Beruflich Dokumente
Kultur Dokumente
Biological Cell Membranes
Hochgeladen von
Mohammed Shaik0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
14 Ansichten44 Seitenbiological
Copyright
© © All Rights Reserved
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenbiological
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
14 Ansichten44 SeitenBiological Cell Membranes
Hochgeladen von
Mohammed Shaikbiological
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 44
II.
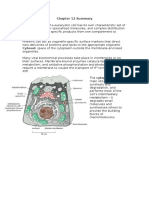
Biological Cell Membranes
The cell membrane forms a
selectively permeable barrier
between the cytoplasm and the
external milieu.
Each cell is bounded by a cell membrane (also
known as the plasma membrane or plasmalemma)
that functions in :
Maintaining the structural integrity of the cell
Controlling movements of substances in and out of the
cell (selective permeability)
Regulating cell–cell interactions
Recognizing via receptors, antigens and foreign cells as
well as altered cells
Acting as an interface between the cytoplasm and the
external milieu
Establishing transport systems for specific molecules
Sustaining a potential difference between the
intracellular and extracellular aspects of the membrane
Transducing extracellular physical or chemical signals
into intracellular events
Cell membranes are not visible with the
light microscope (LM).
In electron micrographs (EM), the
plasmalemma is about 7.5 nm thick and
appears as a trilaminar structure of 2 thin,
dense lines with an intervening light area.
Each layer is about 2.5 nm in width, and the
entire structure is known as the unit
membrane (Fig. 2–7).
◦ The inner (cytoplasmic) dense line is its inner
leaflet;
◦ the outer dense line is its outer leaflet
Figure 2-7 A junction between two cells demonstrates
the trilaminar structures
Molecular Composition
The plasmalemma is composed of a phospholipid
bilayer and associated integral and peripheral
proteins.
Each leaflet is composed of a single layer of
phospholipids and associated proteins, usually in
a 1 : 1 proportion by weight. In certain cases, such
as myelin sheaths, however, the lipid component
outweighs the protein component by a ratio of
4 : 1. The two leaflets, composing a lipid bilayer in
which proteins are suspended, constitute the basic
structure of all membranes of the cell (Fig. 2–8).
Although as viewed by the EM the two leaflets
appear indistinguishable from each other, it is
known that their phospholipid compositions are
different and, therefore, the two leaflets are said to
be asymmetrical.
Figure 2-8
Three-dimensional diagrammatic representation of
the fluid mosaic model of the cell membrane.
Each phospholipid molecule of the lipid
bilayer is composed of a polar head, located
at the surface of the membrane, and two long
nonpolar fatty acyl tails, usually consisting of
chains of 16 to 18 carbon atoms, projecting
into the center of the plasmalemma (see Fig.
2–8).
The nonpolar fatty acyl tails of the two layers
face each other within the membrane and
form weak noncovalent bonds with each other,
holding the bilayer together.
Because the phospholipid molecule is
composed of a hydrophilic head and a
hydrophobic tail, the molecule is said to be
amphipathic.
The polar heads are composed of glycerol, to which a
positively charged nitrogenous group is attached by a
negatively charged phosphate group. The two fatty
acyl tails, only one of which is usually saturated, are
covalently bound to glycerol. Other amphipathic
molecules, such as glycolipids, glycosphingolipids,
and cholesterol, are also present in the cell membrane.
The unsaturated fatty acyl molecules increase membrane
fluidity, whereas cholesterol decreases it (although
cholesterol concentrations much lower than normal
increase membrane fluidity).
In fact, certain regions of the plasmalemma are so well
endowed with glycosphingolipids and cholesterol that
they create a bulge in the cell membrane. These
thickened microdomains are known as lipid rafts, and
they form a slight bulge into the extracellular space..
Frequently, lipid rafts possess protein components that
participate in diverse signaling events (discussed in the
next paragraph). Therefore, lipid rafts appear to facilitate
and enhance the possibilities of communications between
and among a variety of cells
The protein components of the plasmalemma either span
the entire lipid bilayer as integral proteins or are
attached to the cytoplasmic aspect (and, at times, the
extracellular aspect) of the lipid bilayer as peripheral
proteins.
Because most integral proteins pass through the entire
thickness of the membrane, they are also referred to as
transmembrane proteins. Those regions of
transmembrane proteins that project into the cytoplasm
or the extracellular space are composed of hydrophilic
amino acids, whereas the intramembrane region consists
of hydrophobic amino acids.
Transmembrane proteins frequently form ion
channels and carrier proteins that facilitate
the passage of specific ions and molecules
across the cell membrane.
Many of these transmembrane proteins are
quite long and are folded so that they make
several passes through the membrane and
thus are known as multipass proteins and
are frequently attached to the inner leaflet
(and infrequently to the outer leaflet) by
prenyl groups or fatty acyl groups. The
cytoplasmic and extracytoplasmic aspects of
these proteins commonly possess receptor
sites that are specific for particular signaling
molecules.
Once these molecules are recognized at
these receptor sites, the integral proteins
can alter their conformation and can
perform a specific function.
Because the same integral membrane
proteins have the ability to float like
icebergs in the sea of phospholipids, this
model is referred to as the fluid mosaic
model of membrane structure. However,
the integral proteins frequently possess
only limited mobility, especially in polarized
cells, in which particular regions of the cell
serve specialized functions.
Peripheral proteins do not usually form
covalent bonds with either the integral
proteins or the phospholipid components of
the cell membrane. Although they are usually
located on the cytoplasmic aspect of the cell
membrane, they may also be on the
extracellular surface.
These proteins may form bonds either with
the phospholipid molecules or with the
transmembrane proteins.
Frequently, they are associated with the
secondary messenger system of the cell (see
section on signaling molecules) or with the
cytoskeletal apparatus.
Using freeze-fracture techniques, one can
cleave the plasma membrane into its two
leaflets in order to view the hydrophobic
surfaces (Figs. 2–9 and 2–10).
The outer surface of the inner leaflet is
referred to as the P-face (closer to the
protoplasm); the inner surface of the outer
leaflet is known as the E-face (closer to the
extracellular space).
EM of freeze-fractured plasma membranes
show that the integral proteins, visualized by
shadowing replica, are more numerous on the
P-face than on the E-face (see Fig. 2–10).
Figure 2-9
Schematic diagram of the E-face and the P-face of the cell
membrane.
Figure 2-10
Freeze-fracture replica of a cell membrane. The E-face (closer to the
extracellular space) is on the right, and the P-face (closer to the protoplasm)
is on the left. Note that the integral proteins are more numerous on the P-
face (left-hand side) than on the E-face (right-hand side). (×168000)
(From Leeson TS, Leeson CR, Papparo AA. Text/Atlas of Histology. Philadelphia: WB Saunders; 1988.)
Glycocalyx
Glycocalyx, composed usually of carbohydrate chains,
coats the cell surface.
A fuzzy coat, referred to as the cell coat or
glycocalyx, is often evident in EM of the cell
membrane. This coat is usually composed of
carbohydrate chains that are covalently attached to
transmembrane proteins and/or phospholipid
molecules of the outer leaflet (see Fig. 2–8).
Additionally, some of the extracellular matrix
molecules, adsorbed to the cell surface, also
contribute to its formation. Its intensity and
thickness vary, but it may be as thick as 50 nm on
some epithelial sheaths, such as those lining regions
of the digestive system.
Because of its numerous negatively charged sulfate
and carboxyl groups, the glycocalyx stains intensely
with lectins as well as with dyes, such as ruthenium
red and Alcian blue, permitting its visualization with
light microscopy. The most important function of the
glycocalyx
is protection of the cell from interaction with
inappropriate proteins, from chemical injury, and from
physical injury.
Other cell coat functions include cell–cell recognition
and adhesion, as occurs between endothelial cells and
neutrophils as well as T cells and antigen-presenting
cells (APCs);
facilitation of blood clotting and inflammatory
responses;
and assistance in reducing friction between blood and
the endothelial cells lining blood vessels.
Membrane Transport Proteins
Membrane transport proteins are of two types,
channel proteins and
carrier proteins, and
they facilitate the movement of aqueous
molecules and ions across the plasmalemma.
Although the hydrophobic components of
the plasma membrane limit the movement of
polar molecules across it, the presence and
activities of specialized transmembrane
proteins facilitate the transfer of these
hydrophilic molecules across this barrier.
These transmembrane proteins and protein
complexes form channel proteins and
carrier proteins, which are specifically
concerned with the transfer of ions and
small molecules across the plasma
membrane.
A number of small nonpolar molecules
(e.g., benzene, oxygen, nitrogen) and
uncharged polar molecules (e.g., water,
glycerol) can move across the cell
membrane by simple diffusion down their
concentration gradients. Even when driven
by a concentration gradient, however,
significant movement of most ions and
small molecules across a membrane
requires the aid of either channel proteins
or carrier proteins. This process is known
as facilitated diffusion.
Because both types of diffusion occur
without any input of energy other than that
inherent in the concentration gradient, they
represent passive transport (Fig. 2–11). By
expending energy, cells can transport ions and
small molecules against their concentration
gradients.
Only carrier proteins can mediate such
energy-requiring active transport. The
several channel proteins involved in facilitated
diffusion are discussed first, and the more
versatile carrier proteins are considered
afterward.
Figure 2-11 Types of transport. A, Passive transport: diffusion, ion channel-mediated diffusion,
and carrier-mediated diffusion. B, Active transport: coupled transport. Symport and antiport.
Channel Proteins
Channel proteins may be gated or
ungated;
they are incapable of transporting
substances against a concentration
gradient.
Channel proteins participate in the
formation of hydrophilic pores, called ion
channels, across the plasmalemma. In
order to form hydrophilic channels, the
proteins are folded so that the
hydrophobic amino acids are positioned
peripherally, interacting with the fatty acyl
tails of the phospholipid molecules of the
lipid bilayer; whereas the hydrophilic
amino acids face inward, forming a polar
inner lining for the channel.
There are more than 100 different types of ion
channels; of these, some are specific for one
particular ion, but others permit the passage of
several different ions and small water-soluble
molecules.
Although these ions and small molecules follow
chemical or electrochemical concentration
gradients for the direction of their passage, cells
have the capability of preventing these substances
from entering these hydrophilic tunnels by means of
controllable gates that block their opening.
Most channels are gated channels; only a few are
ungated. Gated channels are classified according to
the control mechanism required to open the gate.
Voltage-Gated Channels
Voltage-gated channels go from the closed to
the open position, permitting the passage of ions
from one side of the membrane to the other. The
most common example is depolarization in the
transmission of nerve impulses. In some channels,
such as Na+ channels, the open position is
unstable, and the channel goes from an open to
an inactive position, in which the passage of the
ion is blocked, and, for a short time (a few
milliseconds), the gate cannot be opened again.
This is the refractory period (ex. on the
nervous system). The velocity of response to
depolarization may also vary, and some of those
channels are referred to as velocity-dependent.
Ligand-Gated Channels
Channels that require the binding of a
ligand (signaling molecule) to the channel
protein to open their gate are known as
ligand-gated channels. Unlike voltage-
gated channels, these channels remain open
until the ligand dissociates from the channel
protein; they are referred to as ion
channel–linked receptors. Some of the
ligands controlling these gates are
neurotransmitters, whereas others are
nucleotides
Neurotransmitter-gated channels are usually
located on the postsynaptic membrane. The
neurotransmitter binds to a specific site on the protein,
altering its molecular conformation, thus opening the
channel or gate and permitting the influx of a specific
ion into the cell. Some neurotransmitters are
excitatory, whereas others are inhibitory. Excitatory
neurotransmitters (e.g., acetylcholine) facilitate
depolarization, whereas inhibitory neurotransmitters
facilitate hyperpolarization of the membrane
In nucleotide-gated channels, the signal molecule is
a nucleotide (e.g., cyclic adenosine
monophosphate [cAMP] in olfactory receptors and
cyclic guanosine monophosphate [cGMP] in rods
of the retina) that binds to a site on the protein and, by
altering the conformation of the protein complex,
permits the flow of a particular ion through the ion
channel.
Mechanically Gated Channels
In mechanically gated channels, an actual
physical manipulation is required to open the gate.
An example of this mechanism is found in the hair
cells of the inner ear. These cells, located on the
basilar membrane, possess stereocilia that are
embedded in a matrix known as the tectorial
membrane. Movement of the basilar membrane
causes a shift in the positions of the hair cells,
resulting in the bending of the stereocilia. This
physical distortion opens the mechanically gated
channels of the stereocilia located in the inner
ear, permitting the entry of cations into the cell,
depolarizing it. This event generates impulses that
the brain interprets as sound.
G-Protein–Gated Ion Channels
Certain gated ion channels (e.g.,
muscarinic acetylcholine receptors of
cardiac muscle cells) require the
interaction between a receptor molecule
and a G-protein complex (see section on
G-protein–linked receptors) with the
resultant activation of the G protein. The
activated G protein then interacts with
the channel protein modulating the ability
of the channel to open or close.
Ungated Channels
One of the most common forms of an
ungated channel is the potassium (K+)
leak channel, which permits the movement
of K+ ions across it and is instrumental in
the creation of an electrical potential
(voltage) difference between the two
sides of the cell membrane. Because this
channel is ungated, the transit of K+ ions is
not under the cell’s control; rather, the
direction of ion movement reflects its
concentration on the two sides of the
membrane.
Aquaporins
Currently, there are twelve different types of
aquaporins that are known; these are a family of
multipass proteins that form channels designed for
the passage of water from one side of the cell
membrane to the other. Some of these channels are
pure water transporters (e.g., AqpZ), whereas others
transport glycerol (e.g., GlpF). These aquaporins
discriminate in the transport of the two molecules by
restricting the pore sizes in such a fashion that
glycerol is too large to pass through pores of the
AqpZ channel.
An interesting property of aquaporins is that they are
completely impermeable to protons, so that streams
of protons cannot traverse the channel even though
they readily pass through water molecules via the
process of donor-acceptor configurations.
Aquaporins interfere with this donor-acceptor
model by forcing the water molecules to flip-
flop half way along the channel, so that water
molecules enter the channel face up (hydrogen
side up and oxygen side down, that, is the
oxygen enters first followed by the two
hydrogens), flip over, and leave the channel face
down (so that the oxygen leaves first, followed
by the two hydrogens).
ex: in kidney
in the properly functioning aquaporins may
transport as much as 20 L of water per hour,
whereas
improperly functioning aquaporins may result in
diseases such as:
diabetes insipidus and
congenital cataracts of the eye.
Carrier Proteins
Carrier proteins can use ATP-driven
transport mechanisms to ferry specific
substances across the plasmalemma against
a concentration gradient.
Carrier proteins are multipass membrane transport proteins
that possess binding sites for specific ions or molecules on both
sides of the lipid bilayer. When an ion or molecule specific to the
particular carrier protein binds to the binding site, the carrier
protein undergoes reversible conformational changes; as the ion
or molecule is released on the other side of the membrane, the
carrier protein returns to its previous conformation
As stated previously, transport by carrier proteins may be
passive, along an electrochemical concentration gradient, or
active, against a gradient, thereby requiring energy
expenditure by the cell.
Transport may be
uniport, a single molecule moving in one direction,
or coupled, two different molecules moving in the
same (symport)
or opposite (antiport) directions (see Fig. 2–11). Coupled
transporters convey the solutes either simultaneously or
sequentially.
Primary Active Transport by Na+-K+ Pump
Normally, the concentration of Na+ is much greater
outside of the cell than inside, and the concentration
of K+ is much greater inside of the cell than outside.
The cell maintains this concentration differential by
expending adenosine triphosphate (ATP) to
drive a coupled antiport carrier protein known as
the Na+-K+ pump. This pump transports K+ ions
into and Na+ ions out of the cell, each against a
steep concentration gradient. Because this
concentration differential is essential for the survival
and normal functioning of practically every animal
cell, the plasma membrane of all animal cells
possesses a large number of these pumps.
The Na+- K+ pump possesses 2 binding sites for K+ on its
extracellular aspect and 3 binding sites for Na+ on its
cytoplasmic aspect;
thus, for every 2 K+ ions conveyed into the cell, 3 Na+
ions are transported out of the cell.
The constant operation of this pump reduces the intracellular ion
concentration, resulting in decreased intracellular osmotic pressure.
If the osmotic pressure within the cell were not reduced by the
Na+-K+ pump, water would enter the cell in large quantities, causing
the cell to swell and eventually to succumb to osmotic lysis (i.e., the
cell would burst). Hence, it is through the operation of this pump
that the cell is able to regulate its osmolarity and, consequently, its
volume.
Additionally, this pump supplies minor assistance to the K+ leak
channels in the maintenance of the cell membrane potential.
Because the binding sites on the external aspect of the pump bind
not only K+ but also the glycoside ouabain, this glycoside inhibits
the Na+-K+ pump.
Secondary Active Transport by Coupled
Carrier Proteins
The ATP-driven transport of Na+ out of the
cell establishes a low intracellular
concentration of that ion. The energy reservoir
inherent in the sodium ion gradient can be
used by carrier proteins to transport ions or
other molecules against a concentration
gradient. Frequently, this mode of active
transport is referred to as secondary active
transport, distinct from the primary active
transport, which uses the energy released
from hydrolysis of ATP.
The carrier proteins that participate in
secondary active transport are either
symports or antiports. As a Na+ ion binds to
the extracellular aspect of the carrier
protein, another ion or small molecule (e.g.,
glucose) also binds to a region on the same
aspect of the carrier protein, inducing in it a
conformational alteration. The change in
conformation results in the transfer and
subsequent release of both molecules on the
other side of the membrane.
ATP-Binding Cassette Transporters (ABC-
Transporters)
These highly conserved transporters occur
in the largest numbers of all carrier proteins.
They are present in both prokaryotic
organisms, such as bacteria, as well as in all
eukaryotic organisms. The major difference is
that, in prokaryotic organisms, the ABC-
transporters move substances in both
directions (into and out of the cell), whereas,
in eukaryotic cells, the transport is in a single
direction only, namely out of the cell; only
the eukaryotic transporters are discussed.
ABC-transporters are transmembrane proteins, thus
protruding through the entire cell membrane. The
intracellular portion of the transporters possesses
binding sites (known as ATP-binding cassettes) for two
ATP molecules. When ATP is not present, the
intracellular binding sites for specific molecules are
exposed, and the particular ion or molecule adheres to
the binding site. When the ATP molecules bind to the
ATP-binding cassettes, the transporter’s conformation
becomes altered, and the ion or molecule is permitted
to leave at the transporter’s extracellular surface. It
should be stated that not all ABC-transporters are
located on the plasmalemma; many are present on the
membranes of intracellular membranous organelles,
such as the trans-Golgi network (TGN), rough
endoplasmic reticulum (ER), and mitochondrion. The
transfer of epitopes into the TGN or into the rough ER
depends on the activities of specialized ABC-
transporters (see Chapter 12, Lymphoid [Immune]
System).
Many ABC-transporters transport various
hydrophobic toxic substances and drugs
out of the cell. Many cancer cells possess
specific ABC-transporters, known as
multidrug resistance proteins (MDR
proteins), that drive anticancer drugs out
of the cell, thus providing the malignant
cells with increased resistance to
chemotherapeutic agents.
Clinical Correlations
A member of the ABC-transporters, the cystic fibrosis
transmembrane conductance regulator protein
(CFTR protein, coded for by a mutated form of the
CFTR gene), is responsible for the formation of
abnormal chloride channel proteins, especially in the
respiratory system. These channels formed by these
proteins do not permit Cl– ions to pass through them to
leave the cell, thereby the increased negative charges
due to the increased concentration of chloride ions in
the cytoplasm attract Na+ ions into the cells. The
elevated NaCl content of the cell attracts water from
the extracellular milieu into the cell, increasing the
viscosity of the mucus lining the respiratory tract. The
thickened mucus blocks the smaller bronchioles leading
to infection, debilitated lung function, and, eventually,
death.
Das könnte Ihnen auch gefallen
- The Cell MembraneDokument36 SeitenThe Cell Membraneanulorance98Noch keine Bewertungen
- LO Week 7Dokument22 SeitenLO Week 7Matthew ChristopherNoch keine Bewertungen
- 3 Cytology Membran JunctionDokument48 Seiten3 Cytology Membran JunctionSamNoch keine Bewertungen
- Cell MembraneDokument15 SeitenCell MembraneAyesha SaleemNoch keine Bewertungen
- Zoology Lecture 4Dokument18 SeitenZoology Lecture 4Stephen OcheaNoch keine Bewertungen
- Components of Plasma Membranes: Learning ObjectivesDokument14 SeitenComponents of Plasma Membranes: Learning ObjectivesAlthea Junsan BernabeNoch keine Bewertungen
- Plasma Membrane in DetailsDokument24 SeitenPlasma Membrane in DetailsSyeda Kaneez Mohammadi MohammadiNoch keine Bewertungen
- Membrane StructureDokument20 SeitenMembrane StructureErnitaNoch keine Bewertungen
- Plasma MembraneDokument6 SeitenPlasma Membraneparashakthi PRNoch keine Bewertungen
- Unit 2 ReviewerDokument4 SeitenUnit 2 Revieweraaleah moscaNoch keine Bewertungen
- This Learning Competency Is Extracted From The Deped Curriculum GuideDokument6 SeitenThis Learning Competency Is Extracted From The Deped Curriculum GuideZuzuNoch keine Bewertungen
- Module 3B - The Plasma MembraneDokument17 SeitenModule 3B - The Plasma MembraneDiane Balaba OsingNoch keine Bewertungen
- Unit 3: How Are Eukaryotic Cells Organized Into Smaller Parts?Dokument6 SeitenUnit 3: How Are Eukaryotic Cells Organized Into Smaller Parts?juan mondaNoch keine Bewertungen
- SF - MD.0145.T.22 Ally Omary MwambelaDokument10 SeitenSF - MD.0145.T.22 Ally Omary MwambelaAlly OmaryNoch keine Bewertungen
- Study Exercise Biology 10-12 97.03Dokument7 SeitenStudy Exercise Biology 10-12 97.03Fabiola Vania FeliciaNoch keine Bewertungen
- Lesson 4: Cell Membrane and Its PermeabilityDokument12 SeitenLesson 4: Cell Membrane and Its PermeabilityremshopNoch keine Bewertungen
- BIO 315. NotesDokument42 SeitenBIO 315. NotesKevin KipropNoch keine Bewertungen
- 16.plasma membr-WPS OfficeDokument4 Seiten16.plasma membr-WPS OfficeMohamed RizwanNoch keine Bewertungen
- B&B Medical Physiology - II. Physiology of Cells and Molecules. 2. Functional Organization of The Cell. BDokument4 SeitenB&B Medical Physiology - II. Physiology of Cells and Molecules. 2. Functional Organization of The Cell. BbahlasaNoch keine Bewertungen
- Cell-Cell Adhesion and Cell Junction: Submitted by Ashish Palodkar Msc. Biotechnology 1 SemDokument70 SeitenCell-Cell Adhesion and Cell Junction: Submitted by Ashish Palodkar Msc. Biotechnology 1 SemGovinda BiswasNoch keine Bewertungen
- 3 B Cytology-Membrane StructuresDokument19 Seiten3 B Cytology-Membrane Structuresm.mansoor3377Noch keine Bewertungen
- Structural Biochemistry/Cell Organelles: Pictorial Representation of Organelles in A Typical Animal CellDokument8 SeitenStructural Biochemistry/Cell Organelles: Pictorial Representation of Organelles in A Typical Animal CellPrincess Angie GonzalesNoch keine Bewertungen
- Cell & Its FunctionsDokument14 SeitenCell & Its Functionsamber tariqNoch keine Bewertungen
- 2 4 Plasma MembraneDokument22 Seiten2 4 Plasma Membraneıamnıkolaı 4Noch keine Bewertungen
- Basic Cell Structure & FunctionDokument27 SeitenBasic Cell Structure & FunctionJawaad AsifNoch keine Bewertungen
- Membrane Structure and Function: Lecture OutlineDokument11 SeitenMembrane Structure and Function: Lecture OutlineJohn Westly S. SabueroNoch keine Bewertungen
- Revision 2Dokument56 SeitenRevision 2Biology BảoNoch keine Bewertungen
- Plasma Membrane DeutschDokument5 SeitenPlasma Membrane DeutschSafeer SefiNoch keine Bewertungen
- Chapter 4-Jnu Biomembranes-1 - 複本Dokument48 SeitenChapter 4-Jnu Biomembranes-1 - 複本Wai Kwong ChiuNoch keine Bewertungen
- Plant Cell Structure and Importance of Cell Organelles I Post Harvest PhysiologyDokument10 SeitenPlant Cell Structure and Importance of Cell Organelles I Post Harvest Physiologychristom karungariNoch keine Bewertungen
- Chapter7 - Membrane Structure and FunctionDokument13 SeitenChapter7 - Membrane Structure and FunctionKrizziane Ivy CuajotorNoch keine Bewertungen
- Animal Physiology Theory Lec1Dokument24 SeitenAnimal Physiology Theory Lec1ao868598Noch keine Bewertungen
- Kuliah MembranDokument61 SeitenKuliah Membrannikmah nuur rochmahNoch keine Bewertungen
- Chapter 12 SummaryDokument7 SeitenChapter 12 SummaryCharlotteNoch keine Bewertungen
- Structure and Organization of MembranesDokument20 SeitenStructure and Organization of MembranesronojoysenguptaNoch keine Bewertungen
- Bio MembranesDokument7 SeitenBio MembranesSwasti Agnihotri100% (1)
- Integrasi Sel Dalam Jaringan (Kuliah) - 2 - 2Dokument125 SeitenIntegrasi Sel Dalam Jaringan (Kuliah) - 2 - 2komang nickoNoch keine Bewertungen
- Concise Histology Gartner, Leslie PDFDokument478 SeitenConcise Histology Gartner, Leslie PDFLexsshNoch keine Bewertungen
- Membrane Structure and Function: Lecture OutlineDokument11 SeitenMembrane Structure and Function: Lecture Outlinehaha_le12Noch keine Bewertungen
- Basic Cell Theory - StructureDokument32 SeitenBasic Cell Theory - StructureRuby SamarNoch keine Bewertungen
- Anatomy and Physiology Chapter 3Dokument44 SeitenAnatomy and Physiology Chapter 3Stephanie FoleyNoch keine Bewertungen
- The Structural Components of The Cell MembraneDokument5 SeitenThe Structural Components of The Cell MembraneJanine CambaNoch keine Bewertungen
- Cell+ +Cell+MembraneDokument26 SeitenCell+ +Cell+Membraneemancipation1506Noch keine Bewertungen
- Cell Membrane: Navigation SearchDokument10 SeitenCell Membrane: Navigation SearchMuhassanah Abd AzizNoch keine Bewertungen
- Anatomic Sciences PDFDokument656 SeitenAnatomic Sciences PDFFatima Nurjanna AwaliNoch keine Bewertungen
- The Plasma (Cytoplasmic) MembraneDokument6 SeitenThe Plasma (Cytoplasmic) Membraneprism1702Noch keine Bewertungen
- 2structure and Functions of Cell MembraneDokument19 Seiten2structure and Functions of Cell MembraneedriansamaNoch keine Bewertungen
- CellDokument8 SeitenCellvjaNoch keine Bewertungen
- CropDokument11 SeitenCropHall OwnNoch keine Bewertungen
- Module 10 ACTDokument5 SeitenModule 10 ACTLeighNoch keine Bewertungen
- Cell MebraneDokument10 SeitenCell MebraneChandra SihombingNoch keine Bewertungen
- Cell Structure: Prepared by Dr. Sana AslamDokument41 SeitenCell Structure: Prepared by Dr. Sana Aslamsajjad Ali balouch100% (1)
- Morphological Function of The Cell Presentation PHDokument52 SeitenMorphological Function of The Cell Presentation PHDoc HamsNoch keine Bewertungen
- CytoplasmDokument13 SeitenCytoplasmTanveerNoch keine Bewertungen
- Cell OrganellesDokument8 SeitenCell OrganellesMuqadas khanNoch keine Bewertungen
- All Epithelial Cells in Contact With Subjacent Connective Tissue Have at Their Basal Surfaces A FeltDokument8 SeitenAll Epithelial Cells in Contact With Subjacent Connective Tissue Have at Their Basal Surfaces A FeltMegan LewisNoch keine Bewertungen
- Membrane Structure and FunctionDokument53 SeitenMembrane Structure and FunctionRenel BendoyNoch keine Bewertungen
- Plasma MembraneDokument18 SeitenPlasma MembraneJemuel Bucud LagartoNoch keine Bewertungen
- Chapter 1doc 3Dokument4 SeitenChapter 1doc 3Enju ChipepeNoch keine Bewertungen
- Healthy Lifestyle and EatingDokument4 SeitenHealthy Lifestyle and EatingMohammed ShaikNoch keine Bewertungen
- Bones of The Foot - Tarsal Bones - Metatarsal Bone - Geeky MedicsDokument9 SeitenBones of The Foot - Tarsal Bones - Metatarsal Bone - Geeky MedicsMohammed ShaikNoch keine Bewertungen
- Anatomy of The Limbic SystemDokument32 SeitenAnatomy of The Limbic SystemMohammed ShaikNoch keine Bewertungen
- ChromosomesDokument11 SeitenChromosomesMohammed ShaikNoch keine Bewertungen
- All Graphs and Charts Available in Show MeDokument16 SeitenAll Graphs and Charts Available in Show MeGANGA TAGRANoch keine Bewertungen
- CISCO Router Software - Configuration PDFDokument408 SeitenCISCO Router Software - Configuration PDFasalihovicNoch keine Bewertungen
- V7R3 Recovery Guide Sc415304Dokument560 SeitenV7R3 Recovery Guide Sc415304gort400Noch keine Bewertungen
- Baulkham Hills 2012 2U Prelim Yearly & SolutionsDokument11 SeitenBaulkham Hills 2012 2U Prelim Yearly & SolutionsYe ZhangNoch keine Bewertungen
- DLP Mathematics 6 q1 w6Dokument5 SeitenDLP Mathematics 6 q1 w6Kathlyn PerezNoch keine Bewertungen
- 0606 Additional Mathematics: MARK SCHEME For The May/June 2015 SeriesDokument9 Seiten0606 Additional Mathematics: MARK SCHEME For The May/June 2015 Serieswai yanNoch keine Bewertungen
- HydrometerDokument13 SeitenHydrometerShubhrajit MaitraNoch keine Bewertungen
- LTE Rach ProcedureDokument4 SeitenLTE Rach ProcedureDeepak JammyNoch keine Bewertungen
- Ch-21 Runoff TrianglesDokument9 SeitenCh-21 Runoff TrianglesIsha TachhekarNoch keine Bewertungen
- Backup Olt CodigoDokument5 SeitenBackup Olt CodigoCarlos GomezNoch keine Bewertungen
- Number Patterns and SequencesDokument10 SeitenNumber Patterns and SequencesMohamed Hawash80% (5)
- Acceleration InquiryDokument4 SeitenAcceleration Inquiryapi-240343522Noch keine Bewertungen
- Computer Dictionary PDFDokument95 SeitenComputer Dictionary PDFMarjenneilNoch keine Bewertungen
- Pelod Vs Sofa Scoring in PediatricDokument6 SeitenPelod Vs Sofa Scoring in PediatricAdrian KhomanNoch keine Bewertungen
- Microgrid Modeling and Grid Interconnection StudiesDokument71 SeitenMicrogrid Modeling and Grid Interconnection StudiesVeeravasantharao BattulaNoch keine Bewertungen
- MA201 Mechanical Vertical Machining Center 133-134Dokument2 SeitenMA201 Mechanical Vertical Machining Center 133-134Ali HashmiNoch keine Bewertungen
- Agfa CR 10XDokument4 SeitenAgfa CR 10Xwisateru Inti niagaNoch keine Bewertungen
- 144S... - PCB Series: Signal Conditioned Precision Pressure TransducersDokument4 Seiten144S... - PCB Series: Signal Conditioned Precision Pressure TransducersAnish KumarNoch keine Bewertungen
- FST 3000Dokument4 SeitenFST 3000ariksyaiful82Noch keine Bewertungen
- HST TrainingDokument11 SeitenHST TrainingRamesh BabuNoch keine Bewertungen
- Gps Vehicle Tracker User Manual: PrefaceDokument13 SeitenGps Vehicle Tracker User Manual: PrefaceFedericoNoch keine Bewertungen
- Odp-090r16bv 17KV PDFDokument1 SeiteOdp-090r16bv 17KV PDFAlberto LinaresNoch keine Bewertungen
- API ISCAN-LITE ScannerDokument4 SeitenAPI ISCAN-LITE Scannergrg_greNoch keine Bewertungen
- Detection of Repetitive Forex Chart PatternsDokument8 SeitenDetection of Repetitive Forex Chart PatternsDwight ThothNoch keine Bewertungen
- Strain STREMADokument6 SeitenStrain STREMAChavin StormNoch keine Bewertungen
- Lec 4 Second Order Linear Differential EquationsDokument51 SeitenLec 4 Second Order Linear Differential EquationsTarun KatariaNoch keine Bewertungen
- 10048-PAVE-RPT-0101 Types of The Vehicle Considered, Vehicle Details and Single Equivalent Load and Reprtions of LoadDokument14 Seiten10048-PAVE-RPT-0101 Types of The Vehicle Considered, Vehicle Details and Single Equivalent Load and Reprtions of LoaddarshanNoch keine Bewertungen
- Signal Integrity Modeling and Measurement of TSV in 3D ICDokument4 SeitenSignal Integrity Modeling and Measurement of TSV in 3D IC張志榮Noch keine Bewertungen
- Measures of Central Tendency: Mean Median ModeDokument20 SeitenMeasures of Central Tendency: Mean Median ModeRia BarisoNoch keine Bewertungen
- PC - Section 1.3 - Worksheet PDFDokument2 SeitenPC - Section 1.3 - Worksheet PDFAnabbNoch keine Bewertungen