Beruflich Dokumente
Kultur Dokumente
Jurnal Imnisasi PDF 6
Hochgeladen von
Anggi Nugi0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
4 Ansichten19 SeitenJurnal
Copyright
© © All Rights Reserved
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenJurnal
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
4 Ansichten19 SeitenJurnal Imnisasi PDF 6
Hochgeladen von
Anggi NugiJurnal
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 19
Clinical and Experimental Immunology ORIGINAL ARTICLE doi:10.1111/cei.
12634
Prime-boost vaccination strategy with bacillus Calmette–Gu ́erin
(BCG) and liposomized alpha-crystalline protein 1 reinvigorates BCG
potency
K. F. Siddiqui, M. Amir, N. Khan, G. Rama Krishna, J. A. Sheikh, K. Rajagopal1 and J. N. Agrewala Immunology
Laboratory, CSIR-Institute of Microbial Technology, Chandigarh, India
1Current address: CSIR-Central Food and Technology Research Institute, Mysore, India
Accepted for publication 25 March 2015 Correspondence: J. N. Agrewala, CSIR- Institute of Microbial Technology,
Chandigarh-160036, India. Email: javed@imtech.res.in
Summary
Bacillus Calmette–Gu ́erin (BCG) remains the only available and most widely administered vaccine against
Mycobacterium tuberculosis (Mtb), yet it fails to protect vaccinated individuals either from primary infection or
reactivation of latent tuberculosis (TB). Despite BCG’s variable efficacy against TB, the fact remains that BCG
imparts protection in children against the disease, indicating that BCG possesses a wide protective antigenic
repertoire. However, its failure to impart protection in adulthood can be linked to its failure to generate long-lived
memory response and elicitation of an inadequate immune response against latency-associated antigens. Therefore,
to improve the protective efficacy of BCG, a novel vaccination strategy is required. Consequently, in the present
study, we have exploited the vaccination potential of liposomized a-crystalline 1 (Acr1L), a latency-associated
antigen to induce enduring protective immunity against Mtb in BCG-primed animals. It is noteworthy that an
increase in the multi-functional [interferon (IFN)-ghi/tumour necrosis factor (TNF)-ahi] CD4 and CD8 T cells were
observed in BCG-primed and Acr1L-boosted (BCG-Acr1L) animals, compared to BCG alone. Further, substantial
expansion of both central memory (CD44hi/CD62Lhi) and effector memory (CD44hi/CD62Llo) populations of CD4
and CD8 T cells was noted. Importantly, BCG-Acr1L exhibited significantly better protection than BCG, as
evidenced by a reduction in the bacterial burden and histopathological data of the lungs. In essence, BCG-Acr1L
could be a potent future vaccination strategy to reinvigorate BCG potency.
Keywords: Acr1, BCG, CD4 cells, CD8 cells, prime boost, tuberculosis, vaccine
Introduction
Bacillus Calmette–Gu ́erin (BCG) is the only vaccine approved against tuberculosis (TB). Unfortunately, it fails to generate an
enduring memory T cell response against Mycobacterium tuberculosis (Mtb), as indicated by the fact that it protects childhood
but not the adult manifestation of the disease [1–3]. Further, it does not protect immu- nized individuals either from Mtb primary
infection or reactivation of latent infection [4–7]. This failure may be due to BCG being cleared by the host immunity before its
antigens could optimally prime the immune system to gen- erate an enduring memory T cell response against latency- associated
antigens. These antigens are crucial in imparting protection against Mtb [8,9]. Several proteins of Mtb are
expressed during latency; one such antigen is alpha- crystalline protein 1 (Acr1) (16 kDa antigen; HspX; a- crystallin1; Rv2031c)
[10–12]. This antigen is considered to be a potent vaccine candidate against dormant Mtb. Interestingly, Acr1 elicits higher
interferon (IFN)-g release in individuals with latent TB compared to those with active disease [8]. This signifies that Acr1 helps
in the mainte- nance of a disease-free state in such subjects, thus making Acr1 an attractive target for the development of vaccine
against TB [12]. Recently, substantial progress has been made in developing vaccines against TB [13]. However, most of the
vaccines are based on immunodominant anti- gens that are recognized during the early stages of Mtb infection [14–17]. In
addition, there are very few vaccine studies based on latency-associated antigens [9,18–20].
286 V
C
2015 Institute of Microbial Technology, Clinical and Experimental Immunology, 181: 286–296
Acr1 reinvigorates BCG potency
V C
A potentially successful vaccine should have the ability to induce and maintain long-term antigen-specific endur- ing memory
T cells, which should be expanded easily on re-exposure to the pathogen. Antigen-specific memory T cells express cytokines
which activate the cells of the immune system, thereby helping in rapid clearance of the invading bacterium and protecting the
host from subse- quent infections. Apart from the well-documented role of CD4 T cells, several reports have indicated the
protective role of CD8 T cells in TB [21–23]. The antigens delivered to antigen-presenting cells (APCs) normally undergo an
exogenous pathway for processing and presentation to CD4 T cells, but not to CD8 T cells. However, fusogenic liposomes
prepared from the yeast lipids can deliver anti- gen into the cytosol of APCs, leading to the generation of not only
antigen-specific CD4 but also CD8 T cells [24]. In addition, liposomes are not only effective adjuvants or vehicles to deliver
antigens, but can successfully evoke CD4 T helper type 1 (Th1) and Th2 immunity and enhance memory T cell responses
[25,26].
Following the above-mentioned approaches, we adopted a prime-boost vaccination strategy using BCG and liposomized-Acr1
(BCG-Acr1L) antigen to invigorate the protective efficacy of BCG against Mtb. BCG-Acr1L vaccination proved advantageous in
significantly improv- ing BCG potency, as evidenced by augmentation in the immune response and decline in the mycobacterial
bur- den in animals exposed to Mtb.
Materials and methods
Mice
Female C3H/HeN mice (6–8 weeks) were procured from the CSIR Institute of Microbial Technology (CSIR- IMTECH) animal
facility after approval from the Insti- tute’s Animal Ethics Committee. For immunization and infection experiments, animals were
housed in the Biosaf- ety level-3 facility of CSIR-IMTECH. Animals were offered commercial diet and water ad libitum.
Mycobacterial strains
Mtb (H37Rv) and M. bovis (BCG, Danish strain) were a kind gift from Dr V. M. Katoch, NJIL and OMD, Agra. Mycobacteria
were cultured in 7H9 medium containing 0405% Tween-80 and supplemented with 10% oleic albu- min dextrose catalase
(OADC).
Protection studies
Mice (six to eight per group) were primed subcutane- ously with BCG (1 3 106 CFU/animal) and 21 days later were administered
a booster dose of Acr1L (50 μg/mice) (BCG-Acr1L). Similarly, the control groups were primed with either Acr1L, Acr1 or BCG
and boosted with Acr1L
(Acr1L-Acr1L) or Acr1 (Acr1-Acr1) or Acr1 (BCG-Acr1), respectively. In addition, groups were kept inoculated with BCG and
placebo [phosphate-buffered saline (PBS)]. A dose of 50 μg/mouse free Acr1 or entrapped in lipo- somes was used. The animals
were rested for 160 days and then aerosol-challenged with a low dose of H37Rv using an inhalation exposure system (Glas-Col,
Terre Haute, IN, USA) to deposit approximately 100 live bacte- ria in the lungs [as checked by colony-forming unit (CFU)
plating after 24 h of exposure]. After 35 days, ani- mals were killed and lungs were harvested. Serially diluted lung homogenates
were plated on Middlebrook 7H11 medium supplemented with 2-thiophene carboxylic hydrazide (TCH, 2 μg/ml) and OADC.
Colonies were counted after 3–4 weeks of incubation at 378C.
Entrapment of Acr1 in liposomes (Acr1L)
The yeast lipids were isolated from Saccharomyces cerevisiae and liposomes were prepared as described earlier [27]. Briefly,
yeast lipids were reduced to thin dry film. The film was hydrated followed by sonication in a bath-type sonica- tor for 15–30 min.
The liposomes formed were mixed at this stage with an equal volume of Acr1. This mixture was flash- frozen, thawed and then
lyophilized. The lyophilized powder was reconstituted in PBS. It was washed a further three times with PBS to remove the traces
of the unentrapped sol- ute. The protein entrapped in the liposomes was estimated by lysing with 1% Triton X-100 solution
followed by the addition of bicinchoninic acid (BCA) reagent to lysed lipo- somes and then incubating at 378C for 45 min.
Finally, the absorbance was measured at 570 nm wavelength.
Isolation of lymphocytes from spleen and lungs
Mice immunized as indicated in protection studies were killed 35 days after aerosol Mtb challenge. Spleens and lymph nodes
were removed aseptically and a single-cell sus- pension was prepared. Red blood cells (RBCs) were lysed using an ACK lysis
buffer, washed three times with PBS and resuspended in complete medium [RPMI-1640110% fetal bovine serum (FBS)]. Viable
cells were counted using the trypan blue dye-exclusion method. Lung cells were prepared as described elsewhere [28]. Briefly,
the lungs were perfused through the right ventricle with chilled PBS. Once lungs became white, they were removed, chopped and
incubated with digestion mixture collagenase (047 mg/ml) and DNase (30 μg/ml) in media at 378C for 1 h. Digested tissues were
disrupted and passed through 70-μm pore size nylon cell strainers. The RBCs were lysed by ACK lysis buffer. The resultant
single-cell suspension was washed three times, resuspended in complete media and used for cultures.
Proliferation assays
Cell proliferation assays were set as described previously [28–30]. Briefly, lymphocytes (2 3 105 cells/well) isolated
2015 Institute of Microbial Technology, Clinical and Experimental Immunology, 181: 286–296 287
K. F. Siddiqui et al.
288 V
C
Immunoglobulin (Ig)G1 and IgG2a isotype ELISA
Serum samples were collected from Mtb aerosol- challenged mice after 35 days. Acr1-specific antibodies were determined in the
serum samples, as described else- where [28]. Briefly, diluted serum samples (3100) were added on Acr1 (10 μg/ml) precoated
plates. Acr1-specific IgG1 and IgG2a were detected using biotinylated anti- mouse IgG1 or IgG2a antibodies, followed by the
addi- tion of avidin-horseradish peroxidase (HRP). Colour was developed by adding o-phenylenediamine (OPD)-H
2
from spleen and lymph nodes were cultured in triplicate in 200 μl of complete RPMI-1640 10% FCS with optimal concentrations
of Acr1L, Acr1 and purified protein deriv- ative (PPD) in 96-well U-bottomed plates. After 72 h, the cells were pulsed with
[methyl-3H]-thymidine (045 μCi/ well) and plates were harvested 16 h later using the Tomtec-Harvester-96 (Tomtec, Hamden,
CT, USA). The incorporated radioactivity was measured using the Wallac 1450 Microbeta Trilux b-scintillation counter (Perkin
Elmer, Waltham, MA, USA).
Immunophenotyping
Cells were stimulated with Acr1, Acr1L and PPD, as described earlier, for the proliferation assay. After 48 h, cells were
harvested and incubated with Fc block and then stained for CD4, CD8, CD44, CD62L and their isotype-matched controls for 30
min on ice. After wash- ing three times, cells were fixed with paraformaldehyde and acquired using the fluorescence activated
cell sorter (FACS) Aria II cell sorter (BD Biosciences, San Jose, CA, USA). Data were analysed with Diva software (version
64142).
Cytokines enzyme-linked immunosorbent assay (ELISA)
Cultures were set as described for the proliferation assay. The supernatants (SNs) for interleukin (IL)-4 and IFN-g were collected
after 48 h. Cytokine levels were estimated by sandwich ELISA, as per the manufacturer’s instruc- tions, and the results were
expressed in pg/ml.
Intracellular staining
Lymphocytes (2 3 105 cells/ml) were cultured with Acr1, Acr1L (25 μg/ml) and PPD (25 μg/ml) in 96-well U-bot- tomed plates
for 48 h. Cells were pooled and washed three times with buffer (PBS–FBS 1%). Cells were restimulated with phorbol myristate
acetate (PMA) (50 ng/ml) and ionomycin (1 μg/ml) for 6 h and brefeldin A (10 μg/ml) was added in cultures for the last 4 h.
After stimulation, cells were washed three times with staining buffer [bovine serum albumin (BSA) 1%, NaN
3
O
2 and reaction was stopped using 7% H
2
SO
4
. Plates were read at 492 nm. The usual procedures of incubation and washing
were followed after each step. Results are expressed as the ratio of IgG2a to IgG1.
Histopathological analysis
Mice were killed and lung tissues were fixed in 10% buf- fered formalin. Histological sections were stained using haematoxylin
and eosin, as described elsewhere [28]. Pho- tomicrographs were captured on Olympus IX71 micro- scope at either 310 or 320
magnifications.
Results
Characterization of Acr1 antigen of Mtb entrapped in yeast liposomes
To elicit both CD4 and CD8 T cell responses, we entrapped Acr1 antigens of Mtb in liposomes (Acr1L), prepared from fusogenic
lipids isolated from yeast. The size of the lipo- somes was determined by differential light scattering (DLS) to be of an average
diameter of 22744 nm. Little difference was observed between the size of Acr1L and the empty lip- osomes. Further, we also
measured the poly disparity index and diffusion coefficient (Fig. 1a,b). The shape of the lipo- somes was spherical, as determined
by scanning electron microscopy (SEM); (Fig. 1c). The majority of the lipo- somes were much smaller at the edges of detection
under the magnification used. The transmission electron micro- scope (TEM) images indicated that the liposomes were spherical
and unilamellar (Fig. 1d,e). The entrapment effi- ciency was approximately 50%, as examined by protein estimation (data not
shown). Further, efficacy of entrap- ment was substantiated by fluorescence microscopy data using fluorescein isothiocyanate
(FITC)-labelled antigen (Fig. 1f,g). This preparation of Acr1L was used in the prime-boost vaccination study with BCG.
Immunization with BCG-Acr1L induces long-lasting memory Th1 response
The mice primed with BCG were boosted with liposom- ized Acr1 (BCG-Acr1L). The animals were rested for 160 days to
generate a bona fide memory T cell response.
0.01% in PBS]. Fc receptors were blocked and then stained with
fluorochrome-labelled anti-CD4 and CD8 antibodies. Cells were washed three times with staining buffer and fixed in 2%
paraformaldehyde. Cells were then permeabil- ized with buffer (0401% saponin PBS–FCS 1%). Further, cells were incubated
with fluorochrome-labelled anti-cyto- kine monoclonal antibodies (mAbs) (or isotype-matched control antibodies) in
permeabilization buffer. The incu- bation period for each step was 30 min at 48C, and after every incubation the usual washing
steps were followed. Later, cells were fixed in paraformaldehyde and acquired on a FACS Aria II, followed by data analysis
using FACS Diva (BD Biosciences, San Jose, CA, USA).
2015 Institute of Microbial Technology, Clinical and Experimental Immunology, 181: 286–296
Acr1 reinvigorates BCG potency
Fig. 1. Characterization of liposomes for shape, size and entrapment of alpha-crystalline protein 1 (Acr1) antigen. Liposomes
prepared from fusogenic yeast lipids were characterized by (a) intensity distribution curve indicating the size of empty liposomes
by differential light scattering (DLS); (b) intensity distribution curve of protein entrapped in the liposomes; (c) scanning electron
microscopy (SEM) images at magnification 20X; (d,e) transmission electron microscope (TEM) images of empty and protein
entrapped liposomes, respectively; (f,g) differential interference contrast (DIC) and fluorescence images of fluorescein
isothiocyanate (FITC)-tagged Acr1 entrapped in liposomes. Arrows indicate FITC-Acr1 entrapped in liposomes. The data
represented are of three independent experiments.
V C
2015 Institute of Microbial Technology, Clinical and Experimental Immunology, 181: 286–296 289
K. F. Siddiqui et al.
Fig. 2. Elicitation of immune responses after prime boost with bacillus Calmette–Gu ́erin (BCG)-liposomized alpha-crystalline
protein 1 (Acr1L). Mice (six to eight per group) were primed subcutaneously (s.c.) with BCG [1 3 106 colony-forming units
(CFU)/animal] and 21 days later were administered a booster dose of Acr1L (BCG-Acr1L). Similarly, the control groups were
primed with Acr1L and boosted with Acr1L (Acr1L- Acr1L) or Acr1-Acr1 or BCG-Acr1 or BCG-phosphate-buffered saline
(PBS) or PBS-PBS (placebo). Animals were rested for 160 days before aerosol challenge with Mycobacterium tuberculosis
(Mtb). Thirty-five days after challenge, mice were killed and in-vitro cell cultures were set. T cell proliferation was studied after
in-vitro stimulation of cultures with (a) purified protein derivative (PPD) or (b) Acr1L. The proliferation was measured by
[methyl-3H]-thymidine incorporation. The results are expressed as a stimulation index (SI), calculated by dividing counts per
minute (cpm) of antigen-stimulated cultures with unstimulated cells. Interferon (IFN)-g was estimated by enzyme-linked
immunosorbent assay (ELISA) in the supernatants (SNs) of the cells cultured for 48 h in the presence of (c) Acr1L or (d) PPD.
The data are expressed as pg/ml; (e) Acr1-specific immunoglobulin (Ig)G1 and IgG2a isotypes were detected in the serum and
expressed as ratio of IgG2a/IgG1. Data represented as mean6standard error of the mean (s.e.m.) are of two independent
experiments, with six to eight mice per group. Statistical analysis was performed by Tukey–Kramer multiple comparison tests. *P
<0405; **P <0401; ***P< 04001.
290 V
C
2015 Institute of Microbial Technology, Clinical and Experimental Immunology, 181: 286–296
Acr1 reinvigorates BCG potency
Fig. 3. Vaccination with bacillus Calmette–Gu ́erin (BCG)-liposomized alpha-crystalline protein 1 (Acr1L) induces enduring
memory CD4 and CD8 T cells. Lymphocytes were obtained from BCG-Acr1L-administered mice, which were challenged with
Mycobacterium tuberculosis (Mtb). The lymphocytes were isolated from spleens and lymph nodes and cultured with purified
protein derivative (PPD) and Acr1L. After 48 h, cells were harvested, stained for the expression of memory markers CD44 and
CD62L and analysed by flow cytometry on (a) CD4 T cells and (b) CD8 T cells. (c) Lymphocytes isolated from the lungs were
stimulated with PPD and stained for memory T cell markers CD44 and CD62L. Numbers in the inset indicate percentage of cells
expressing CD44hi/CD62Lhi. Data are representative of two independent experiments with six to seven mice in each group.
[Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Later, the animals were sacrificed and the Mtb-specific T cell response was monitored. Compared to BCG, BCG- Acr1L
exhibited significantly better T cell proliferation on in-vitro priming cells with either PPD (P < 04001) or Acr1 (P < 0401) (Fig.
2a,b). Control groups immunized with placebo (PBS) or Acr1 alone failed to improve the T cell recall response (Fig. 2a). Among
the subsets of CD4 T cells, Th1 cells play an important role in the protection against Mtb infection. Therefore, we monitored the
release of IFN-g, a Th1 cytokine. A significantly (P < 04001) higher production of IFN-g was observed in BCG-
Acr1L-vaccinated animals compared to BCG alone on in- vitro exposure of cells with either Acr1L or PPD (Fig. 2c,d). The
IFN-g release was comparatively higher in cul- tures exposed in vitro to Acr1L than controls. It has been reported that Acr1 and
its epitopes predominantly indu- ces secretion of IFN-g [12,31–33]. This was suggestive of
V C
2015 Institute of Microbial Technology, Clinical and Experimental Immunology, 181: 286–296 291
the fact that BCG-Acr1L immunization induced a robust Th1 memory response.
It has been well established that when B cells interact with Th1 cells they produce mainly IgG2a, while Th2 cells secrete
primarily IgG1. The significant (P < 04001) increase in the ratio of Acr1-specific IgG2a/IgG1 further substantiated the
predominance of Th1 cells upon BCG-Acr1L immunization (Fig. 2e). The proliferation data for cytokine secretion sig- nify that
vaccination with BCG-Acr1L significantly evokes long-lasting (160 days) Th1 immunity against Mtb.
BCG-Acr1L induces enduring CD4 and CD8 T cell memory response
Generation of long-lasting memory T cell response is a hallmark of a successful vaccine. Compared to BCG, BCG-Acr1L
considerably expanded the pool of both
K. F. Siddiqui et al.
Fig. 4. Bacillus Calmette–Gu ́erin (BCG)-liposomized alpha-crystalline protein 1 (Acr1L) vaccination induces multi-functional
T helper type 1 (Th1) cells. Lymphocytes were isolated from the lungs of animals immunized with BCG-Acr1L, BCG-Acr1,
Acr1L, Acr1, BCG and phosphate- buffered saline (PBS), which were later challenged with Mycobacterium tuberculosis (Mtb).
The cells were cultured with purified protein derivative (PPD) for 48 h. Later, supernatants (SNs) were harvested and
enzyme-linked immunosorbent assay (ELISA) for the estimation of (a) interferon (IFN)-g and (b) interleukin (IL)-4. Data
expressed as pg/ml in bar diagrams were analysed with the Tukey–Kramer test. **P < 0401; ***P <04001. Intracellular
expression of tumour necrosis factor (TNF)-a and IFN-g was monitored by flow cytometry in (c,e) CD4 T cells and (d,f) CD8 T
cells in (c,d) lung cells; (e,f) splenocytes. Figures in the contour plots indicate percentage of IFN-g and TNF-a-expressing T cells.
The results are representative of two independent experiments with six to eight mice per group. [Color figure can be viewed in
the online issue, which is available at wileyonlinelibrary.com.]
central memory (CD44hi/CD62Lhi) (BCG versus BCG- Acr1L: 18% versus 31% and 28 versus 35%, when chal- lenged in vitro
with PPD and Acr1L, respectively) and effector memory (CD44hi/CD62Llo) (BCG versus BCG- Acr1L: 10 versus 16% and 10
versus 15%, when challenged in vitro with PPD and Acr1L, respectively) CD4 T cells (Fig. 3a). Importantly, in-vitro challenge
of the cells with Acr1L showed better expansion of central memory pool of CD4 T cells than PPD. A similar trend was apparent
in the case of effector memory CD8 T cells (Fig. 3b). Fur- ther, the cells isolated from the lungs showed a sizeable increase in the
total number of central memory CD4 and CD8 T cells expressing CD44hi/CD62Lhi (Fig. 3c). These findings suggest that
BCG-Acr1L can effectively evoke the generation of enduring memory CD4 and CD8 T cells.
292 V
C
2015 Institute of Microbial Technology, Clinical and Experimental Immunology, 181: 286–296
Immunization with BCG-Acr1L augments lung immunity
The adaptive immune response to Mtb is initiated in the draining lymph nodes and effector T cells migrate subsequently to the
site of infection [34–36]. The adapt- ive immunity in the lungs plays a decisive role in imparting protection against Mtb. CD4 T
cells isolated from the lungs of mice immunized with BCG-Acr1L exhibited substantial (P < 0401) release of IFN-g (Fig. 4a). In
contrast, a significant (P < 0405) decrease in the secretion of IL-4 was observed (Fig. 4b) and an increase in the frequency of
polyfunctional [IFN-ghi/tumour necrosis factor (TNF)-ahi] CD4 (Fig. 4c,e) and CD8 T (Fig. 4d,f) cells was noted in the lungs
(Fig. 4c,d) and
Acr1 reinvigorates BCG potency
Fig. 5. Bacillus Calmette–Gu ́erin (BCG)- liposomized alpha-crystalline protein 1 (Acr1L) provides significantly better
protection than BCG. Mice (six to eight per group) were immunized with BCG-Acr1L, BCG-Acr1, Acr1L, Acr1, BCG and
placebo, which were later aerosol-challenged with Mycobacterium tuberculosis (Mtb). After 35 days, animals were killed. (a)
Mtb load is represented as mean 6standard error of log
10 colony-forming units (CFU)/g of the lung. Statistical analysis was performed by
Student– Newman–Keuls multiple-comparisons post-test to compare the significance between the two groups. *P <0405; ***P
<04001. (b) Lungs were fixed in formalin and sections were stained with haematoxylin and eosin. Photomicrographs (320)
display the lung sections. Arrows indicate small or large developing follicular granulomas.
spleen cells (Fig. 4e,f) of BCG-Acr1L-vaccinated mice. Furthermore, TNF-ahi expressing CD4 and CD8 T cells were seen to
predominate over IFN-g1 cells (Fig. 4c,d). Little difference was observed in the control groups of animals immunized with
placebo (PBS), Acr1 alone or BCG. These results (Fig. 4a–f) demonstrate the predom- inance of multi-functional
(IFN-ghi/TNF-ahi) CD4 and CD8 T cells in both the lungs and spleen in the BCG- Acr1-immunized mice. Both IFN-g and
TNF-a are con- sidered to play potent roles in conferring immunity against Mtb.
V C
2015 Institute of Microbial Technology, Clinical and Experimental Immunology, 181: 286–296 293
BCG-Acr1L vaccination significantly protects mice from Mtb
The ultimate protective efficacy of any vaccine against TB is established by enumerating the mycobacterial burden in the lungs
of the vaccinated animals. Compared to BCG alone, BCG-Acr1L prime-boost vaccination resulted in a significant (P < 0405)
decline in the mycobacterial load in the lungs. Further, compared to placebo or Acr1, a consid- erably higher decline in CFU was
perceived (P < 04001) (Fig. 5a), and a substantial amelioration in the histopatho- logical changes was noted in the lungs. This
was evidenced
K. F. Siddiqui et al.
by a reduction in the size and number of granulomas and
vaccination augmented: (i) the generation of both CD4 less
consolidated and comparatively normal alveolar struc-
and CD8 T cells; (ii) the pool of mainly Th1 cells; (iii) ture
of the lungs (Fig. 5b), thus establishing the potent role
the frequency of multi-functional (IFN-g1/TNF-a1) CD4 of
BCG-AcrL1 in eliciting protection against TB.
and CD8 T cells; (iv) the proportion of enduring memory CD4 and CD8 T cells; (v) robust immunity in the lung
Discussion
cells; and (vi) clearance of the mycobacterial burden in the lungs and reduced pathology. The failure to protect adulthood TB
signifies the inability
One of the fundamental features of a successful vaccine
of BCG to elicit enduring memory T cells and therefore
is its ability to elicit long-lasting T cell memory. We have
long-lasting immunity [1]. Recently, BCG vaccination has
demonstrated that vaccination with BCG-Acr1L induces a
been demonstrated to evoke a weak central memory T
better memory T cells response than BCG alone, both in
cell response, which is hypothesized as a fundamental
the lungs and spleen. The memory T cell response gener-
basis for the failure of the BCG vaccine in humans [37].
ated was bona fide, as evidenced by the fact that mice In
addition to this, BCG is also incapable of providing
prime-boosted with BCG-Acr1L were rested for 160 days
sterilizing immunity against primary Mtb infection due
before the memory response was monitored. BCG-Acr1L to
an inadequate immune response against latency-
generated a vast pool of effector and central memory T
associated antigens [8]. Latency-associated antigens have
cells in CD4 and CD8 T cells. We also observed that Mtb-
been projected to be potential candidates for vaccine
specific T cells were principally of the Th1 phenotype, as
development against TB [4,9,38]. Acr1 protein of Mtb is
seen by the predominant production of IFN-g. To further
one of the most immunogenic antigens, which is
validate the generation of the Th1 response, we also
expressed predominantly at the time of latency [10]. We
monitored the levels of IgG1 and IgG2a isotypes. A pre-
recently reported that this protein inhibits the maturation
dominant secretion of Mtb-specific IgG2a was noted in and
differentiation of immature dendritic cells (DCs) by
the mice vaccinated with BCG-Acr1L, a hallmark of the
inducing a tolerogenic phenotype [39]. Once DCs
Th1 phenotype [42]. The CFU and pathology data further
mature, this protein activates the DCs and induces the
supported the protective potential of BCG-Acr1L vaccina-
release of potent proinflammatory cytokines (unpublished
tion. Reports in the literature indicate that predominance
data). Thus, introduction of a prime-boost vaccination
of the Th2 response is detrimental in achieving protection
strategy using BCG and Acr1 could emerge as a success-
against TB. Thus, to gain protective immunity against ful
vaccination approach. A DNA-based booster vaccine
TB, it is not only essential to have a predominant Th1
expressing 16 kDa has been tested effectively [40] but,
response but also a limited Th2 response [43]. We
considering the clinical relevance and ethical issues of
observed a significant decrease in IL-4 secretion in BCG-
DNA immunization, we evaluated a protein-based
Acr1L-vaccinated animals compared to BCG alone. How-
booster approach. We observed Acr1 to be ineffective in
ever, a decline in the Th2 response could alleviate pathol-
evoking a T cell response or secretion of IFN-g (Fig. 2).
ogy during Mtb infection [44,45], but histological analysis
However, liposomes are known to assist the immuno-
of infected lungs in animals immunized with BCG-Acr1L
genic potential of antigens [24–27]. Hence, we encapsu-
showed minimal consolidation and infiltration with a lated
Acr1 in the fusogenic liposomes prepared from
comparatively normal alveolar structure. yeast lipids. There
is a distinct advantage of making lipo-
BCG vaccine alone was unable to induce optimal CD8
somes from fusogenic lipids, as they can induce and
T cells necessary for the clearance of Mtb [46,47]. In con-
enhance the generation of both CD4 and CD8 T cells.
trast, vaccination with BCG-Acr1L results in an enhanced
Consequently, we used liposomes prepared from fuso-
pool of multi-functional (IFN-g1/TNF-a1) CD4 and genic
lipids to entrap Acr1 (Acr1L). In a prime-boost
CD8 T cells in both lungs and secondary lymphoid
regimen, Acr1L significantly bolstered the vaccination
organs. Recent reports have shown that T cells producing
potential of BCG.
multiple cytokines, such as the concomitant release of Besides
the importance of CD4 T cells, a recent study
IFN-g and TNF-a, are functionally superior to their also
demonstrated a major role for CD8 T cells in anti-
single-positive counterparts [48]. We observed a markedly
TB immunity [23], indicating that CD8 T cells should be
higher frequency of Acr1-specific multi-functional CD4
included in strategies for the development of new TB vac-
and CD8 T cells in BCG-Acr1L-immunized animals. cines.
Antigen-entrapped liposomes have been used to
Multi-functional T cells form a reservoir of effector and
generate antigen-specific memory T cells [24,41]; hence,
central memory CD4 and CD8 T cells and therefore may in
the current study, mice were primed with BCG and
be mediating an efficient and sustained protection against
boosted with Acr1L. Further, to study the bona fide long-
Mtb. term memory T cells, mice were rested for 160 days
after
We found that BCG-Acr1L was more efficient than
vaccination before being challenged with Mtb. The major
BCG in reducing the bacterial burden in the lungs even
findings arising from this study were that BCG-Acr1L
after 160 days of immunization. This establishes an
294 V
C
2015 Institute of Microbial Technology, Clinical and Experimental Immunology, 181: 286–296
Acr1 reinvigorates BCG potency
important role for BCG-Acr1L vaccination in imparting enduring protective memory CD4 and CD8 T cells response against Mtb.
Our approach of encapsulating Acr1 antigen of Mtb in fusogenic liposomes prepared from yeast lipids bolstered the induction
and enhance- ment of memory CD4 and CD8 T cells. Currently, it is difficult to elucidate precisely the mechanism involved in
enhancing the memory T cell response by BCG-Acr1L. However, it is known that liposomes boost the formation of memory T
cells by releasing memory-enhancing cyto- kines IL-1, IL-6, IL-7 and IL-15 [49]. Moreover, it is important to mention here that
this strategy is beneficial in reinvigorating the potency of the BCG vaccine in enhancing long-lasting immunity, because it is
known that BCG fails to generate long-lasting immunity, as shown by the fact that it can protect only children, but not adults,
from TB [1–3].
In conclusion, a prime-boost regimen employing BCG and Acr1 entrapped in fusogenic liposomes has overcome the snags
associated with BCG failure to generate endur- ing memory T cell immunity. This is evidenced by a sig- nificant improvement in
inducing the generation of the long-lasting protective efficacy of BCG by BCG-Acr1L immunization. Therefore, vaccination
with BCG-Acr1 may be an important future strategy to reinvigorate BCG efficacy as a vaccine to control TB.
Acknowledgements
The authors are thankful to Dr Manoj Raje and Mr Anil Theophillus for electron microscopy and Council of Scien- tific and
Industrial Research and Department of Biotechnol- ogy, India for financial support. K. F. S., M. A. and N. K. are the recipients of
fellowship of the Department of Biotech- nology and G. R. K. and J. A. S. of the Council of Scientific and Industrial Research,
India.
Disclosure
The authors declare that they have no conflicts of interest.
References
1 Colditz GA, Brewer TF, Berkey CS et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the
published literature. JAMA 1994; 271:698–702. 2 Comstock GW. Efficacy of BCG vaccine. JAMA 1994; 272:766. 3 Fine PE.
Variation in protection by BCG: implications of and
for heterologous immunity. Lancet 1995; 346:1339–45. 4 Aagaard C, Hoang T, Dietrich J et al. A multistage tuberculosis
vaccine that confers efficient protection before and after expo- sure. Nat Med 2011; 17:189–94. [CrossRef] 5 Corbett EL, Watt
CJ, Walker N et al. The growing burden of tuberculosis: global trends and interactions with the HIV epi- demic. Arch Intern Med
2003; 163:1009–21.
V C
6 Cunningham AF, Spreadbury CL. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and
localization of the 16-kilodalton alpha-crystallin homolog. J Bacteriol 1998; 180:801–8. 7 Sherman DR, Voskuil M,
Schnappinger D, Liao R, Harrell MI, Schoolnik GK. Regulation of the Mycobacterium tuberculosis hypoxic response gene
encoding alpha-crystallin. Proc Natl Acad Sci USA 2001; 98:7534–9. 8 Vekemans J, Ota MO, Sillah J et al. Immune responses to
myco- bacterial antigens in the Gambian population: implications for vaccines and immunodiagnostic test design. Infect Immun
2004; 72:381–8. 9 Roupie V, Romano M, Zhang L et al. Immunogenicity of eight dormancy regulon-encoded proteins of
Mycobacterium tubercu- losis in DNA-vaccinated and tuberculosis-infected mice. Infect Immun 2007; 75:941–9. 10 Siddiqui
KF, Amir M, Agrewala JN. Understanding the biology of 16 kDa antigen of Mycobacterium tuberculosis: scope in diag- nosis,
vaccine design and therapy. Crit Rev Microbiol 2011; 37: 349–57. 11 Demissie A, Leyten EM, Abebe M et al. Recognition of
stage- specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis. Clin
Vaccine Immunol 2006; 13:179–86. [CrossRef] 12 Geluk A, Lin MY, van Meijgaarden KE et al. T-cell recognition of the HspX
protein of Mycobacterium tuberculosis correlates with latent M. tuberculosis infection but not with M. bovis BCG vaccination.
Infect Immun 2007; 75:2914–21. 13 Andersen P. Tuberculosis vaccines – an update. Nat Rev Micro-
biol 2007; 5:484–7. 14 Brandt L, Skeiky YA, Alderson MR et al. The protective effect of the Mycobacterium bovis BCG
vaccine is increased by coadmi- nistration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M.
tuberculosis-infected guinea pigs. Infect Immun 2004; 72:6622–32. 15 Dietrich J, Andersen C, Rappuoli R, Doherty TM, Jensen
CG, Andersen P. Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and
boosts prior bacillus Calmette–Guerin immunity. J Immunol 2006; 177:6353–60. 16 Ly LH, McMurray DN. Tuberculosis:
vaccines in the pipeline.
Exp Rev Vaccines 2008; 7:635–50. 17 McShane H, Pathan AA, Sander CR et al. Recombinant modi- fied vaccinia virus
Ankara expressing antigen 85A boosts BCG- primed and naturally acquired antimycobacterial immunity in humans. Nat Med
2004; 10:1240–4. [CrossRef] 18 Spratt JM, Britton WJ, Triccas JA. In vivo persistence and pro- tective efficacy of the bacille
Calmette Guerin vaccine overex- pressing the HspX latency antigen. Bioeng Bugs 2010; 1:61–5. 19 Khera A, Singh R, Shakila H
et al. Elicitation of efficient, pro- tective immune responses by using DNA vaccines against tuber- culosis. Vaccine 2005;
23:5655–65. 20 Shi C, Chen L, Chen Z et al. Enhanced protection against tuber- culosis by vaccination with recombinant BCG
over-expressing HspX protein. Vaccine 2010; 28:5237–44. 21 Cho S, Mehra V, Thoma-Uszynski S et al. Antimicrobial activity
of MHC class I-restricted CD81 T cells in human tuberculosis. Proc Natl Acad Sci USA 2000; 97:12210–5. 22 Flynn JL, Chan J.
Tuberculosis: latency and reactivation. Infect
Immun 2001; 69:4195–201.
2015 Institute of Microbial Technology, Clinical and Experimental Immunology, 181: 286–296 295
K. F. Siddiqui et al.
23 Chen CY, Huang D, Wang RC et al. A critical role for CD8 T
36 Swain SL, Agrewala JN, Brown DM et al. CD41 T-cell
memory: cells in a nonhuman primate model of tuberculosis. PLOS
generation and multi-faceted roles for CD41 T cells in
protec- Pathog 2009; 5:e1000392.
tive immunity to influenza. Immunol Rev 2006;
211:8–22. 24 Owais M, Masood AK, Agrewala JN, Bisht D, Gupta CM. Use of
37 Henao-Tamayo MI, Ordway DJ, Irwin SM, Shang S,
Shanley liposomes as an immunopotentiating delivery system: in perspec-
C, Orme IM. Phenotypic definition of effector and memory
tive of vaccine development. Scand J Immunol 2001; 54:125–32.
T-lymphocyte subsets in mice chronically infected with
25 Agrewala JN, Owais M, Gupta CM, Mishra GC. Antigen incor-
Mycobacterium tuberculosis. Clin Vaccine Immunol 2010;
17: poration into liposomes results in the enhancement of IL-4 and
618–25. IgG1 secretion: evidence for preferential expansion
of Th-2 cells.
38 Schuck SD, Mueller H, Kunitz F et al. Identification of
T-cell Cytokines Mol Ther 1996; 2:59–65.
antigens specific for latent Mycobacterium tuberculosis
infection. 26 Mishra V, Mahor S, Rawat A et al. Development of novel fuso-
PLOS ONE 2009; 4:e5590. genic vesosomes for
transcutaneous immunization. Vaccine
39 Siddiqui KF, Amir M, Gurram RK et al. Latency-associated
pro- 2006; 24:5559–70.
tein Acr1 impairs dendritic cell maturation and
functionality: a 27 Owais M, Gupta CM. Liposome-mediated cytosolic delivery of
possible mechanism of immune evasion by Mycobacterium
macromolecules and its possible use in vaccine development.
tuberculosis. J Infect Dis 2013; 209:1436–45. Eur J
Biochem 2000; 267:3946–56.
40 Dey B, Jain R, Gupta UD, Katoch VM, Ramanathan
VD, Tyagi 28 Singh V, Gowthaman U, Jain S et al. Coadministration of inter-
AK. A booster vaccine expressing a latency-associated
antigen leukins 7 and 15 with bacille Calmette–Guerin mounts enduring
augments BCG induced immunity and confers enhanced
protec- T cell memory response against Mycobacterium tuberculosis.
tion against tuberculosis. PLoS One 2011; 6:e23360. J
Infect Dis 2010; 202:480–9.
41 Wang C, Liu P, Zhuang Y et al. Lymphatic-targeted
cationic lip- 29 Singh V, Jain S, Gowthaman U et al. Co-administration of IL-
osomes: a robust vaccine adjuvant for promoting long-term
11IL-61TNF-alpha with Mycobacterium tuberculosis infected
immunological memory. Vaccine 2014; 32:5475–83.
macrophages vaccine induces better protective T cell memory
42 Stevens TL, Bossie A, Sanders VM et al. Regulation of
antibody than BCG. PLOS ONE 2011; 6:e16097.
isotype secretion by subsets of antigen-specific helper T
cells. 30 Gowthaman U, Singh V, Zeng W et al. Promiscuous peptide of
Nature 1988; 334:255–8. 16 kDa antigen linked to
Pam2Cys protects against Mycobacte-
43 Rook GA, Dheda K, Zumla A. Immune responses to
tuberculo- rium tuberculosis by evoking enduring memory T-cell response.
sis in developing countries: implications for new vaccines.
Nat J Infect Dis 2011; 204:1328–38.
Rev Immunol 2005; 5:661–7. 31 Agrewala JN,
Wilkinson RJ. Differential regulation of Th1 and
44 Buccheri S, Reljic R, Caccamo N et al. IL-4 depletion
enhances Th2 cells by p91-110 and p21-40 peptides of the 16-kD alpha-
host resistance and passive IgA protection against
tuberculosis crystallin antigen of Mycobacterium tuberculosis. Clin Exp
infection in BALB/c mice. Eur J Immunol 2007;
37:729–37. Immunol 1998; 114:392–7.
45 Rook GA. Th2 cytokines in susceptibility to
tuberculosis. Curr 32 Agrewala JN, Wilkinson RJ. Influence of HLA-DR on the phe-
Mol Med 2007; 7:327–37. notype of CD41 T lymphocytes
specific for an epitope of the
46 Grode L, Seiler P, Baumann S et al. Increased vaccine
efficacy 16-kDa alpha-crystallin antigen of Mycobacterium tuberculosis.
against tuberculosis of recombinant Mycobacterium bovis
bacille Eur J Immunol 1999; 29:1753–61.
Calmette–Guerin mutants that secrete listeriolysin. J
Clin Invest 33 Rueda CM, Marin ND, Garcia LF, Rojas M. Characterization of
2005; 115:2472–9. CD4 and CD8 T cells producing
IFN-gamma in human latent
47 Schaible UE, Winau F, Sieling PA et al. Apoptosis
facilitates and active tuberculosis. Tuberculosis 2010; 90:346–53.
antigen presentation to T lymphocytes through MHC-I
and 34 Agrewala JN, Brown DM, Lepak NM, Duso D, Huston G,
CD1 in tuberculosis. Nat Med 2003; 9:1039–46. Swain SL.
Unique ability of activated CD41 T cells but not
48 Darrah PA, Patel DT, De Luca PM et al. Multifunctional
TH1 rested effectors to migrate to non-lymphoid sites in the absence
cells define a correlate of vaccine-mediated protection
against of inflammation. J Biol Chem 2007; 282:6106–15.
Leishmania major. Nat Med 2007; 13:843–50. 35 Reiley
WW, Calayag MD, Wittmer ST et al. ESAT-6-specific
49 Steers NJ, Peachman KK, McClain S, Alving CR, Rao M.
Lipo- CD4 T cell responses to aerosol Mycobacterium tuberculosis
some-encapsulated HIV-1 Gag p24 containing lipid A
induces infection are initiated in the mediastinal lymph nodes. Proc
effector CD41 T-cells, memory CD81 T-cells, and pro-
Natl Acad Sci USA 2008; 105:10961–6.
inflammatory cytokines. Vaccine 2009; 27:6939–49.
296 V
C
2015 Institute of Microbial Technology, Clinical and Experimental Immunology, 181: 286–296
Das könnte Ihnen auch gefallen
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- 2009 2011 DS Manual - Club Car (001-061)Dokument61 Seiten2009 2011 DS Manual - Club Car (001-061)misaNoch keine Bewertungen
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- Analysis of Electric Machinery Krause Manual Solution PDFDokument2 SeitenAnalysis of Electric Machinery Krause Manual Solution PDFKuldeep25% (8)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- CH 1 - Democracy and American PoliticsDokument9 SeitenCH 1 - Democracy and American PoliticsAndrew Philip ClarkNoch keine Bewertungen
- Dokumen - Pub - Bobs Refunding Ebook v3 PDFDokument65 SeitenDokumen - Pub - Bobs Refunding Ebook v3 PDFJohn the First100% (3)
- Lesson 5 Designing and Developing Social AdvocacyDokument27 SeitenLesson 5 Designing and Developing Social Advocacydaniel loberizNoch keine Bewertungen
- Project Scheduling and TrackingDokument47 SeitenProject Scheduling and TrackingArun VinodhNoch keine Bewertungen
- Modulo EminicDokument13 SeitenModulo EminicAndreaNoch keine Bewertungen
- Mark Garside Resume May 2014Dokument3 SeitenMark Garside Resume May 2014api-199955558Noch keine Bewertungen
- Mcom Sem 4 Project FinalDokument70 SeitenMcom Sem 4 Project Finallaxmi iyer75% (4)
- Week 7Dokument24 SeitenWeek 7Priyank PatelNoch keine Bewertungen
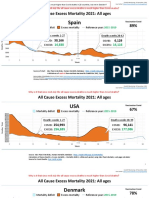
- Countries EXCESS DEATHS All Ages - 15nov2021Dokument21 SeitenCountries EXCESS DEATHS All Ages - 15nov2021robaksNoch keine Bewertungen
- An Exploration of The Ethno-Medicinal Practices Among Traditional Healers in Southwest Cebu, PhilippinesDokument7 SeitenAn Exploration of The Ethno-Medicinal Practices Among Traditional Healers in Southwest Cebu, PhilippinesleecubongNoch keine Bewertungen
- Ozone Therapy - A Clinical Review A. M. Elvis and J. S. EktaDokument5 SeitenOzone Therapy - A Clinical Review A. M. Elvis and J. S. Ektatahuti696Noch keine Bewertungen
- MGMT Audit Report WritingDokument28 SeitenMGMT Audit Report WritingAndrei IulianNoch keine Bewertungen
- Linguistics Is Descriptive, Not Prescriptive.: Prescriptive Grammar. Prescriptive Rules Tell You HowDokument2 SeitenLinguistics Is Descriptive, Not Prescriptive.: Prescriptive Grammar. Prescriptive Rules Tell You HowMonette Rivera Villanueva100% (1)
- Crown WF-3000 1.2Dokument5 SeitenCrown WF-3000 1.2Qirat KhanNoch keine Bewertungen
- DP 2 Human IngenuityDokument8 SeitenDP 2 Human Ingenuityamacodoudiouf02Noch keine Bewertungen
- Chapter3 Elasticity and ForecastingDokument25 SeitenChapter3 Elasticity and ForecastingGee JoeNoch keine Bewertungen
- Article An Incident and Injury Free Culture Changing The Face of Project Operations Terra117 2Dokument6 SeitenArticle An Incident and Injury Free Culture Changing The Face of Project Operations Terra117 2nguyenthanhtuan_ecoNoch keine Bewertungen
- Post Appraisal InterviewDokument3 SeitenPost Appraisal InterviewNidhi D100% (1)
- Floating Oil Skimmer Design Using Rotary Disc MethDokument9 SeitenFloating Oil Skimmer Design Using Rotary Disc MethAhmad YaniNoch keine Bewertungen
- De Thi Hoc Ki 1 Mon Tieng Anh Lop 5 Co File NgheDokument10 SeitenDe Thi Hoc Ki 1 Mon Tieng Anh Lop 5 Co File Nghetuyen truongNoch keine Bewertungen
- Turning PointsDokument2 SeitenTurning Pointsapi-223780825Noch keine Bewertungen
- CX Programmer Operation ManualDokument536 SeitenCX Programmer Operation ManualVefik KaraegeNoch keine Bewertungen
- Chemistry Form 4 Daily Lesson Plan - CompressDokument3 SeitenChemistry Form 4 Daily Lesson Plan - Compressadila ramlonNoch keine Bewertungen
- RSA - Brand - Guidelines - 2019 2Dokument79 SeitenRSA - Brand - Guidelines - 2019 2Gigi's DelightNoch keine Bewertungen
- Blue Prism Data Sheet - Provisioning A Blue Prism Database ServerDokument5 SeitenBlue Prism Data Sheet - Provisioning A Blue Prism Database Serverreddy_vemula_praveenNoch keine Bewertungen
- Opc PPT FinalDokument22 SeitenOpc PPT FinalnischalaNoch keine Bewertungen
- WL-80 FTCDokument5 SeitenWL-80 FTCMr.Thawatchai hansuwanNoch keine Bewertungen
- WEB DESIGN WITH AUSTINE-converted-1Dokument9 SeitenWEB DESIGN WITH AUSTINE-converted-1JayjayNoch keine Bewertungen