Beruflich Dokumente
Kultur Dokumente
12 Chemistry Imp Ch4 3
Hochgeladen von
Gopal PenjarlaCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
12 Chemistry Imp Ch4 3
Hochgeladen von
Gopal PenjarlaCopyright:
Verfügbare Formate
Chemical Formula Models - Acids, Bases and Polyatomic Ions
H H Cl
H PO4
H NO3
H
CH3COO
H
H
SO4 Na OH
H
NH3
K OH
H
CO3
H H OH
Content may be reproduced for educational use with acknowledgement © www.chemicalformula.org
The Chemical Formula of Acids, Bases and Polyatomic Ions
1. Acids 3. Ions (charged particles) 4. Chemical Equations
Phosphoric acid H3PO4 Phosphate ion PO43- H3PO4 -> 3H+ + PO43-
Sulfuric acid H2SO4 Sulfate ion SO42- H2SO4 -> 2H+ + SO42-
Carbonic acid H2CO3 Carbonate ion CO32- H2CO3 -> 2H+ + CO32-
Hydrochloric acid HCl Chloride ion Cl- HCl -> H+ + Cl-
Nitric acid HNO3 Nitrate ion NO3- HNO3 -> H+ + NO3-
Acetic acid CH3COOH Acetate ion CH3COO- CH3COOH -> CH3COO- + H+
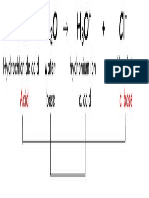
Acids produce hydrogen ions in solution, H+ Hydrogen ion H+
2. Alkalis (soluble bases)
Sodium hydroxide NaOH Hydroxide ion OH- NaOH -> Na+ + OH-
Potassium hydroxide KOH Hydroxide ion OH- KOH -> K+ + OH-
Ammonia NH3 Ammonium ion NH4+ NH3 + H2O -> NH4+ + OH-
Bases produce hydroxide ions in solution, OH-
Content may be reproduced for educational use with acknowledgement © www.chemicalformula.org
Das könnte Ihnen auch gefallen
- Everything Is Fucked by Mark Manson PDFDokument6 SeitenEverything Is Fucked by Mark Manson PDFGopal PenjarlaNoch keine Bewertungen
- Forensic Medicine and ToxicologyDokument8 SeitenForensic Medicine and ToxicologyGopal PenjarlaNoch keine Bewertungen
- CHAPTER 3 - Concept of Acid-Base NeutralizationDokument49 SeitenCHAPTER 3 - Concept of Acid-Base NeutralizationRichie BobbyNoch keine Bewertungen
- Mysterious Mixtures ExperimentDokument13 SeitenMysterious Mixtures ExperimentJerneth Nyka FloresNoch keine Bewertungen
- Monohydric Alcohols Their Ethers and Esters Sulphur Analogues Nitrogen Derivatives Organometallic Compounds: A Modern Comprehensive TreatiseVon EverandMonohydric Alcohols Their Ethers and Esters Sulphur Analogues Nitrogen Derivatives Organometallic Compounds: A Modern Comprehensive TreatiseNoch keine Bewertungen
- Oxoacids of Chlorine by H To O ChemistryDokument44 SeitenOxoacids of Chlorine by H To O ChemistryRitu JoharNoch keine Bewertungen
- Cleaning Validation For Biopharmaceutical Manufacturing at GenentechDokument4 SeitenCleaning Validation For Biopharmaceutical Manufacturing at GenentechLisa Denysa SigarNoch keine Bewertungen
- Carboxylic Acids and Its Derivatives NotesDokument26 SeitenCarboxylic Acids and Its Derivatives NotesAyush Gangwani50% (2)
- Acids and Bases NotesDokument17 SeitenAcids and Bases NotesNap DoNoch keine Bewertungen
- Ammonia Cracker PDFDokument8 SeitenAmmonia Cracker PDFpinky_y2kNoch keine Bewertungen
- View CertificateDokument1 SeiteView CertificateGopal PenjarlaNoch keine Bewertungen
- Control of Flexural Cracks by Jack C. McCormacDokument9 SeitenControl of Flexural Cracks by Jack C. McCormacbig_one214Noch keine Bewertungen
- Regulation of Acid-Base Balance: ElizabethDokument35 SeitenRegulation of Acid-Base Balance: ElizabethGeorge LusanaNoch keine Bewertungen
- PH Test Lab SheetDokument4 SeitenPH Test Lab Sheetnor5903Noch keine Bewertungen
- Ionic EquilibriumDokument60 SeitenIonic EquilibriumVermavinay3940 vinay394080% (5)
- Alcohols, Ethers, Phenols: - Structure and BondingDokument32 SeitenAlcohols, Ethers, Phenols: - Structure and Bondingalshaeri999Noch keine Bewertungen
- Chemistry 1302: CHAPTER 3.3 - Acid Base ChemistryDokument15 SeitenChemistry 1302: CHAPTER 3.3 - Acid Base Chemistryzak mahmoudNoch keine Bewertungen
- Phenols 3 PDFDokument58 SeitenPhenols 3 PDFNova sounds - No copyright musicNoch keine Bewertungen
- Chemical Formula - Common Compunds - Typs of Chemical FormulaDokument7 SeitenChemical Formula - Common Compunds - Typs of Chemical FormulaSHAMS QUAMARNoch keine Bewertungen
- 1 - Introduction To Acid-Base PDFDokument4 Seiten1 - Introduction To Acid-Base PDFJron Victor Smith SamsonNoch keine Bewertungen
- CBSE Class 12 Chem Notes Question Bank Alcohols Phenols and Ethers PDFDokument19 SeitenCBSE Class 12 Chem Notes Question Bank Alcohols Phenols and Ethers PDFParam MNoch keine Bewertungen
- Hydro Hydro Hydro: + Nonmetal+ Ic + Acid Nonmetal + Ic + Acid Nonmetal + + AcidDokument21 SeitenHydro Hydro Hydro: + Nonmetal+ Ic + Acid Nonmetal + Ic + Acid Nonmetal + + AcidHani TamimiNoch keine Bewertungen
- Chap 02cDokument10 SeitenChap 02cRCNoch keine Bewertungen
- Chem Tech Review Acids and BasesDokument69 SeitenChem Tech Review Acids and BasesClintNoch keine Bewertungen
- Acid Base ConceptDokument20 SeitenAcid Base Conceptyadavamlesh045Noch keine Bewertungen
- PPP1A 8.1 Arrhenius and NamingDokument13 SeitenPPP1A 8.1 Arrhenius and NamingRichard LindemannNoch keine Bewertungen
- Acizi Si BazeDokument4 SeitenAcizi Si BazeKristanna123Noch keine Bewertungen
- Ionic Equilibrium Lecture 9 (1st January 2023) Handout and HomeworkDokument402 SeitenIonic Equilibrium Lecture 9 (1st January 2023) Handout and Homeworktanishq yadavNoch keine Bewertungen
- Acids and BasesDokument17 SeitenAcids and Basespadidela swarochishraoNoch keine Bewertungen
- Chapter 3 - Concept of Acid-Base NeutralisationDokument58 SeitenChapter 3 - Concept of Acid-Base NeutralisationIkmal FikriNoch keine Bewertungen
- Chapter 7 Acid-Base ReactionDokument111 SeitenChapter 7 Acid-Base ReactionUMMU MARDHIAH ABDUL HALIMNoch keine Bewertungen
- Base Practice PDFDokument4 SeitenBase Practice PDFabdelrahmanadelm2008Noch keine Bewertungen
- Chapter 3 Acid - BaseDokument98 SeitenChapter 3 Acid - BasePHƯƠNG ĐẶNG YẾNNoch keine Bewertungen
- FI-Medicinal Chemistry 1-Smsrt Genap - TerbaruDokument24 SeitenFI-Medicinal Chemistry 1-Smsrt Genap - TerbaruSahabat BaljaiNoch keine Bewertungen
- Organi and Inorganic AcidDokument7 SeitenOrgani and Inorganic AcidSHWETA GUPTANoch keine Bewertungen
- Chapter 3 Acid - BaseDokument96 SeitenChapter 3 Acid - BaseAnh NhamNoch keine Bewertungen
- PhenolsDokument34 SeitenPhenolsLoran Prelya TengayNoch keine Bewertungen
- Chapter 3 Acid - BaseDokument98 SeitenChapter 3 Acid - BaseKhoa Nguyen Viet DangNoch keine Bewertungen
- 1288 PhenolsDokument29 Seiten1288 PhenolsX ThrxNoch keine Bewertungen
- Chapter 4 Reactions in SolutionsDokument29 SeitenChapter 4 Reactions in SolutionsMohamed AlQallafNoch keine Bewertungen
- Alcohols, Phenols and Ethers - Short Notes - Prayas JEE 2024Dokument6 SeitenAlcohols, Phenols and Ethers - Short Notes - Prayas JEE 2024yash vardhanNoch keine Bewertungen
- Conjugate Acid and Base PairsDokument1 SeiteConjugate Acid and Base Pairsfelisita wisangNoch keine Bewertungen
- Kimi1055 L4 Acid Base Equilibria SlidesDokument30 SeitenKimi1055 L4 Acid Base Equilibria Slidesmurad8rahimovNoch keine Bewertungen
- Acid Nomenclature KEY1Dokument2 SeitenAcid Nomenclature KEY1Jose ArenasNoch keine Bewertungen
- Dicarboxylic Acids: - Bromoadipic AcidDokument32 SeitenDicarboxylic Acids: - Bromoadipic AcidByakuya BleachNoch keine Bewertungen
- Chapter 3 Acid - BaseDokument98 SeitenChapter 3 Acid - BaseNhan PhướcNoch keine Bewertungen
- AcidBase Equilibrium Ch101EEEDokument343 SeitenAcidBase Equilibrium Ch101EEEA. F. M. Mahfuzul KabirNoch keine Bewertungen
- Phenols and Ethers NotesDokument9 SeitenPhenols and Ethers NotesDhanaranjani BNoch keine Bewertungen
- Acids & Bases in Organic ChemistryDokument24 SeitenAcids & Bases in Organic ChemistryBaban BaidyaNoch keine Bewertungen
- Phenols: Bio-Organic Chemistry-I BBT-202 Lecture-13Dokument37 SeitenPhenols: Bio-Organic Chemistry-I BBT-202 Lecture-13Sate AhmadNoch keine Bewertungen
- CH 35 Titrimetry Acid BaseDokument46 SeitenCH 35 Titrimetry Acid BaseFarhan Muhammad IskandarNoch keine Bewertungen
- Esterfication MechanismDokument1 SeiteEsterfication MechanismrasikamuhandiramgeNoch keine Bewertungen
- Acids Answer ChemistryDokument1 SeiteAcids Answer ChemistryW BNoch keine Bewertungen
- Carboxylic Acid 13-02-2019Dokument29 SeitenCarboxylic Acid 13-02-2019Sania KhanNoch keine Bewertungen
- 3.ii. BASES AND ACIDS OF CHOICEDokument110 Seiten3.ii. BASES AND ACIDS OF CHOICEKeith OmwoyoNoch keine Bewertungen
- APChemDokument8 SeitenAPChemMacie CareyNoch keine Bewertungen
- Unit 1 Activity 7 - Notes TypedDokument3 SeitenUnit 1 Activity 7 - Notes TypedSijie LiNoch keine Bewertungen
- Handout - Acids and Bases - v2 - 101Dokument2 SeitenHandout - Acids and Bases - v2 - 101maheenkhan1605Noch keine Bewertungen
- Chem 222 Lecture Note (1) - 1Dokument11 SeitenChem 222 Lecture Note (1) - 1estherorjimkd1Noch keine Bewertungen
- Carboxylic Acids, Anhydrides, Esters, and AmidesDokument35 SeitenCarboxylic Acids, Anhydrides, Esters, and AmidesHarshwardhan PhatakNoch keine Bewertungen
- Hanifa KimiaDokument3 SeitenHanifa KimiaTengku FarhanNoch keine Bewertungen
- Country's Best Online Test PlatformDokument63 SeitenCountry's Best Online Test PlatformSubhrasankar RaychaudhuryNoch keine Bewertungen
- Bronst Acid Base QsDokument8 SeitenBronst Acid Base QsIsta EgbetoNoch keine Bewertungen
- Asam Basa - 16Dokument157 SeitenAsam Basa - 16Yulia KevinNoch keine Bewertungen
- Alcohols & Ethers TheoryDokument15 SeitenAlcohols & Ethers TheorySaif KhanNoch keine Bewertungen
- Acid Ionic EqulbrmDokument21 SeitenAcid Ionic EqulbrmsheenajerryNoch keine Bewertungen
- Organometallic Chemistry: Plenary Lectures Presented at the Fourth International Conference on Organometallic ChemistryVon EverandOrganometallic Chemistry: Plenary Lectures Presented at the Fourth International Conference on Organometallic ChemistryF. G. A. StoneNoch keine Bewertungen
- MONTHLY - PORTFOLIO - AXISMF-Oct 2019Dokument117 SeitenMONTHLY - PORTFOLIO - AXISMF-Oct 2019Gopal PenjarlaNoch keine Bewertungen
- Stoichiometry and Redox Reactions PDFDokument66 SeitenStoichiometry and Redox Reactions PDFGopal PenjarlaNoch keine Bewertungen
- 1st Year Chemistry Model Paper 1 PDFDokument1 Seite1st Year Chemistry Model Paper 1 PDFGopal PenjarlaNoch keine Bewertungen
- Atomic StructureDokument1 SeiteAtomic StructureGopal PenjarlaNoch keine Bewertungen
- Equivalent Weight and NormalityDokument6 SeitenEquivalent Weight and NormalityGopal PenjarlaNoch keine Bewertungen
- Balancing Chemical Equations Worksheet Student Instructions: Acids Alkalis (Bases)Dokument5 SeitenBalancing Chemical Equations Worksheet Student Instructions: Acids Alkalis (Bases)Gopal PenjarlaNoch keine Bewertungen
- Section 27 Dash 3Dokument2 SeitenSection 27 Dash 3Gopal PenjarlaNoch keine Bewertungen
- IIT JEE Main Advanced Physical Chemistry 12th Volumetric Analysis PDFDokument38 SeitenIIT JEE Main Advanced Physical Chemistry 12th Volumetric Analysis PDFGopal PenjarlaNoch keine Bewertungen
- Mini Project Reaction Engineering GROUP 9 (Stage 2)Dokument41 SeitenMini Project Reaction Engineering GROUP 9 (Stage 2)Syahmi Asraaf100% (3)
- Bovine BOVINE - SERUM - ALBUMINSerum AlbuminDokument3 SeitenBovine BOVINE - SERUM - ALBUMINSerum AlbuminHaaelNoch keine Bewertungen
- Sheet 1Dokument3 SeitenSheet 1Kwaku AkostikNoch keine Bewertungen
- Ion Exchange Resin, Ionac C-240 by Sybron Chemicals IncDokument3 SeitenIon Exchange Resin, Ionac C-240 by Sybron Chemicals IncEnngelpberttNoch keine Bewertungen
- TSI - Lab Guidelines and Standards (2003)Dokument36 SeitenTSI - Lab Guidelines and Standards (2003)chritopherNoch keine Bewertungen
- Section 1: Identification: Safety Data Sheet: Simple Green® All-Purpose CleanerDokument5 SeitenSection 1: Identification: Safety Data Sheet: Simple Green® All-Purpose CleanerHARIS SHAHIDNoch keine Bewertungen
- A Study On Physico-Chemical Properties of Ground Water Quality of Various Locations of Kanpur CityDokument3 SeitenA Study On Physico-Chemical Properties of Ground Water Quality of Various Locations of Kanpur CityNeerja ShuklaNoch keine Bewertungen
- Heats Sodium Hydroxide Solutions: SpecificDokument4 SeitenHeats Sodium Hydroxide Solutions: SpecificTJ ArciagaNoch keine Bewertungen
- Molecular Basis Off InheritanceDokument5 SeitenMolecular Basis Off InheritanceShivam Kumar PathakNoch keine Bewertungen
- Pengaruh Tegangan Listrik Dan Ukuran Awal Fragment Pada Metode BiorockDokument11 SeitenPengaruh Tegangan Listrik Dan Ukuran Awal Fragment Pada Metode BiorockEko Sulkhani YuliantoNoch keine Bewertungen
- Liquid-Liquid Equilibrium: Ternary SystemDokument29 SeitenLiquid-Liquid Equilibrium: Ternary SystemaaaNoch keine Bewertungen
- Discrimination Between Roasted Coffee, Roasted Corn and Coffee Husks by DiffuseDokument8 SeitenDiscrimination Between Roasted Coffee, Roasted Corn and Coffee Husks by DiffuseJavier OrtizNoch keine Bewertungen
- Astm B 237 - 01Dokument2 SeitenAstm B 237 - 01kaminaljuyu100% (2)
- Metallurgy of Iron and SteelmakingDokument13 SeitenMetallurgy of Iron and SteelmakingAgustine SetiawanNoch keine Bewertungen
- Fuel Cells Module ManualDokument85 SeitenFuel Cells Module ManualstarofdseaNoch keine Bewertungen
- Literature ReviewDokument7 SeitenLiterature ReviewDeepa Chitralekha RanaNoch keine Bewertungen
- CR Lab ReportDokument6 SeitenCR Lab ReportslowteeNoch keine Bewertungen
- Accurate Rapid Analysis of Alkali Contents in Portland CementDokument4 SeitenAccurate Rapid Analysis of Alkali Contents in Portland CementyinglvNoch keine Bewertungen
- EE669 Lecture Slides Module 1Dokument46 SeitenEE669 Lecture Slides Module 1sivanaresh14Noch keine Bewertungen
- Assignment 2Dokument4 SeitenAssignment 2eja70Noch keine Bewertungen
- CalculationDokument7 SeitenCalculationTiger RaoNoch keine Bewertungen
- Fluid Mechanics Chapter 1 5 PDFDokument32 SeitenFluid Mechanics Chapter 1 5 PDFPotLopez100% (1)
- Basic Tools of Analytical Chemistry: Chapter OverviewDokument28 SeitenBasic Tools of Analytical Chemistry: Chapter OverviewReza LaurinaNoch keine Bewertungen
- UV Curing Glass Glue - Guangdong Hengda New Materials Technology-HTB1h3fHHXXXXXXOXFXX - prxfXXX4Dokument6 SeitenUV Curing Glass Glue - Guangdong Hengda New Materials Technology-HTB1h3fHHXXXXXXOXFXX - prxfXXX4ugo_rossiNoch keine Bewertungen