell depletion by express- or TLR2 ligands did not induce MHC class II expres-
Macropinocytosis
ing DTR under the control of the promoter of the gene sion61–63. However, subsequent studies suggested that
A type of endocytosis (or
phagocytosis) that occurs encoding langerin (also known as CD207) had no effect human basophils could express MHC class II in certain
during the engulfment of on TH2 cell induction, it was concluded that dermal DCs disease states (for example, in patients with lupus neph
apoptotic cells. During were responsible for antigen presentation in this set- ritis), in inflamed tissues that had high levels TH2‑type
macropinocytosis, large ting51. Dermal DCs incorporated the largest amount of cytokines64, and in response to culture with IL‑3 (REF. 65).
droplets of fluid are trapped
within the membrane
injected antigen and were the most potent at inducing However, even these cultured human basophils could
protrusions (ruffles) or T cell proliferation in ex vivo co‑culture experiments51. not present allergens61 or exogenous peptides to induce
phagocytic arms. However, DCs alone were unable to skew T cells to a the activation of human T cells, perhaps because they
NATURE REVIEWS | IMMUNOLOGY VOLUME 14 | NOVEMBER 2014 | 723
© 2014 Macmillan Publishers Limited. All rights reserved
REVIEWS
lacked expression of relevant co-stimulatory molecules65. obtained using mice adoptively transferred with antigen-
It remains possible that the bone marrow-derived and specific naive T cell receptor (TCR)-transgenic T cells,
tissue basophils that are purified from mice are not well suggesting that eosinophils could also activate naive
represented among the blood-derived human basophils T cells87. The activation of T cells by eosinophils seemed
that were used in these studies. Nonetheless, there is not to be MHC class II dependent, as the intraperitoneal
yet any compelling evidence that human basophils can injection of Strongyloides stercoralis antigen-loaded
present antigens to CD4+ T cells. wild-type eosinophils, but not MHC class II-deficient
eosinophils, resulted in the increased production of
Eosinophils as APCs TH2 cell-associated cytokines91.
Eosinophils are a population of circulating granulocytes However, some studies have argued that eosinophils
that have long been associated with TH2 cell responses. might be incapable of efficiently processing protein
They are elicited in response to IL‑5 production dur- antigens. Although human eosinophils expressed MHC
ing allergic inflammation and parasitic infections66–69, class II after IL‑3 stimulation in vitro and could stimulate
and they are equipped with an arsenal of cationic pro- antigen-specific primed T cells when pulsed with pep-
teins and inflammatory mediators with antihelminth tides, they were unable to do so when pulsed with whole
effector functions70. In addition to their effector role, antigen80. Similarly, whole protein-pulsed mouse eosino-
eosinophils may also be involved in the modula- phils could not stimulate antigen-specific naive T cells,
tion of TH2 cell immune responses. Similar to baso- although some proliferation of T cells was observed
phils, eosinophils have constitutive activity at the IL4 when the eosinophils were pulsed with peptides84.
locus38,40,71 and they are the major source of IL‑4 upon A recent study contradicted these findings and showed
challenge with Schistosoma mansoni eggs69. Moreover, that mouse eosinophils were in fact efficient APCs for
eosinophils release chemokines that are crucial for the naive antigen-specific T cells both in vitro and in vivo91.
recruitment of T cells to the lungs during allergic airway They attributed the previously reported lack of antigen-
hyperresponsiveness72–74. processing ability of eosinophils to the use of NH4Cl
Like mast cells and basophils, eosinophils can also to lyse red blood cells. In their experiments, eosino-
express MHC class II. Expression of MHC class II by phils were unable to activate antigen-specific T cells if
eosinophils was first reported in sputum and broncho they were exposed to NH4Cl, potentially owing to its
alveolar lavage samples from patients with asthma in the lysos omotropic activity. Although this method was
early 1990s75,76. Although freshly isolated eosinophils used by one of the previous studies84, red blood cells
from blood were devoid of MHC class II expression, its were removed by density gradients and centrifugation
expression could be induced following in vitro culture in the other 80. Alternatively, differences in the human
with activated T cell-conditioned media, GM‑CSF, IL‑3, and mouse systems may contribute to the discrepancies
or a combination of IL‑3 and IFNγ77–80. Similar observa- that were seen in the latter study.
tions have been made in mice, where airway or lymph Although some controversy still remains as to their
node eosinophils constitutively express MHC class II81–84. antigen-processing ability, many studies have convinc-
Although mouse eosinophils in the peritoneal cavity ingly demonstrated that MHC class II and co‑stimulatory
are MHC class II negative, expression of MHC class II molecules are expressed by airway-associated and lymph
is induced following the culture of these cells with node eosinophils, and by cytokine-activated circulating
GM‑CSF85–87. HLA‑DR in human eosinophils localizes to eosinophils77–84. Moreover, eosinophils localize to the
detergent-resistant lipid rafts that have been suggested same areas as classical APCs, as they migrate to T cell
to enhance antigen presentation by professional APCs88 zones in the lymph nodes during airway hypersensitivity
and, in both humans and mice, MHC class II‑expressing reactions81,87. Recently, mice that express DTR driven
eosinophils are capable of presenting superantigens, by eosinophilic peroxidase have been generated, but
peptides and protein antigens to T cell hybridomas and T cell-dependent responses in such mice have only been
T cell lines78,79,85,86,89. These data suggest that eosinophils analysed in an asthma model. At least in an allergic air-
have the potential to function as APCs. way disease model, eosinophil depletion had no effect
Evidence supporting a role of eosinophils in antigen during the antigen sensitization phase92, suggesting that
presentation in vivo came from studies demonstrating T cell responses are intact in the absence of eosinophils.
that eosinophils elicited by repeated airway antigen The generation of new methods to selectively ablate
challenge migrate from endobronchial areas to the MHC class II expression on eosinophils will be helpful
draining mediastinal lymph nodes81. These eosino- to further investigate the in vivo role of eosinophils as
phils were predominantly found in the T cell zones of APCs in other disease settings.
the draining lymph nodes and they formed clusters
with antigen-specific T cells87. Lymph node eosinophils APC functions of neutrophils
expressed MHC class II, CD80 and CD86 and could So far, we have examined the potential antigen-
restimulate memory T cells from antigen-challenged presenting functions of mast cells, basophils and eosino-
mice81,90. Moreover, intratracheal transfer of antigen- phils. However, neutrophils are the most abundant type
Superantigens pulsed eosinophils resulted in the enhanced prolifera- of granulocyte and are worth considering. Neutrophils
Proteins that bind to and
activate all T cells that express
tion of antigen-experienced CD4+ T cells in the draining are rapidly recruited to sites of tissue damage where they
a particular set of Vβ T cell lymph nodes, suggesting that eosinophils could induce extrude neutrophil extracellular traps (NETs), produce
receptor genes. antigen-specific T cell proliferation. Similar results were antimicrobial peptides, phagocytose microorganisms
724 | NOVEMBER 2014 | VOLUME 14 www.nature.com/reviews/immunol
© 2014 Macmillan Publishers Limited. All rights reserved
REVIEWS
and recruit other immune cells to clear pathogens. expression of MHC class II and could acquire, process
Thus, neutrophils are generally regarded as professional and present exogenous antigens in vitro but did not
phagocytes that are involved early in the response to induce proliferation of CD4+ T cells. It was suggested
tissue injury and infection. However, there is increas- that ILCs probably do not activate naive CD4+ T cells;
ing evidence that neutrophils may also modulate the nevertheless, cell-specific deletion of MHC class II
adaptive immune response through the production expression in retinoic acid receptor-related orphan
of chemokines and cytokines that recruit DCs to sites of receptor-γt (RORγt)-expressing ILC3s led to the dysreg-
inflammation93. Thioglycollate-elicited peritoneal neutro ulation of CD4+ T cells specific for commensal bacteria
phils in mice express CD80, but neither CD86 nor MHC and the loss of intestinal epithelial integrity 102. Thus,
class II94. However, co-culture with CD4+ T cells may lead these authors proposed that MHC class II expression
to a minimal level of cell-surface MHC class II expres- on ILCs contributed to intestinal homeostasis by coun-
sion and the ability to process OVA protein and activate tering professional APCs to prevent the inappropriate
OVA-specific CD4+ T cells94. Similarly, neutrophils puri- activation of commensal-specific CD4+ T cells. In con-
fied from the colons of colitic mice were MHC class II+ trast with these initial observations, a second laboratory
and, when pulsed with OVA peptide, could also activate examined the function of splenic ILC3s from the same
CD4+ T cells. Human neutrophils could express HLA‑DR strain of mice lacking expression of MHC class II in
and could stimulate superantigen-dependent T cell ILC3s103. Interestingly, in a different animal facility, this
activation; however, they could not re‑activate tetanus strain of mice did not develop any intestinal pathology,
toxoid-specific T cells95. suggesting a crucial role for microbial exposure103. They
Despite the suggestion that neutrophils may be also reported that, in contrast to ‘tolerizing’ intestinal
professional APCs, the interpretation of these data is ILC3s, splenic ILC3s respond to IL‑1β by upregulating
quite complicated. First, although neutrophil prepara- both MHC class II and co-stimulatory molecules, and
tions in these studies are 95–98% pure, the presence thus contribute to CD4+ T cell proliferation. Perhaps
of only a few contaminating DCs in either the neutro- ILC3s localized to different environments have different
phil or T cell preparation could be sufficient to medi- effects on CD4+ T cell activation and differentiation.
ate DC‑driven T cell activation. Second, neutrophils In agreement with these observations, we recently
induced by Pseudomonas aeruginosa airway infection lack showed that CD4+ T cells regulate the number and func-
MHC class II expression, despite expressing CD80 and tion of ILC3s in the mouse intestinal lamina propria in
CD86 (REF. 96). Finally, it is notable that in many of these an MHC class II‑dependent manner104. Although we did
reports, MHC class II expression occurs in GM‑CSF-rich not show that this was directly regulated by MHC class II
environments. In this setting, these ‘differentiated’ neutro expression on the ILCs, we did find that the level of
phils could easily be confused with inflammatory TNF MHC class II expressed by ILC3s in the intestinal lamina
and iNOS producing (TIP)-DCs97 or a recently described propria was determined by the presence of functional
‘neutrophil–DC hybrid’ cell that shows characteristics of CD4+ T cells104. Thus, MHC class II‑expressing ILC3s
both cell types98. Thus, there is not compelling evidence may regulate and also be regulated by CD4+ T cells.
that neutrophils can function as true APCs. There may also be such cross-regulation between
ILC2s and TH2 cells. ILC2s were first characterized as
ILCs and CD4+ T cells as APCs cells that expressed MHC class II and inducible T cell co-
In recent years, there has been growing interest in ILCs, stimulator (ICOS)105. They are resident in the lungs and
which function as rapid sources of cytokines early dur- other mucosal tissues, and IL‑2 produced by TH2 cells
ing immune responses. ILCs lack rearranged antigen may enhance cytokine production by ILC2s106. More
receptors but otherwise resemble CD4+ T cells in their importantly, purified lung ILC2s were shown to present
developmental pathways, transcription factor profiles peptide to TCR-transgenic CD4+ T cells in vitro106; how-
and cytokine-producing patterns. Type 1 ILCs (ILC1s), ever, ILC2s could not present OVA protein in this study.
ILC2s and ILC3s produce TH1-, TH2- and TH17‑type A more recent examination found that peptide-loaded
cytokines, respectively. Recent evidence implicates ILCs MHC class II+ ILC2s induced TH2 cell differentiation
as crucial regulators of innate immunity and inflamma- in vitro; ILC2s could also internalize and process pro-
tion at barrier surfaces, including the skin, airways and tein antigens, but in vitro CD4+ T cell proliferation could
gastrointestinal tract (reviewed in REF. 99). not be detected107. Nonetheless, MHC class II+ ILCs con-
In addition to their early effects on innate immune tributed to the clearance of intestinal helminths107. The
responses, there is increasing evidence that ILCs may study of MHC class II‑dependent functions of ILC2s
directly interact with CD4+ T cells. One group reported and ILC3s is a rapidly evolving area and the data remain
that ILC3s can express CXC-chemokine receptor 5 contradictory. Importantly, the systems studied to date
Neutrophil extracellular
(CXCR5) and CC-chemokine receptor 7 (CCR7), and involve ablation of cell-specific MHC class II expression
traps
(NETs). A set of extracellular localize to secondary lymphoid organs where they may rather than directly assaying the APC function of ILC3s
fibres produced by activated control the maintenance of memory CD4+ T cells100,101. in the absence of antigen presentation by DCs.
neutrophils to ensnare More directly, genome-wide transcriptional profiling of Among other non‑B cell lymphocytes, CD4+ T cells
invading microorganisms. ILC3s showed that they express genes that encode MHC also have the ability to express MHC class II and pre-
NETs enhance neutrophil
killing of extracellular
class II structural components, as well as proteins that sent antigens to other CD4+ T cells. Although CD4+
pathogens, while minimizing are necessary for antigen presentation, including Ii and T cells from mice do not express MHC class II, human
damage to host cells. H2‑DM102. In this report, a subset of ILC3s had surface CD4+ T cells express HLA‑DR molecules upon TCR
NATURE REVIEWS | IMMUNOLOGY VOLUME 14 | NOVEMBER 2014 | 725
© 2014 Macmillan Publishers Limited. All rights reserved
REVIEWS
Lymph node
stromal cell
Endothelial cell
Intestinal epithelial cell
MHC
class II
Constitutive MHC class II Inducible MHC class II Inducible MHC class II
expression expression expression
Function of MHC class II expression unclear
but may have a role in tolerance induction
Figure 2 | Epithelial cells and stromal cells express MHC class II molecules. Vascular endothelial cells, multiple
types of epithelial cells (including intestinal and pulmonary epithelial cells) and lymph nodeNature
stromalReviews
cells can| Immunology
express
MHC class II molecules. Although these cells have been shown to activate CD4 T cells in vitro, their expression
+
of MHC class II molecules in vivo is thought to be more relevant for the induction and maintenance of immune tolerance.
activation108,109. Antigen presentation by CD4+ T cells (as reviewed in REF. 112) by concentrating antigens in the
seems to have a dominant tolerance-inducing effect on lymph nodes and directing DC trafficking. Additionally,
other CD4+ T cells. Peptide-loaded T cell clones can multiple groups have demonstrated that LNSCs ectopi-
activate each other to induce proliferation. However, cally express tissue-specific antigens, including transgene-
these T cells are defective in response to restimula- directed neoantigens and endogenous tyrosinase,
tion110, suggesting that antigen-presenting CD4+ T cells and that they can induce the deletion of self-reactive
induce anergy. As MHC class II molecules on CD4+ CD8+ T cells113–117. The mechanisms for the expression of
T cells are loaded with self peptides111, it has been pos- tissue-specific antigens are not completely clear. Thymic
tulated that activation by HLA‑DR molecules expressed medullary epithelial cells express autoimmune regulator
by CD4 + T cells may serve to limit self peptide- (AIRE), which is a transcriptional activator that promotes
reactive CD4+ T cells. The lack of MHC class II expres- the expression of tissue-specific antigens to regulate cen-
sion by mouse CD4+ T cells has hampered progress in tral deletional tolerance. The Anderson laboratory used
investigating the antigen-presenting role of CD4+ T cells. an Aire-driven transgenic reporter to identify a popula-
tion of lymph node cells that expressed AIRE and tissue-
Non-haematopoietic cells as APCs specific antigens117. Interestingly, these AIRE+ cells
Up to this point, we have focused on haematopoietic expressed low levels of CD45 and CD11c, and were radio-
cells — predominantly myeloid cells — that might act sensitive, suggesting that they were a haematopoietic pop-
as APCs. However, there is an extensive literature on the ulation that was distinct from other LNSCs117. It is not clear
antigen-presenting abilities of radioresistant endothelial if AIRE is expressed at significant levels in LNSCs, although
cells and epithelial cells in particular clinical settings, a related transcriptional regulator, deformed epidermal
especially autoimmunity and transplant tolerance (FIG. 2). autoregulatory factor 1 (DEAF1), may be114,115.
LNSCs could either be intrinsically tolerogenic cells
Lymph node stromal cells. The T cell–APC interactions or may simply express MHC class I in the absence of
that mediate T cell activation do not occur in free space co-stimulatory molecules and, as such, induce the dele-
but in tightly regulated anatomic regions of lymph nodes, tion of self-reactive CD8+ T cells113,114. However, the
which are defined by the presence of non-haematopoietic ImmGen consortium has shown that FRCs, LECs and
stromal cells of mesenchymal and endothelial origin. BECs can upregulate surface expression of MHC class II
LNSCs can be divided into subclasses on the basis of molecules under inflammatory or infectious condi-
their surface expression of the glycoproteins CD31 (also tions118. In this setting, it has been demonstrated that
known as PECAM1) and podoplanin (also known as peripheral AIRE+ cells, but not radioresistant LNSCs,
GP38), and their localization within lymph nodes. These can also mediate deletional tolerance of autoreactive
subclasses include fibroblastic reticular cells (FRCs), folli CD4+ T cells117. Importantly, there are no data suggest-
cular DCs (FDCs), lymphatic endothelial cells (LECs), ing that CD4+ T cell deletion induced by either LNSCs or
blood endothelial cells (BECs), pericytes and a small AIRE+ cells is mediated independently of DCs. Indeed,
proportion (<5%) of otherwise undefined stromal cells. a recent report suggested that LNSC-mediated deletion
For many years, it was presumed that the sole func- of CD4+ T cells is dependent on peptide–MHC class II
tion on non-FDC LNSCs was to provide the structural complexes that are acquired from DCs119. Thus, it is
‘backbone’ for secondary lymphoid organs. However, possible that these cells are similar to basophils in
the stroma clearly contributes to adaptive responses modulating antigen presentation by DCs.
726 | NOVEMBER 2014 | VOLUME 14 www.nature.com/reviews/immunol
© 2014 Macmillan Publishers Limited. All rights reserved
REVIEWS
Endothelial cells and epithelial cells. Both human and but not by IECs, could drive CD4+ T cell-dependent intes-
mouse vascular endothelial cells and tissue-resident tinal inflammation137. The requirement for MHC class II
epithelial cells can express MHC class I and class II was examined by selectively deleting expression of CIITA
molecules, and there is some evidence that interactions in non-haematopoietic cells, including IECs138. In these
with CD4+ T cells may be involved in some autoimmune mice, colitis induced by IL‑10 blockade was much more
diseases and in graft rejection. Vascular endothelial cells in severe than in co‑housed wild-type littermates; disease
human allografts can express both MHC class I and class II was attributed to increased numbers of local TH1 cells
molecules, and can present processed protein antigens to and an imbalance in the relative numbers of effector
T cell clones120,123,124. However, there is no evidence either T cells and TReg cells. Similar to investigations of IECs in
in vitro or in vivo that human endothelial cells can express the small intestine (the focus of most published work),
CD80 or CD86, which are necessary to stimulate naive it was also found that MHC class II+ colonic epithelial
alloreactive T cells120. Both rat and mouse allograft models cells could not stimulate naive CD4+ T cells in vitro;
suggest that DCs in the graft (‘passenger leukocytes’) are rather, they suppressed the activation of CD4+ T cells
necessary to initiate graft rejection121,122. However, memory by professional APCs138,139. The mechanism for this was
cells have decreased requirements for co-stimulation and not clear, although it was independent of transforming
one group has shown in xenograft models that human growth factor-β.
memory T cells — specifically the effector memory sub- In both mice and humans, as many as half of the MHC
set — can directly respond to graft vascular endothelium class II+ cells in the lungs are of non-haematopoietic
to mediate rejection123,124. origin140,141. Non-haematopoietic cells include vascular
Epithelial cells — including intestinal epithelial cells and alveolar epithelial cells. Most data suggest that adap-
(IECs), airway epithelial cells and keratinocytes — are tive immunity to respiratory pathogens is predominantly
uniquely positioned at the interface between the host mediated by haematopoietic APCs, including migra-
immune system and an environment teeming with anti- tory DCs and alveolar macrophages; however, there
gens, including pathogenic microorganisms and food are data suggesting that epithelial cells might modulate
antigens, as well as commensal microorganisms. Thus, these responses. For example, type II alveolar epithelial
decisions about whether to generate pro-inflammatory cells comprise only 4% of the epithelium140 but they are
or tolerizing responses must continuously be made at strategically located to respond to airborne pathogens,
mucosal surfaces. Similar to vascular endothelial cells, and they produce antimicrobial peptides and pro-
epithelial cells can express MHC class II and may be inflammatory mediators such as complement compo-
uniquely poised to regulate T cell responses to mucosal nents141,142. Type II alveolar epithelial cells in both mice
antigens. Most work has focused on IECs and lung and humans express MHC class II that can be upregulated
alveolar epithelial cells; however, we have previously by IFNγ and microbial stimulation143. Both endogenous
suggested that MHC class II expression restricted to neo-self antigens and exogenous antigens — for example,
keratinocytes could mediate autoimmune skin disease125. from Bacille Calmette–Guérin (BCG) — can be processed
Multiple reports suggest that both mouse and human and presented by type II alveolar epithelial cells143. MHC
ileal IECs constitutively express MHC class II molecules. class II expression on pulmonary non-haematopoietic
Do these cells have the machinery to present antigen? cells may contribute to decreased inflammation and
Much of the work suggesting that IECs can process and acceptance of orthotopic lung transplants144. In trans-
present antigen has been limited to in vitro studies using genic systems, type II alveolar epithelial cells can prime
cell lines or primary cells treated with IFNγ. These stud- antigen-specific CD4+ T cells. Interestingly, the T cell
ies do show that in the presence of IFNγ, the appropriate response is skewed towards differentiation of forkhead
machinery — including Ii, H2‑DM and proteases — box P3 (FOXP3)-expressing CD4+ T cells143. In this regard,
can be expressed by IECs in the small intestine and by type II alveolar epithelial cells may be similar to vascular
oesophageal epithelial cells126. Interestingly, IECs isolated endothelial cells. Thus, endothelial cells and epithelial cells
from patients with inflammatory bowel disease (IBD) may be additional cell types that express MHC class II and
or from animal models of IBD express higher levels of modulate the outcome of DC–CD4+ T cell interactions.
MHC class II127–129. Work using IEC-like cell lines has
shown that these cells can stimulate T cell hybridomas Concluding remarks
or clones in vitro130–135. However, the evidence that naive There are a large number of haematopoietic and
T cells can be stimulated by IECs in vivo is less compel- non-haematopoietic cell types that can express MHC
ling. Indeed, there is no clear evidence that IECs express class II molecules and present antigens to CD4+ T cells.
sufficient levels of the appropriate co-stimulatory However, MHC class II expression alone is not sufficient
molecules to activate naive CD4+ T cells127–129. for full APC function, as APCs need to be able to pro-
There are accumulating data suggesting that IECs may cess antigens, migrate to secondary lymphoid organs and
contribute to the differentiation of TReg cells and the main- express co‑stimulatory molecules. In this regard, pro-
tenance of tolerance. Neoantigen targeted to IECs induced fessional APCs, such as macrophages and B cells, could
the expansion of antigen-specific TReg cell populations potentially replace the function of DCs in certain situ-
in a manner that was independent of DC depletion136. ations. However, the non-professional APCs that have
A more recent in vivo study examining transgenic mice been discussed in this Review are unlikely to replace
in which MHC class II expression was restricted to either DCs, because there is little compelling data (if any) that
IECs or DCs showed that antigen presentation by DCs, these cell types are able to activate naive CD4+ T cells.
NATURE REVIEWS | IMMUNOLOGY VOLUME 14 | NOVEMBER 2014 | 727
© 2014 Macmillan Publishers Limited. All rights reserved
REVIEWS
Rather, we propose that these non-conventional APCs been implicated in the induction of TH2 cell responses,
modulate immune responses that have been initiated whereas CD4+ T cells, and stromal, endothelial and epi-
by DCs. This is especially true for non-haematopoietic thelial cells may contribute to tolerance. Studies in mice
cells that may mediate the deletion of autoreactive T cells clearly require careful analyses of the cell types that are
or may stimulate TReg cells. The requirement for addi- affected by manipulation. Similarly, increasing study
tional non‑DC APCs is quite context dependent — for of human tissues other than blood will be necessary to
example, basophils, mast cells and eosinophils have validate findings from mouse systems.
1. Lemos, M. P., Fan, L., Lo, D. & Laufer, T. M. 20. Kambayashi, T. et al. Inducible MHC class II 37. Arinobu, Y. et al. Developmental checkpoints of
CD8α+ and CD11b+ dendritic cell-restricted expression by mast cells supports effector and the basophil/mast cell lineages in adult murine
MHC class II controls Th1 CD4+ T cell immunity. regulatory T cell activation. J. Immunol. 182, hematopoiesis. Proc. Natl Acad. Sci. USA 102,
J. Immunol. 171, 5077–5084 (2003). 4686–4695 (2009). 18105–18110 (2005).
2. Lemos, M. P., Esquivel, F., Scott, P. & Laufer, T. M. This study shows that MHC class II expression on 38. Voehringer, D., Shinkai, K. & Locksley, R. M.
MHC class II expression restricted to CD8α+ and mast cells is induced by TLR agonists and IFNγ, Type 2 immunity reflects orchestrated recruitment
CD11b+ dendritic cells is sufficient for control of and can support the activation of effector of cells committed to IL‑4 production. Immunity 20,
Leishmania major. J. Exp. Med. 199, 725–730 T cells and TReg cells but not that of naive T cells. 267–277 (2004).
(2004). 21. Razin, E. et al. Interleukin 3: A differentiation and 39. Min, B. et al. Basophils produce IL‑4 and accumulate
3. Sokol, C. L. et al. Basophils function as antigen- growth factor for the mouse mast cell that contains in tissues after infection with a Th2‑inducing parasite.
presenting cells for an allergen-induced T helper chondroitin sulfate E proteoglycan. J. Immunol. 132, J. Exp. Med. 200, 507–517 (2004).
type 2 response. Nature Immunol. 10, 713–720 1479–1486 (1984). 40. Gessner, A., Mohrs, K. & Mohrs, M. Mast cells,
(2009). 22. Tkaczyk, C., Villa, I., Peronet, R., David, B. & basophils, and eosinophils acquire constitutive IL‑4
4. Perrigoue, J. G. et al. MHC class II‑dependent Mecheri, S. FcεRI-mediated antigen endocytosis turns and IL‑13 transcripts during lineage differentiation
basophil‑CD4+ T cell interactions promote TH2 interferon-gamma-treated mouse mast cells from that are sufficient for rapid cytokine production.
cytokine-dependent immunity. Nature Immunol. inefficient into potent antigen-presenting cells. J. Immunol. 174, 1063–1072 (2005).
10, 697–705 (2009). Immunology 97, 333–340 (1999). 41. Karasuyama, H., Mukai, K., Tsujimura, Y. & Obata, K.
5. Gregory, G. D., Robbie-Ryan, M., Secor, V. H., 23. Yoshimoto, T. et al. Basophils contribute to TH2‑IgE Newly discovered roles for basophils: a neglected
Sabatino, J. J. Jr & Brown, M. A. Mast cells are responses in vivo via IL‑4 production and presentation minority gains new respect. Nature Rev. Immunol.
required for optimal autoreactive T cell responses of peptide-MHC class II complexes to CD4+ T cells. 9, 9–13 (2009).
in a murine model of multiple sclerosis. Nature Immunol. 10, 706–712 (2009). 42. Sokol, C. L., Barton, G. M., Farr, A. G. & Medzhitov, R.
Eur. J. Immunol. 35, 3478–3486 (2005). References 3, 4 and 23 show that basophils A mechanism for the initiation of allergen-induced
6. Maurer, M. et al. Skin mast cells control T cell- express MHC class II and present antigens to T helper type 2 responses. Nature Immunol.
dependent host defense in Leishmania major T cells to promote TH2‑type responses. 9, 310–318 (2007).
infections. FASEB J. 20, 2460–2467 (2006). 24. Hammad, H. et al. Inflammatory dendritic cells—not 43. Tsujimura, Y. et al. Basophils play a pivotal role in
7. Frandji, P. et al. Antigen-dependent stimulation by basophils—are necessary and sufficient for induction immunoglobulin-G‑mediated but not immunoglobulin-
bone marrow-derived mast cells of MHC of Th2 immunity to inhaled house dust mite allergen. E‑mediated systemic anaphylaxis. Immunity 28,
class II‑restricted T cell hybridoma. J. Immunol. J. Exp. Med. 207, 2097–2111 (2010). 581–589 (2008).
151, 6318–6328 (1993). This study argues that FcεRI-expressing DCs 44. Le Gros, G., Ben-Sasson, S. Z., Seder, R.,
8. Fox, C. C., Jewell, S. D. & Whitacre, C. C. Rat and not basophils are responsible for antigen Finkelman, F. D. & Paul, W. E. Generation of
peritoneal mast cells present antigen to a PPD-specific presentation in response to HDM allergen. interleukin 4 (IL‑4)-producing cells in vivo and in vitro:
T cell line. Cell. Immunol. 158, 253–264 (1994). 25. Phythian-Adams, A. T. et al. CD11c depletion severely IL‑2 and IL‑4 are required for in vitro generation of
9. Dimitriadou, V. et al. Expression of functional major disrupts Th2 induction and development in vivo. IL‑4‑producing cells. J. Exp. Med. 172, 921–929
histocompatibility complex class II molecules on J. Exp. Med. 207, 2089–2096 (2010). (1990).
HMC‑1 human mast cells. J. Leukoc. Biol. 64, 26. Gong, J. et al. The antigen presentation function of 45. Swain, S. L., Weinberg, A. D., English, M. & Huston, G.
791–799 (1998). bone marrow-derived mast cells is spatiotemporally IL‑4 directs the development of Th2‑like helper
10. Poncet, P., Arock, M. & David, B. MHC restricted to a subset expressing high levels of cell effectors. J. Immunol. 145, 3796–3806 (1990).
class II‑dependent activation of CD4+ T cell surface FcεRI and MHC II. BMC Immunol. 11, 34 46. Else, K. J., Finkelman, F. D., Maliszewski, C. R. &
hybridomas by human mast cells through (2010). Grencis, R. K. Cytokine-mediated regulation of
superantigen presentation. J. Leukoc. Biol. 27. Gaudenzio, N. et al. Cell-cell cooperation at the chronic intestinal helminth infection. J. Exp. Med.
66, 105–112 (1999). T helper cell/mast cell immunological synapse. 179, 347–351 (1994).
11. Frandji, P. et al. Presentation of soluble antigens Blood 114, 4979–4988 (2009). 47. Cohn, L., Homer, R. J., Marinov, A., Rankin, J. &
by mast cells: upregulation by interleukin‑4 and 28. Ito, T. et al. Roles of PU.1 in monocyte- and mast cell- Bottomly, K. Induction of airway mucus production
granulocyte/macrophage colony-stimulating factor specific gene regulation: PU.1 transactivates CIITA pIV By T helper 2 (Th2) cells: a critical role for interleukin 4
and downregulation by interferon-γ. Cell. Immunol. in cooperation with IFN-γ. Int. Immunol. 21, 803–816 in cell recruitment but not mucus production.
163, 37–46 (1995). (2009). J. Exp. Med. 186, 1737–1747 (1997).
12. Frandji, P. et al. Exogenous and endogenous antigens 29. Lu, L. F. et al. Mast cells are essential intermediaries 48. Min, B. Th2 immunity: a step closer to completion.
are differentially presented by mast cells to CD4+ in regulatory T‑cell tolerance. Nature 442, 997–1002 Immunol. Cell Biol. 88, 235 (2010).
T lymphocytes. Eur. J. Immunol. 26, 2517–2528 (2006). 49. Hida, S., Tadachi, M., Saito, T. & Taki, S.
(1996). 30. Eller, K. et al. IL‑9 production by regulatory T cells Negative control of basophil expansion by IRF‑2
13. Skokos, D. et al. Mast cell-derived exosomes induce recruits mast cells that are essential for regulatory critical for the regulation of Th1/Th2 balance.
phenotypic and functional maturation of dendritic T cell-induced immune suppression. J Immunol. 186, Blood 106, 2011–2017 (2005).
cells and elicit specific immune responses in vivo. 83–91 (2011). 50. Oh, K., Shen, T., Le Gros, G. & Min, B. Induction of
J. Immunol. 170, 3037–3045 (2003). 31. Maurer, D. et al. Fcε receptor I on dendritic cells Th2 type immunity in a mouse system reveals a novel
14. Tkaczyk, C. et al. In vitro and in vivo delivers IgE-bound multivalent antigens into a immunoregulatory role of basophils. Blood 109,
immunostimulatory potential of bone marrow-derived cathepsin S‑dependent pathway of MHC class II 2921–2927 (2007).
mast cells on B- and T‑lymphocyte activation. presentation. J. Immunol. 161, 2731–2739 (1998). 51. Allenspach, E. J., Lemos, M. P., Porrett, P. M.,
J. Allergy Clin. Immunol. 105, 134–142 (2000). 32. Shin, J. S., Shelburne, C. P., Jin, C., LeFurgey, E. A. & Turka, L. A. & Laufer, T. M. Migratory and lymphoid-
15. Skokos, D. et al. Mast cell-dependent B and T Abraham, S. N. Harboring of particulate allergens resident dendritic cells cooperate to efficiently
lymphocyte activation is mediated by the secretion within secretory compartments by mast cells following prime naive CD4 T cells. Immunity 29, 795–806
of immunologically active exosomes. J. Immunol. IgE/FcεRI-lipid raft-mediated phagocytosis. (2008).
166, 868–876 (2001). J. Immunol. 177, 5791–5800 (2006). 52. Mack, M. et al. Identification of antigen-capturing
16. Raposo, G. et al. Accumulation of major 33. Tkaczyk, C. et al. Specific antigen targeting to surface cells as basophils. J. Immunol. 174, 735–741
histocompatibility complex class II molecules in IgE and IgG on mouse bone marrow-derived mast cells (2005).
mast cell secretory granules and their release upon enhances efficiency of antigen presentation. 53. Tang, H. et al. The T helper type 2 response to
degranulation. Mol. Biol. Cell 8, 2631–2645 Immunology 94, 318–324 (1998). cysteine proteases requires dendritic cell-basophil
(1997). 34. Dvorak, A. M., Newball, H. H., Dvorak, H. F. & cooperation via ROS-mediated signaling.
17. Nakae, S. et al. Mast cells enhance T cell activation: Lichtenstein, L. M. Antigen-induced IgE-mediated Nature Immunol. 11, 608–617 (2010).
importance of mast cell costimulatory molecules and degranulation of human basophils. Lab Invest. 43, This study argues that basophils are important
secreted TNF. J. Immunol. 176, 2238–2248 126–139 (1980). for papain-induced TH2 cell responses not for
(2006). 35. Ishizaka, T. & Ishizaka, K. Immunological events at the their APC function but for their effects on DCs.
18. Kambayashi, T. et al. Indirect involvement of allergen- surface of basophil granulocytes and mast cells which 54. Merad, M. et al. Langerhans cells renew in the skin
captured mast cells in antigen presentation. induce degranulation. Scand. J. Respir. Dis. Suppl. throughout life under steady-state conditions.
Blood 111, 1489–1496 (2008). 98, 13–22 (1977). Nature Immunol. 3, 1135–1141 (2002).
19. Nakano, N. et al. Notch signaling confers antigen- 36. Arinobu, Y., Iwasaki, H. & Akashi, K. Origin of 55. Bursch, L. S. et al. Identification of a novel population
presenting cell functions on mast cells. J. Allergy Clin. basophils and mast cells. Allergol Int. 58, 21–28 of Langerin+ dendritic cells. J. Exp. Med. 204,
Immunol. 123, 74–81.e1 (2009). (2009). 3147–3156 (2007).
728 | NOVEMBER 2014 | VOLUME 14 www.nature.com/reviews/immunol
© 2014 Macmillan Publishers Limited. All rights reserved
REVIEWS
56. Ginhoux, F. et al. Blood-derived dermal langerin+ 80. Celestin, J. et al. IL‑3 induces B7.2 (CD86) expression 102. Hepworth, M. R. et al. Innate lymphoid cells regulate
dendritic cells survey the skin in the steady state. and costimulatory activity in human eosinophils. CD4+ T‑cell responses to intestinal commensal
J. Exp. Med. 204, 3133–3146 (2007). J. Immunol. 167, 6097–6104 (2001). bacteria. Nature 498, 113–117 (2013).
57. Poulin, L. F. et al. The dermis contains langerin+ 81. Shi, H. Z., Humbles, A., Gerard, C., Jin, Z. & This is the first study to suggest that ILC3s in the
dendritic cells that develop and function Weller, P. F. Lymph node trafficking and antigen gut regulate CD4+ T cell responses to commensal
independently of epidermal Langerhans cells. presentation by endobronchial eosinophils. bacteria through MHC class II expression.
J. Exp. Med. 204, 3119–3131 (2007). J. Clin. Invest. 105, 945–953 (2000). 103. von Burg, N. et al. Activated group 3 innate lymphoid
58. Ohnmacht, C. et al. Basophils orchestrate chronic This is the first study to show that mouse cells promote T‑cell-mediated immune responses.
allergic dermatitis and protective immunity against eosinophils from antigen-sensitized airways express Proc. Natl Acad. Sci. USA 111, 12835–12840 (2014).
helminths. Immunity 33, 364–374 (2010). MHC class II and can support T cell activation. This study shows that ILC3s stimulated with IL‑1β
59. Sullivan, B. M. et al. Genetic analysis of basophil 82. Shi, H. Z. et al. Endobronchial eosinophils express MHC class II and co-stimulatory molecules,
function in vivo. Nature Immunol. 12, 527–535 (2011). preferentially stimulate T helper cell type 2 responses. and can stimulate naive CD4+ T cell activation.
60. Otsuka, A. et al. Basophils are required for the Allergy 59, 428–435 (2004). 104. Korn, L. L. et al. Conventional CD4+ T cells regulate
induction of Th2 immunity to haptens and peptide 83. Duez, C. et al. Migration and accumulation of IL‑22‑producing intestinal innate lymphoid cells.
antigens. Nature Commun. 4, 1739 (2013). eosinophils toward regional lymph nodes after airway Mucosal Immunol. 7, 1045–1057 (2014).
61. Eckl-Dorna, J. et al. Basophils are not the key allergen challenge. J. Allergy Clin. Immunol. 114, 105. Neill, D. R. et al. Nuocytes represent a new innate
antigen-presenting cells in allergic patients. 820–825 (2004). effector leukocyte that mediates type‑2 immunity.
Allergy 67, 601–608 (2012). 84. van Rijt, L. S. et al. Airway eosinophils accumulate in Nature 464, 1367–1370 (2010).
62. Kitzmuller, C. et al. Human blood basophils do not act the mediastinal lymph nodes but lack antigen- 106. Mirchandani, A. S. et al. Type 2 innate lymphoid cells
as antigen-presenting cells for the major birch pollen presenting potential for naive T cells. J. Immunol. drive CD4+ Th2 cell responses. J. Immunol. 192,
allergen Bet v 1. Allergy 67, 593–600 (2012). 171, 3372–3378 (2003). 2442–2448 (2014).
References 61 and 62 were the first studies to 85. Tamura, N. et al. Requirement of CD80 and CD86 107. Oliphant, C. J. et al. MHCII-mediated dialog between
propose that basophils might not act as APCs in molecules for antigen presentation by eosinophils. group 2 innate lymphoid cells and CD4+ T cells
the setting of human allergy. Scand. J. Immunol. 44, 229–238 (1996). potentiates type 2 immunity and promotes parasitic
63. Sharma, M. et al. Circulating human basophils lack 86. Del Pozo, V. et al. Eosinophil as antigen-presenting helminth expulsion. Immunity 41, 283–295 (2014).
the features of professional antigen presenting cells. cell: activation of T cell clones and T cell hybridoma by This is the first study to show that ILC2s express
Sci. Rep. 3, 1188 (2013). eosinophils after antigen processing. Eur. J. Immunol. MHC class II and co‑stimulatory molecules, and can
64. Charles, N., Hardwick, D., Daugas, E., Illei, G. G. & 22, 1919–1925 (1992). support peptide-mediated naive CD4+ T cell
Rivera, J. Basophils and the T helper 2 environment 87. Wang, H. B., Ghiran, I., Matthaei, K. & Weller, P. F. activation.
can promote the development of lupus nephritis. Airway eosinophils: allergic inflammation recruited 108. Ko, H. S., Fu, S. M., Winchester, R. J., Yu, D. T. &
Nature Med. 16, 701–707 (2010). professional antigen-presenting cells. J. Immunol. Kunkel, H. G. Ia determinants on stimulated human
65. Voskamp, A. L., Prickett, S. R., Mackay, F., 179, 7585–7592 (2007). T lymphocytes. Occurrence on mitogen- and antigen-
Rolland, J. M. & O’Hehir, R. E. MHC class II 88. Akuthota, P., Melo, R. C., Spencer, L. A. & activated T cells. J. Exp. Med. 150, 246–255 (1979).
expression in human basophils: induction and lack of Weller, P. F. M. H. C. Class II and CD9 in human 109. Evans, R. L. et al. Peripheral human T cells sensitized
functional significance. PLoS ONE 8, e81777 (2013). eosinophils localize to detergent-resistant membrane in mixed leukocyte culture synthesize and express
66. Foster, P. S., Hogan, S. P., Ramsay, A. J., microdomains. Am. J. Respir. Cell. Mol. Biol. 46, Ia‑like antigens. J. Exp. Med. 148, 1440–1445 (1978).
Matthaei, K. I. & Young, I. G. Interleukin 5 deficiency 188–195 (2012). 110. LaSalle, J. M., Tolentino, P. J., Freeman, G. J.,
abolishes eosinophilia, airways hyperreactivity, and 89. Handzel, Z. T. et al. Eosinophils bind rhinovirus and Nadler, L. M. & Hafler, D. A. Early signaling defects
lung damage in a mouse asthma model. J. Exp. Med. activate virus-specific T cells. J. Immunol. 160, in human T cells anergized by T cell presentation of
183, 195–201 (1996). 1279–1284 (1998). autoantigen. J. Exp. Med. 176, 177–186 (1992).
67. Collins, P. D., Marleau, S., Griffiths-Johnson, D. A., 90. MacKenzie, J. R., Mattes, J., Dent, L. A. & Foster, P. S. This is the first study to fully characterize the
Jose, P. J. & Williams, T. J. Cooperation between Eosinophils promote allergic disease of the lung by potential of human CD4+ T cells to anergize other
interleukin‑5 and the chemokine eotaxin to induce regulating CD4+ Th2 lymphocyte function. CD4+ T cells through antigen presentation.
eosinophil accumulation in vivo. J. Exp. Med. 182, J. Immunol. 167, 3146–3155 (2001). 111. Costantino, C. M., Spooner, E., Ploegh, H. L. &
1169–1174 (1995). 91. Padigel, U. M. et al. Eosinophils act as antigen- Hafler, D. A. Class, I. I. MHC self-antigen presentation
68. Hogan, S. P., Koskinen, A. & Foster, P. S. Interleukin‑5 presenting cells to induce immunity to Strongyloides in human B and T lymphocytes. PLoS ONE 7, e29805
and eosinophils induce airway damage and bronchial stercoralis in mice. J. Infect. Dis. 196, 1844–1851 (2012).
hyperreactivity during allergic airway inflammation in (2007). 112. Card, C. M., Yu, S. S. & Swartz, M. A. Emerging roles
BALB/c mice. Immunol. Cell Biol. 75, 284–288 (1997). 92. Jacobsen, E. A. et al. Eosinophil activities modulate the of lymphatic endothelium in regulating adaptive
69. Sabin, E. A., Kopf, M. A. & Pearce, E. J. immune/inflammatory character of allergic respiratory immunity. J. Clin. Invest. 124, 943–952 (2014).
Schistosoma mansoni egg-induced early IL‑4 responses in mice. Allergy 69, 315–327 (2014). 113. Cohen, J. N. et al. Lymph node-resident lymphatic
production is dependent upon IL‑5 and eosinophils. 93. Denkers, E. Y., Butcher, B. A., Del Rio, L. & endothelial cells mediate peripheral tolerance via
J. Exp. Med. 184, 1871–1878 (1996). Bennouna, S. Neutrophils, dendritic cells and Aire-independent direct antigen presentation.
70. Hogan, S. P. et al. Eosinophils: biological properties Toxoplasma. Int. J. Parasitol. 34, 411–421 (2004). J. Exp. Med. 207, 681–688 (2010).
and role in health and disease. Clin. Exp. Allergy 38, 94. Abi Abdallah, D. S., Egan, C. E., Butcher, B. A. & 114. Fletcher, A. L. et al. Lymph node fibroblastic
709–750 (2008). Denkers, E. Y. Mouse neutrophils are professional reticular cells directly present peripheral tissue
71. Shinkai, K., Mohrs, M. & Locksley, R. M. Helper T cells antigen-presenting cells programmed to instruct Th1 antigen under steady-state and inflammatory
regulate type‑2 innate immunity in vivo. Nature 420, and Th17 T‑cell differentiation. Int. Immunol. 23, conditions. J. Exp. Med. 207, 689–697 (2010).
825–829 (2002). 317–326 (2011). 115. Lee, J. W. et al. Peripheral antigen display by lymph
72. Jacobsen, E. A. et al. Allergic pulmonary inflammation 95. Fanger, N. A. et al. Activation of human T cells by node stroma promotes T cell tolerance to intestinal
in mice is dependent on eosinophil-induced recruitment major histocompatability complex class II expressing self. Nature Immunol. 8, 181–190 (2007).
of effector T cells. J. Exp. Med. 205, 699–710 (2008). neutrophils: proliferation in the presence of 116. Tewalt, E. F. et al. Lymphatic endothelial cells induce
73. Walsh, E. R. et al. Strain-specific requirement for superantigen, but not tetanus toxoid. Blood 89, tolerance via PD‑L1 and lack of costimulation leading
eosinophils in the recruitment of T cells to the lung 4128–4135 (1997). to high-level PD‑1 expression on CD8 T cells.
during the development of allergic asthma. This study shows that human neutrophils can Blood 120, 4772–4782 (2012).
J. Exp. Med. 205, 1285–1292 (2008). express MHC class II after stimulation with GM‑CSF 117. Gardner, J. M. et al. Extrathymic Aire-expressing cells
74. Fulkerson, P. C. et al. A central regulatory role for and IFNγ, and can support superantigen-mediated are a distinct bone marrow-derived population that
eosinophils and the eotaxin/CCR3 axis in chronic but not peptide-mediated T cell activation. induce functional inactivation of CD4+ T cells.
experimental allergic airway inflammation. Proc. Natl 96. Yamamoto, S. et al. Cutting edge: Pseudomonas Immunity 39, 560–572 (2013).
Acad. Sci. USA 103, 16418–16423 (2006). aeruginosa abolishes established lung transplant This study suggests that some APCs termed LNSCs
75. Hansel, T. T. et al. Sputum eosinophils from asthmatics tolerance by stimulating B7 expression on neutrophils. are actually a bone marrow-derived population of
express ICAM‑1 and HLA‑DR. Clin. Exp. Immunol. 86, J. Immunol. 189, 4221–4225 (2012). cells that induce T cell anergy.
271–277 (1991). 97. Serbina, N. V., Salazar-Mather, T. P., Biron, C. A., 118. Malhotra, D. et al. Transcriptional profiling of stroma
This is the first study to show that sputum but Kuziel, W. A. & Pamer, E. G. TNF/iNOS-producing from inflamed and resting lymph nodes defines
not blood eosinophils from a large proportion dendritic cells mediate innate immune defense against immunological hallmarks. Nature Immunol. 13,
of patients with asthma express HLA‑DR. bacterial infection. Immunity 19, 59–70 (2003). 499–510 (2012).
76. Mengelers, H. J. et al. Immunophenotyping of 98. Matsushima, H. et al. Neutrophil differentiation into a This transcriptional analysis dissects which LNSCs
eosinophils recovered from blood and BAL of allergic unique hybrid population exhibiting dual phenotype can and do express MHC class II that is inducible
asthmatics. Am. J. Respir. Crit. Care Med. 149, and functionality of neutrophils and dendritic cells. by inflammatory signals.
345–351 (1994). Blood 121, 1677–1689 (2013). 119. Dubrot, J. et al. Lymph node stromal cells acquire
77. Beninati, W. et al. Pulmonary eosinophils express 99. Hepworth, M. R. & Sonnenberg, G. F. Regulation of peptide-MHCII complexes from dendritic cells and
HLA‑DR in chronic eosinophilic pneumonia. the adaptive immune system by innate lymphoid cells. induce antigen-specific CD4+ T cell tolerance.
J. Allergy Clin. Immunol. 92, 442–449 (1993). Curr. Opin. Immunol. 27C, 75–82 (2014). J. Exp. Med. 211, 1153–1166 (2014).
78. Hansel, T. T. et al. Induction and function of eosinophil 100. Withers, D. R. et al. Cutting edge: lymphoid tissue This paper suggests that LNSCs express
intercellular adhesion molecule‑1 and HLA‑DR. inducer cells maintain memory CD4 T cells within endogenous MHC class II molecules and can also
J. Immunol. 149, 2130–2136 (1992). secondary lymphoid tissue. J. Immunol. 189, acquire peptide–MHC class II complexes from DCs.
79. Mawhorter, S. D., Kazura, J. W. & Boom, W. H. 2094–2098 (2012). 120. Hughes, C. C., Savage, C. O. & Pober, J. S.
Human eosinophils as antigen-presenting cells: 101. Lane, P. J., Gaspal, F. M., McConnell, F. M., Endothelial cells augment T cell interleukin 2
relative efficiency for superantigen- and antigen- Withers, D. R. & Anderson, G. Lymphoid tissue inducer production by a contact-dependent mechanism
induced CD4+ T‑cell proliferation. Immunology 81, cells: pivotal cells in the evolution of CD4 immunity and involving CD2/LFA‑3 interaction. J. Exp. Med. 171,
584–591 (1994). tolerance? Front. Immunol. 3, 24 (2012). 1453–1467 (1990).
NATURE REVIEWS | IMMUNOLOGY VOLUME 14 | NOVEMBER 2014 | 729
© 2014 Macmillan Publishers Limited. All rights reserved
REVIEWS
121. Lechler, R. I. & Batchelor, J. R. Restoration of 134. Hershberg, R. M. et al. Intestinal epithelial cells use 149. Zinkernagel, R. M. & Doherty, P. C. Restriction of
immunogenicity to passenger cell-depleted kidney two distinct pathways for HLA class II antigen in vitro T cell-mediated cytotoxicity in lymphocytic
allografts by the addition of donor strain dendritic processing. J. Clin. Invest. 100, 204–215 (1997). choriomeningitis within a syngeneic or semiallogeneic
cells. J. Exp. Med. 155, 31–41 (1982). 135. Hershberg, R. M. et al. Highly polarized HLA class II system. Nature 248, 701–702 (1974).
122. Lakkis, F. G., Arakelov, A., Konieczny, B. T. & Inoue, Y. antigen processing and presentation by human 150. Banchereau, J. & Steinman, R. M. Dendritic cells and
Immunologic ‘ignorance’ of vascularized organ intestinal epithelial cells. J. Clin. Invest. 102, the control of immunity. Nature 392, 245–252
transplants in the absence of secondary lymphoid 792–803 (1998). (1998).
tissue. Nature Med. 6, 686–688 (2000). 136. Westendorf, A. M. et al. CD4+Foxp3+ regulatory T cell 151. Mosier, D. E. A requirement for two cell types
123. Shiao, S. L., McNiff, J. M. & Pober, J. S. expansion induced by antigen-driven interaction with for antibody formation in vitro. Science 158,
Memory T cells and their costimulators in human intestinal epithelial cells independent of local dendritic 1573–1575 (1967).
allograft injury. J. Immunol. 175, 4886–4896 (2005). cells. Gut 58, 211–219 (2009). 152. Steinman, R. M. & Cohn, Z. A. Identification of a
124. Shiao, S. L. et al. Human effector memory CD4+ T cells 137. Maggio-Price, L. et al. Lineage targeted MHC‑II novel cell type in peripheral lymphoid organs of mice.
directly recognize allogeneic endothelial cells in vitro transgenic mice demonstrate the role of dendritic cells I. Morphology, quantitation, tissue distribution.
and in vivo. J. Immunol. 179, 4397–4404 (2007). in bacterial-driven colitis. Inflamm. Bowel Dis. 19, J. Exp. Med. 137, 1142–1162 (1973).
References 123 and 124 are two of many articles 174–184 (2013). 153. Steinman, R. M. & Witmer, M. D. Lymphoid dendritic
from the Pober laboratory examining the function 138. Thelemann, C. et al. Interferon-γ induces expression cells are potent stimulators of the primary mixed
of MHC class II+ endothelial cells as APCs during of MHC class II on intestinal epithelial cells and leukocyte reaction in mice. Proc. Natl Acad. Sci. USA
graft acceptance or rejection. Many of the articles protects mice from colitis. PLoS ONE 9, e86844 75, 5132–5136 (1978).
from this laboratory examine the mechanisms for (2014). 154. Blum, J. S., Wearsch, P. A. & Cresswell, P.
stimulating memory but not naive CD4+ T cells. 139. Cruickshank, S. M., McVay, L. D., Baumgart, D. C., Pathways of antigen processing. Annu. Rev. Immunol.
125. Fan, L. et al. Antigen presentation by keratinocytes Felsburg, P. J. & Carding, S. R. Colonic epithelial cell 31, 443–473 (2013).
directs autoimmune skin disease. Proc. Natl Acad. Sci. mediated suppression of CD4 T cell activation. 155. Van Niel, G. et al. Intestinal epithelial exosomes
USA 100, 3386–3391 (2003). Gut 53, 678–684 (2004). carry MHC class II/peptides able to inform the
126. Mulder, D. J. et al. Antigen presentation and MHC 140. Ward, H. E. & Nicholas, T. E. Alveolar type I and immune system in mice. Gut 52, 1690–1697
class II expression by human esophageal epithelial type II cells. Aust. N. Z. J. Med. 14, 731–734 (2003).
cells: role in eosinophilic esophagitis. Am. J. Pathol. (1984). This study suggests that IECs may generate
178, 744–753 (2011). 141. Fehrenbach, H. Alveolar epithelial type II cell: immunologically active exosomes.
127. Sanderson, I. R., Ouellette, A. J., Carter, E. A., defender of the alveolus revisited. Respir. Res. 156. Thery, C. et al. Indirect activation of naive CD4+ T cells
Walker, W. A. & Harmatz, P. R. Differential 2, 33–46 (2001). by dendritic cell-derived exosomes. Nature Immunol.
regulation of B7 mRNA in enterocytes and 142. Strunk, R. C., Eidlen, D. M. & Mason, R. J. 3, 1156–1162 (2002).
lymphoid cells. Immunology 79, 434–438 (1993). Pulmonary alveolar type II epithelial cells synthesize This is one of the first articles demonstrating
128. Framson, P. E., Cho, D. H., Lee, L. Y. & and secrete proteins of the classical and alternative that exosomes may contribute to CD4+ T cell
Hershberg, R. M. Polarized expression and function of complement pathways. J. Clin. Invest. 81, activation.
the costimulatory molecule CD58 on human intestinal 1419–1426 (1988). 157. Thery, C. et al. Molecular characterization of dendritic
epithelial cells. Gastroenterology 116, 1054–1062 143. Gereke, M., Jung, S., Buer, J. & Bruder, D. cell-derived exosomes. Selective accumulation of the
(1999). Alveolar type II epithelial cells present antigen to heat shock protein Hsc73. J. Cell Biol. 147, 599–610
129. Nakazawa, A. et al. Functional expression of CD4+ T cells and induce Foxp3+ regulatory T cells. (1999).
costimulatory molecule CD86 on epithelial cells in Am. J. Respir. Crit. Care Med. 179, 344–355 158. Mallegol, J., van Niel, G. & Heyman, M. Phenotypic
the inflamed colonic mucosa. Gastroenterology 117, (2009). and functional characterization of intestinal epithelial
536–545 (1999). 144. Kreisel, D. et al. Cutting edge: MHC class II exosomes. Blood Cells Mol. Dis. 35, 11–16 (2005).
130. Kaiserlian, D., Vidal, K. & Revillard, J. P. Murine expression by pulmonary nonhematopoietic cells 159. Mallegol, J. et al. T84‑intestinal epithelial exosomes
enterocytes can present soluble antigen to specific plays a critical role in controlling local inflammatory bear MHC class II/peptide complexes potentiating
class II‑restricted CD4+ T cells. Eur. J. Immunol. 19, responses. J. Immunol. 185, 3809–3813 (2010). antigen presentation by dendritic cells.
1513–1516 (1989). 145. Steinman, R. M. Dendritic cells: understanding Gastroenterology 132, 1866–1876 (2007).
131. Buning, J. et al. Antigen targeting to MHC immunogenicity. Eur. J. Immunol. 37, S53–S60
class II‑enriched late endosomes in colonic epithelial (2007). Acknowledgements
cells: trafficking of luminal antigens studied in vivo in 146. McDevitt, H. O. & Sela, M. Genetic control of the Work in the laboratory of T.M.L. is supported by a VA Merit
Crohn’s colitis patients. FASEB J. 20, 359–361 (2006). antibody response. I. Demonstration of determinant- Award. Research in the laboratory of T.K. is supported by fund‑
132. Bland, P. W. & Warren, L. G. Antigen presentation by specific differences in response to synthetic ing from the US National Institutes of Health and the American
epithelial cells of the rat small intestine. I. Kinetics, polypeptide antigens in two strains of inbred mice. Asthma Foundation.
antigen specificity and blocking by anti‑Ia antisera. J. Exp. Med. 122, 517–531 (1965).
Immunology 58, 1–7 (1986). 147. McDevitt, H. O. et al. Genetic control of the immune Competing interests statement
133. Mayer, L. & Shlien, R. Evidence for function of Ia response. Mapping of the IR‑1 locus. J. Exp. Med. The authors declare no competing interests.
molecules on gut epithelial cells in man. J. Exp. Med. 135, 1259–1278 (1972).
166, 1471–1483 (1987). 148. Grumet, F. C. & McDevitt, H. O. Genetic control of
References 130, 132 and 133 are among the first the immune response. Relationship between the DATABASES
manuscripts to demonstrate that IECs express immune response‑1 gene(s) and individual H-2 Immgen Consortium: http://www.immgen.org/
MHC class II and present protein antigen to CD4+ antigenic specificities. Transplantation 13, 171–173 ALL LINKS ARE ACTIVE IN THE ONLINE PDF
T cell hybridomas. (1972).
730 | NOVEMBER 2014 | VOLUME 14 www.nature.com/reviews/immunol
© 2014 Macmillan Publishers Limited. All rights reserved
Das könnte Ihnen auch gefallen
- Assighment 1 Antigen Presenting Cells (APCs)Dokument5 SeitenAssighment 1 Antigen Presenting Cells (APCs)irtaza abbasNoch keine Bewertungen
- Antigen PresentationDokument41 SeitenAntigen PresentationDavid lufafaNoch keine Bewertungen
- Antigen-Presenting CellsDokument9 SeitenAntigen-Presenting Cellsnur fitrianaNoch keine Bewertungen
- HLA and Antigen Presentation: Rania Bakry, MDDokument45 SeitenHLA and Antigen Presentation: Rania Bakry, MDRashed LabNoch keine Bewertungen
- Basic Immunology From The Dermatologicpointofviewbasicimmunology From The Dermatologic Point of ViewDokument84 SeitenBasic Immunology From The Dermatologicpointofviewbasicimmunology From The Dermatologic Point of ViewHendAbdelazizNoch keine Bewertungen
- 4 ImmunityDokument5 Seiten4 Immunityziadnasef186Noch keine Bewertungen
- Immune System - Why?Dokument39 SeitenImmune System - Why?Neria HaroehNoch keine Bewertungen
- Stevens (Science) Med TechDokument31 SeitenStevens (Science) Med TechnotsoninjaninjaNoch keine Bewertungen
- P Fihir Imun HLADokument32 SeitenP Fihir Imun HLAKantorCamat TaliwangNoch keine Bewertungen
- Abul Abbas Keynote Lecture PDFDokument33 SeitenAbul Abbas Keynote Lecture PDFVegan IstaNoch keine Bewertungen
- Cellular ComponentDokument10 SeitenCellular ComponentHeran TeferiNoch keine Bewertungen
- Imunology Review Mention Two Component of The Acquired Immune System!Dokument6 SeitenImunology Review Mention Two Component of The Acquired Immune System!Fatmala Umi MaisarahNoch keine Bewertungen
- HY ImmunologyDokument42 SeitenHY ImmunologyNick RajmaNoch keine Bewertungen
- Theoretical Basis For Immunological Specificity and MemoryDokument18 SeitenTheoretical Basis For Immunological Specificity and MemoryRakin Bin RaihanNoch keine Bewertungen
- DR - Irma-Dasar Imunologi Dan Reaksi HipersensitivitasDokument89 SeitenDR - Irma-Dasar Imunologi Dan Reaksi Hipersensitivitasvandhani79Noch keine Bewertungen
- Week 1 - Jan 27 Lesson Plan: Innate Immunity Adaptive ImmunityDokument40 SeitenWeek 1 - Jan 27 Lesson Plan: Innate Immunity Adaptive ImmunitychocobuniNoch keine Bewertungen
- MHC (Major Histocompatibility Complex)Dokument19 SeitenMHC (Major Histocompatibility Complex)Kayshu Grover100% (2)
- LymphocytesDokument6 SeitenLymphocytesZahraNoch keine Bewertungen
- Immunopathology: Normal Immune ResponseDokument13 SeitenImmunopathology: Normal Immune ResponseChinedu H. DuruNoch keine Bewertungen
- Adaptive ImmunityDokument34 SeitenAdaptive ImmunityMohamed Mido100% (1)
- L1b MHCDokument33 SeitenL1b MHCashutoshrath209Noch keine Bewertungen
- Immunology in Haematology (Part 2)Dokument55 SeitenImmunology in Haematology (Part 2)kiedd_04100% (4)
- NRIreviewpdfDokument15 SeitenNRIreviewpdfnur fitrianaNoch keine Bewertungen
- Lect 2 Molekul Pengenal AntigenDokument36 SeitenLect 2 Molekul Pengenal AntigenRidwan FebrianNoch keine Bewertungen
- General Microbiology and Immunology Competency-MI1.8: Prof Pradyot PrakashDokument40 SeitenGeneral Microbiology and Immunology Competency-MI1.8: Prof Pradyot PrakashKotha Chaitanya AbhiramNoch keine Bewertungen
- Abdullah Assighment APCsDokument5 SeitenAbdullah Assighment APCsirtaza abbasNoch keine Bewertungen
- Antigen Capture and PresentationDokument37 SeitenAntigen Capture and PresentationTutde SedanaNoch keine Bewertungen
- Understanding Cancer and Related TopicsDokument40 SeitenUnderstanding Cancer and Related TopicsSalsabilaNoch keine Bewertungen
- Células Dendríticas: Edgar Garavito, M.DDokument54 SeitenCélulas Dendríticas: Edgar Garavito, M.DGINA STEFANY GUATAQUI DELGADONoch keine Bewertungen
- Abdullah Assighment APCsDokument5 SeitenAbdullah Assighment APCsMuhammad AbdullahNoch keine Bewertungen
- Adaptive ImmunityDokument59 SeitenAdaptive ImmunityRowie WanawanNoch keine Bewertungen
- 11.2 Development of ImmunityDokument90 Seiten11.2 Development of ImmunityDaksha yashaNoch keine Bewertungen
- HAT HAS TO Happen TO Clear A Viral InfectionDokument57 SeitenHAT HAS TO Happen TO Clear A Viral InfectionStella Michelle XuNoch keine Bewertungen
- Full Download Basic Immunology Functions and Disorders of The Immune System 4th Edition Abbas Test BankDokument36 SeitenFull Download Basic Immunology Functions and Disorders of The Immune System 4th Edition Abbas Test Bankpasakazinum100% (34)
- Present Yourself!Dokument14 SeitenPresent Yourself!Christian David Hernandez SilvaNoch keine Bewertungen
- Introduction To ImmunologyDokument14 SeitenIntroduction To Immunologykaiyeol exoNoch keine Bewertungen
- Understanding The Immune SystemDokument40 SeitenUnderstanding The Immune Systemlovely858585Noch keine Bewertungen
- Immuno in For MaticsDokument50 SeitenImmuno in For MaticsAbraham Omar Espinoza CulupúNoch keine Bewertungen
- Dendritic Cells in VaccinesDokument35 SeitenDendritic Cells in VaccinesBruno RIvas SantiagoNoch keine Bewertungen
- Presented By:: Rehnuma K. GhoriDokument28 SeitenPresented By:: Rehnuma K. GhoriavaniNoch keine Bewertungen
- Antigen Processing and Presentation Poster: July 2016Dokument2 SeitenAntigen Processing and Presentation Poster: July 2016NOE HERMINIO VELAZQUEZ RECINOSNoch keine Bewertungen
- Immunology LectureDokument79 SeitenImmunology LectureLogical SolutionsNoch keine Bewertungen
- Antigen Presenting CellDokument26 SeitenAntigen Presenting CellSurja DasNoch keine Bewertungen
- Activity 28 - Adaptive ImmunityDokument6 SeitenActivity 28 - Adaptive ImmunityKaren Joy MagbanuaNoch keine Bewertungen
- Adaptive Immunity IDokument29 SeitenAdaptive Immunity Ixfxw5m5bczNoch keine Bewertungen
- Antigen Presenting Cells: Parbondeep SaikiaDokument20 SeitenAntigen Presenting Cells: Parbondeep SaikiaParbondeep SaikiaNoch keine Bewertungen
- 1 s2.0 S0165247822000645 MainDokument7 Seiten1 s2.0 S0165247822000645 Mainnatacatt13Noch keine Bewertungen
- Antigen Presenting Cell AssighmentDokument4 SeitenAntigen Presenting Cell Assighmentirtaza abbasNoch keine Bewertungen
- Transplant ImmunologyDokument57 SeitenTransplant ImmunologySANDEEP SINGHALNoch keine Bewertungen
- Immuno BiologyDokument111 SeitenImmuno BiologyAsyha KantifaNoch keine Bewertungen
- Class I MCHDokument1 SeiteClass I MCHLumaho Greg Mikael DulawanNoch keine Bewertungen
- NonClassical MHC Class I PDFDokument12 SeitenNonClassical MHC Class I PDFRobMarvinNoch keine Bewertungen
- Linfocitos B y T-DepuratedDokument18 SeitenLinfocitos B y T-DepuratedByron Emerson Gonzales GonzalesNoch keine Bewertungen
- Imunitas AdaptifDokument55 SeitenImunitas AdaptifjagoanpremiumNoch keine Bewertungen
- In The Name of Allah, Most Gracious, Most MercifulDokument24 SeitenIn The Name of Allah, Most Gracious, Most Mercifuljibril channelNoch keine Bewertungen
- Mast Cells Regulate CD4 T Cell Differentiation 2018 Journal of Allergy andDokument22 SeitenMast Cells Regulate CD4 T Cell Differentiation 2018 Journal of Allergy andLuisa FernandaNoch keine Bewertungen
- Clinical Immunology Lecture 6Dokument40 SeitenClinical Immunology Lecture 6jibril channelNoch keine Bewertungen
- Major Histocompatibility Complex - WikipediaDokument57 SeitenMajor Histocompatibility Complex - WikipediaPowell KitagwaNoch keine Bewertungen
- B Cells T Cells IgsDokument84 SeitenB Cells T Cells IgsKhatrinaNoch keine Bewertungen
- Thyroid Disease: Dr. Gusti Hariyadi Maulana, MSC, SPPD-KGH Internist-NephrologistDokument30 SeitenThyroid Disease: Dr. Gusti Hariyadi Maulana, MSC, SPPD-KGH Internist-NephrologistNurul HikmaNoch keine Bewertungen
- SpixDokument6 SeitenSpixAlienformoflifeNoch keine Bewertungen
- Anatomy and Physiology of ThyroidDokument23 SeitenAnatomy and Physiology of ThyroidPonnan DasaiyanNoch keine Bewertungen
- 1 Historical OverviewDokument3 Seiten1 Historical OverviewClair de LuneNoch keine Bewertungen
- Digestive SystemDokument7 SeitenDigestive SystemChristine Joyce S. CruzNoch keine Bewertungen
- Bio 266 Syllabus 2010Dokument2 SeitenBio 266 Syllabus 2010ndnplaya712Noch keine Bewertungen
- ROSELA, ERLAINE MARIE Blood-LabDokument4 SeitenROSELA, ERLAINE MARIE Blood-LabPadoNoch keine Bewertungen
- 1 Basic ConcMouth RehabilitatioDokument5 Seiten1 Basic ConcMouth RehabilitatioMohamed KudaihNoch keine Bewertungen
- Sino Biological Inc. 2013 Product CatalogDokument181 SeitenSino Biological Inc. 2013 Product CatalogMegan MckinneyNoch keine Bewertungen
- English Class 1Dokument9 SeitenEnglish Class 1Jose RamirezNoch keine Bewertungen
- Renal Embryology: Jason Ryan, MD, MPHDokument731 SeitenRenal Embryology: Jason Ryan, MD, MPHFateh BoulounisNoch keine Bewertungen
- Science 10 Q3 - M2Dokument14 SeitenScience 10 Q3 - M2Avha CortesNoch keine Bewertungen
- 11.6.domain Eukarya (Kingdom Animalia 4)Dokument32 Seiten11.6.domain Eukarya (Kingdom Animalia 4)ollie tikaNoch keine Bewertungen
- Ijavol 5no 3sep-Dec20161Dokument161 SeitenIjavol 5no 3sep-Dec20161Ayesha JadoonNoch keine Bewertungen
- Endocrine System NotesDokument6 SeitenEndocrine System NotesHannah Grace CorveraNoch keine Bewertungen
- Circulatory SystemDokument3 SeitenCirculatory SystemjehanNoch keine Bewertungen
- Blood Supply of Head and NeckDokument42 SeitenBlood Supply of Head and NeckhoneyNoch keine Bewertungen
- Hypersensitivity Type IIIDokument16 SeitenHypersensitivity Type IIIRenz CofrerosNoch keine Bewertungen
- Group 4 BladderDokument13 SeitenGroup 4 BladderRuly RamadanaNoch keine Bewertungen
- Papilloedema and Optic AtrophyDokument27 SeitenPapilloedema and Optic AtrophyRobert MontgomeryNoch keine Bewertungen
- Sanathana Sai Sanjeevini: ...... Healing FragrancesDokument25 SeitenSanathana Sai Sanjeevini: ...... Healing Fragrancesamane72Noch keine Bewertungen
- Classsification of Dentomaxillay AnomaliesDokument15 SeitenClasssification of Dentomaxillay AnomaliesAdelina MijaicheNoch keine Bewertungen
- CasePresWeilsDisease Schematic DiagramDokument56 SeitenCasePresWeilsDisease Schematic DiagramLea Mari JavierNoch keine Bewertungen
- Ackerman Proffit 1969Dokument12 SeitenAckerman Proffit 1969FelipeMaldo100% (1)
- Characteristics of NewbornDokument28 SeitenCharacteristics of NewbornChandu Raj100% (4)
- Ortho New MCQs - 16Dokument24 SeitenOrtho New MCQs - 16Chat GptNoch keine Bewertungen
- Blood Supply of Long BonesDokument4 SeitenBlood Supply of Long BonesmainehoonaNoch keine Bewertungen
- R E G U L A T I O N S: JohannesburgDokument24 SeitenR E G U L A T I O N S: JohannesburgdchunNoch keine Bewertungen
- PAR Scoring Sheet - 2017Dokument1 SeitePAR Scoring Sheet - 2017Assem AbdejawwadNoch keine Bewertungen
- Histology Worksheets All in OneDokument15 SeitenHistology Worksheets All in OneGabriel ParasNoch keine Bewertungen
- 10% Human: How Your Body's Microbes Hold the Key to Health and HappinessVon Everand10% Human: How Your Body's Microbes Hold the Key to Health and HappinessBewertung: 4 von 5 Sternen4/5 (33)
- Return of the God Hypothesis: Three Scientific Discoveries That Reveal the Mind Behind the UniverseVon EverandReturn of the God Hypothesis: Three Scientific Discoveries That Reveal the Mind Behind the UniverseBewertung: 4.5 von 5 Sternen4.5/5 (52)
- A Brief History of Intelligence: Evolution, AI, and the Five Breakthroughs That Made Our BrainsVon EverandA Brief History of Intelligence: Evolution, AI, and the Five Breakthroughs That Made Our BrainsBewertung: 4.5 von 5 Sternen4.5/5 (6)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisVon EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisBewertung: 3.5 von 5 Sternen3.5/5 (2)
- Why We Die: The New Science of Aging and the Quest for ImmortalityVon EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityBewertung: 4.5 von 5 Sternen4.5/5 (6)
- Who's in Charge?: Free Will and the Science of the BrainVon EverandWho's in Charge?: Free Will and the Science of the BrainBewertung: 4 von 5 Sternen4/5 (65)
- Gut: the new and revised Sunday Times bestsellerVon EverandGut: the new and revised Sunday Times bestsellerBewertung: 4 von 5 Sternen4/5 (393)
- Masterminds: Genius, DNA, and the Quest to Rewrite LifeVon EverandMasterminds: Genius, DNA, and the Quest to Rewrite LifeNoch keine Bewertungen
- Tales from Both Sides of the Brain: A Life in NeuroscienceVon EverandTales from Both Sides of the Brain: A Life in NeuroscienceBewertung: 3 von 5 Sternen3/5 (18)
- Undeniable: How Biology Confirms Our Intuition That Life Is DesignedVon EverandUndeniable: How Biology Confirms Our Intuition That Life Is DesignedBewertung: 4 von 5 Sternen4/5 (11)
- The Rise and Fall of the Dinosaurs: A New History of a Lost WorldVon EverandThe Rise and Fall of the Dinosaurs: A New History of a Lost WorldBewertung: 4 von 5 Sternen4/5 (597)
- Seven and a Half Lessons About the BrainVon EverandSeven and a Half Lessons About the BrainBewertung: 4 von 5 Sternen4/5 (111)
- A Series of Fortunate Events: Chance and the Making of the Planet, Life, and YouVon EverandA Series of Fortunate Events: Chance and the Making of the Planet, Life, and YouBewertung: 4.5 von 5 Sternen4.5/5 (62)
- Good Without God: What a Billion Nonreligious People Do BelieveVon EverandGood Without God: What a Billion Nonreligious People Do BelieveBewertung: 4 von 5 Sternen4/5 (66)
- The Other Side of Normal: How Biology Is Providing the Clues to Unlock the Secrets of Normal and Abnormal BehaviorVon EverandThe Other Side of Normal: How Biology Is Providing the Clues to Unlock the Secrets of Normal and Abnormal BehaviorNoch keine Bewertungen
- The Ancestor's Tale: A Pilgrimage to the Dawn of EvolutionVon EverandThe Ancestor's Tale: A Pilgrimage to the Dawn of EvolutionBewertung: 4 von 5 Sternen4/5 (812)
- Change Your Brain, Change Your Life (Before 25): Change Your Developing Mind for Real-World SuccessVon EverandChange Your Brain, Change Your Life (Before 25): Change Your Developing Mind for Real-World SuccessBewertung: 4 von 5 Sternen4/5 (18)
- All That Remains: A Renowned Forensic Scientist on Death, Mortality, and Solving CrimesVon EverandAll That Remains: A Renowned Forensic Scientist on Death, Mortality, and Solving CrimesBewertung: 4.5 von 5 Sternen4.5/5 (397)
- The Molecule of More: How a Single Chemical in Your Brain Drives Love, Sex, and Creativity--and Will Determine the Fate of the Human RaceVon EverandThe Molecule of More: How a Single Chemical in Your Brain Drives Love, Sex, and Creativity--and Will Determine the Fate of the Human RaceBewertung: 4.5 von 5 Sternen4.5/5 (517)
- Remnants of Ancient Life: The New Science of Old FossilsVon EverandRemnants of Ancient Life: The New Science of Old FossilsBewertung: 3 von 5 Sternen3/5 (3)
- Darwin's Doubt: The Explosive Origin of Animal Life and the Case for Intelligent DesignVon EverandDarwin's Doubt: The Explosive Origin of Animal Life and the Case for Intelligent DesignBewertung: 4 von 5 Sternen4/5 (19)
- Moral Tribes: Emotion, Reason, and the Gap Between Us and ThemVon EverandMoral Tribes: Emotion, Reason, and the Gap Between Us and ThemBewertung: 4.5 von 5 Sternen4.5/5 (116)
- Buddha's Brain: The Practical Neuroscience of Happiness, Love & WisdomVon EverandBuddha's Brain: The Practical Neuroscience of Happiness, Love & WisdomBewertung: 4 von 5 Sternen4/5 (216)
- The Consciousness Instinct: Unraveling the Mystery of How the Brain Makes the MindVon EverandThe Consciousness Instinct: Unraveling the Mystery of How the Brain Makes the MindBewertung: 4.5 von 5 Sternen4.5/5 (93)
- Civilized To Death: The Price of ProgressVon EverandCivilized To Death: The Price of ProgressBewertung: 4.5 von 5 Sternen4.5/5 (215)