Beruflich Dokumente
Kultur Dokumente
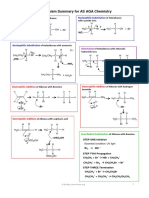
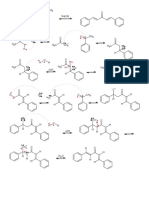
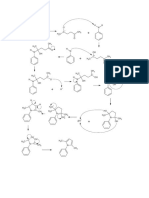
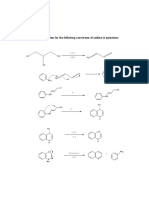
Cope Elimination
Hochgeladen von
Art Julius D. HallazgoOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Cope Elimination
Hochgeladen von
Art Julius D. HallazgoCopyright:
Verfügbare Formate
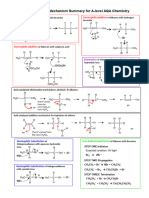
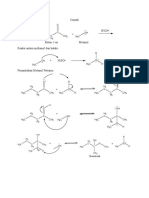
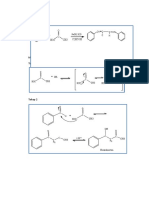
–
H O H
+
N H H N H H H H
H H2O2 heat
+ HO N
H
H H H H H H
H H H H
Note: peroxyacids (e.g. mCPBA) can hydroxyl group is a stronger base than
also be used for the oxidation step the protonated N-oxide
H3C H3C O H3C –
HO OH HO
–
O
CH3 CH3 + H CH3 +
N N N
CH3 CH3 CH3
H2O2
H
+ H2O
H H H
H H H
H H H
"N-oxide"
Tertiary amine
H3C –
O CH3
CH3 +
Bonds Formed: C-C (π), O-H
N H3C
CH3 OH
Bonds Broken C-H, C-N
heat + N
CH3
H hydroxyl amine
H
H
H H
H
N-oxide Alkene
H3C – CH3
O H
CH3 +
H N the C-H bond anti to the N-oxide remains
CH3
H
H
heat
H
H H H
H
H the C-H bond syn to the N-oxide breaks
H
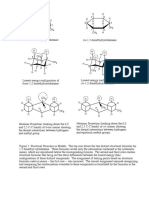
C-H bond anti to the Br breaks (giving Zaitsev) Major Minor
CH3 CH3
CH3 H
H Br
H
H NaOEt +
EtOH, heat H
H H H
H H
H H
H H
H
H C-H bond anti to the Zaitsev product "Hofmann product"
Br breaks (Hofmann) (more substituted alkene) (less substituted alkene)
break C-H form C-C (pi)
H H
H H H H
H H break C-N
H H
H H
H +
H
H
–
N
+
+ HO N
H
N H H
O H
–
O H 11
Transition state
form O-H
H3C H
CH3 H3C H3C
H +
O H
N
H3C –
O H + N
CH3
heat
H H
Bonds Formed: C-C (π), O-H
H H H
H
H Bonds Broken C-H, C-N
H
Das könnte Ihnen auch gefallen
- 6th Central Pay Commission Salary CalculatorDokument15 Seiten6th Central Pay Commission Salary Calculatorrakhonde100% (436)
- 6th Central Pay Commission Salary CalculatorDokument15 Seiten6th Central Pay Commission Salary Calculatorrakhonde100% (436)
- Nucleophilic Substitution and Electrophilic Addition ReactionsDokument4 SeitenNucleophilic Substitution and Electrophilic Addition Reactionsjohn mNoch keine Bewertungen
- Aqa Mechanisms A Level SummaryDokument5 SeitenAqa Mechanisms A Level SummaryRS JNoch keine Bewertungen
- Yaws - Chemical Properties Handbook Physical, Thermodynamics, Engironmental Transport, Safety & Health Related Properties For Organic & Inorganic ChemicalDokument2 SeitenYaws - Chemical Properties Handbook Physical, Thermodynamics, Engironmental Transport, Safety & Health Related Properties For Organic & Inorganic ChemicalRanny NovellaNoch keine Bewertungen
- Summary of All Reactions For Organic ChemistryDokument4 SeitenSummary of All Reactions For Organic Chemistryfoodytang91% (23)
- Coriolis Flow MeasurementDokument19 SeitenCoriolis Flow MeasurementtetiospamNoch keine Bewertungen
- Catalase Enzyme LabDokument3 SeitenCatalase Enzyme LabBruce0% (1)
- Mechanism Summary For A-Level AQA Chemistry: BR BRDokument5 SeitenMechanism Summary For A-Level AQA Chemistry: BR BRamrhkmhNoch keine Bewertungen
- ISO 8217 2017 FUEL STANDARDSDokument2 SeitenISO 8217 2017 FUEL STANDARDSAnton100% (2)
- Analysis of SalivaDokument5 SeitenAnalysis of SalivaYap JackyNoch keine Bewertungen
- Mech. Operations-Particle TechnologyDokument475 SeitenMech. Operations-Particle TechnologyEshwar Iyer100% (3)
- MechanismDokument2 SeitenMechanismRyan BoodramlallNoch keine Bewertungen
- Reaksi Esterifikasi Asam Sederhana Menggunakan NaOHDokument1 SeiteReaksi Esterifikasi Asam Sederhana Menggunakan NaOHSoy Viranda KusumaNoch keine Bewertungen
- Mechanism of the reaction between an amine and an aldehydeDokument1 SeiteMechanism of the reaction between an amine and an aldehydeabnerNoch keine Bewertungen
- A3 ReactionsDokument3 SeitenA3 ReactionshaNoch keine Bewertungen
- Holi4 PDFDokument1 SeiteHoli4 PDFKAREN ROSAS GARCIANoch keine Bewertungen
- HOLI4Dokument1 SeiteHOLI4KAREN ROSAS GARCIANoch keine Bewertungen
- MechanismsDokument5 SeitenMechanismsnajifaahmed223Noch keine Bewertungen
- Organic Chemistry 3 Assignment 1, B1748443Dokument7 SeitenOrganic Chemistry 3 Assignment 1, B1748443zacksNoch keine Bewertungen
- Reductions PPT 29-08-2020Dokument12 SeitenReductions PPT 29-08-2020jkc collegeNoch keine Bewertungen
- Trabajo de Química OrgánicaDokument1 SeiteTrabajo de Química OrgánicaPaulina Mota MacipNoch keine Bewertungen
- Mechanism Summary for AS AQA Chemistry ReactionsDokument3 SeitenMechanism Summary for AS AQA Chemistry ReactionsRS JNoch keine Bewertungen
- Reaksi antara Metanol dan Butan-2-on menghasilkan Ketal 2,2-dimetoksibutanaDokument2 SeitenReaksi antara Metanol dan Butan-2-on menghasilkan Ketal 2,2-dimetoksibutanaElis TianiNoch keine Bewertungen
- Reaksi antara Metanol dan Butan-2-on menghasilkan Ketal 2,2-dimetoksibutanaDokument2 SeitenReaksi antara Metanol dan Butan-2-on menghasilkan Ketal 2,2-dimetoksibutanaElis TianiNoch keine Bewertungen
- Aqa Mechanisms A21Dokument4 SeitenAqa Mechanisms A21Sarah INoch keine Bewertungen
- Heterocyclic ReactionsDokument2 SeitenHeterocyclic Reactionsthat's niceNoch keine Bewertungen
- The Logic of Chemical Synthesis Corey E J Amp Cheng X M 331 463Dokument133 SeitenThe Logic of Chemical Synthesis Corey E J Amp Cheng X M 331 463bann tvNoch keine Bewertungen
- Analisis Sintesis I: Sintesis Vitamin Dan Beberapa AntibiotikDokument12 SeitenAnalisis Sintesis I: Sintesis Vitamin Dan Beberapa AntibiotikNovita Sari AritonangNoch keine Bewertungen
- TABORADA - Act8 (Ketones and Aldehydes)Dokument4 SeitenTABORADA - Act8 (Ketones and Aldehydes)Justin Habaña TaboradaNoch keine Bewertungen
- Ejercicios Axial EcuatorialDokument1 SeiteEjercicios Axial EcuatorialJulio Cesar Boada MartinezNoch keine Bewertungen
- Homework Problems: Structure, Bonding & Hybridization 1. The Molecule Shown Below Is Griseofulvin, An Antifungal CompoundDokument8 SeitenHomework Problems: Structure, Bonding & Hybridization 1. The Molecule Shown Below Is Griseofulvin, An Antifungal CompoundPrachi KaushikNoch keine Bewertungen
- Rumus Gugus KimiaDokument1 SeiteRumus Gugus KimiaBagus WijayaNoch keine Bewertungen
- Dap An Lev BDokument59 SeitenDap An Lev BStormy StudiosNoch keine Bewertungen
- Program Chem Check-Up 2.0: Predicting structures, naming compounds and identifying reactionsDokument2 SeitenProgram Chem Check-Up 2.0: Predicting structures, naming compounds and identifying reactionsanis fazilaNoch keine Bewertungen
- Reaction Mechanism: C C H H H O Hgso H SO CHO H CDokument6 SeitenReaction Mechanism: C C H H H O Hgso H SO CHO H CFATHIMA THANHA T NNoch keine Bewertungen
- Alcohol Phenol Ether (1) 6Dokument9 SeitenAlcohol Phenol Ether (1) 6sdnishacNoch keine Bewertungen
- Dia orDokument8 SeitenDia orNaman MahawarNoch keine Bewertungen
- Coursebook Answers Chapter 18 Asal ChemistryDokument4 SeitenCoursebook Answers Chapter 18 Asal ChemistryMarin PesicNoch keine Bewertungen
- Chemistry - Organic Chemistry MechanismsDokument2 SeitenChemistry - Organic Chemistry Mechanismshelixate100% (3)
- MEKANISMEDokument2 SeitenMEKANISMEEry NourikaNoch keine Bewertungen
- Aldol & Similar Name Reaction - FinalDokument32 SeitenAldol & Similar Name Reaction - Finaldash guptaNoch keine Bewertungen
- Aldol - Similar Name Reaction PDFDokument34 SeitenAldol - Similar Name Reaction PDFSBNoch keine Bewertungen
- 1,3 CycloDokument1 Seite1,3 CycloIsmail ZitouniNoch keine Bewertungen
- Phosphoamidate CouplingDokument1 SeitePhosphoamidate CouplingxrisnicknameNoch keine Bewertungen
- 4.5 Answers To ExercisesDokument4 Seiten4.5 Answers To Exercisesloly62006Noch keine Bewertungen
- Heterosiklis Non AromatisDokument14 SeitenHeterosiklis Non Aromatisdebora mawarsari purbaNoch keine Bewertungen
- Chemistry (Full Test) - Paper 1 - SolutionsDokument6 SeitenChemistry (Full Test) - Paper 1 - SolutionsRavi Kiran KoduriNoch keine Bewertungen
- Associated With HO: H H H H HDokument1 SeiteAssociated With HO: H H H H HKendra ShresthaNoch keine Bewertungen
- Pka BasesDokument1 SeitePka BasesCarlos ArzaluzNoch keine Bewertungen
- Amines NotesDokument9 SeitenAmines NotesMehlam AkbaraliNoch keine Bewertungen
- Alkenes 1 QPDokument7 SeitenAlkenes 1 QPHoaXNoch keine Bewertungen
- Chem DrawDokument7 SeitenChem DrawPutu Ayu WerdhiantyNoch keine Bewertungen
- Tarea x3Dokument1 SeiteTarea x3Ulises DantánNoch keine Bewertungen
- Chem 231Dokument41 SeitenChem 231duruNoch keine Bewertungen
- Functional Group Interconversion Scheme PDFDokument1 SeiteFunctional Group Interconversion Scheme PDFBilal AhmadNoch keine Bewertungen
- Reaksi AlkenaDokument1 SeiteReaksi Alkenajoel13Noch keine Bewertungen
- Ibu Gram CantikDokument2 SeitenIbu Gram CantikgramatolinaNoch keine Bewertungen
- Mekanisme RX DibenzalasetonDokument2 SeitenMekanisme RX DibenzalasetonWulan safitriNoch keine Bewertungen
- Drug Abuse Ag8114en MKDokument56 SeitenDrug Abuse Ag8114en MKA VegaNoch keine Bewertungen
- Moleculas 3Dokument3 SeitenMoleculas 3Eloy Morales perezNoch keine Bewertungen
- Alquinos 13Dokument1 SeiteAlquinos 13Fernando EstradaNoch keine Bewertungen
- Performance Task 3 - Chemical Equations - Diamante Denver-12-MendeleevDokument1 SeitePerformance Task 3 - Chemical Equations - Diamante Denver-12-MendeleevJohn Lloyd Langco100% (1)
- Mecanismo de Los Derivados Muestra 3Dokument5 SeitenMecanismo de Los Derivados Muestra 3MELISSA Peña OrtizNoch keine Bewertungen
- NullDokument2 SeitenNullapi-26498641Noch keine Bewertungen
- Aldehyde & Ketone RXDokument5 SeitenAldehyde & Ketone RXhaNoch keine Bewertungen
- An Advanced and Highly Intelligent Species of LifeDokument1 SeiteAn Advanced and Highly Intelligent Species of LifeArt Julius D. HallazgoNoch keine Bewertungen
- Exercise 3 - Embryology: Art Julius Hallazgo Developmental Biology Laboratory - YaDokument5 SeitenExercise 3 - Embryology: Art Julius Hallazgo Developmental Biology Laboratory - YaArt Julius D. HallazgoNoch keine Bewertungen
- An Advanced and Highly Intelligent Species of LifeDokument1 SeiteAn Advanced and Highly Intelligent Species of LifeArt Julius D. HallazgoNoch keine Bewertungen
- Ecolec Guide QuestionsDokument26 SeitenEcolec Guide QuestionsArt Julius D. Hallazgo100% (1)
- DevlecfruitflyforumDokument1 SeiteDevlecfruitflyforumArt Julius D. HallazgoNoch keine Bewertungen
- Lab Ex 1 - The CellDokument16 SeitenLab Ex 1 - The CellArt Julius D. HallazgoNoch keine Bewertungen
- Devlabdrawings - Exercise 4Dokument8 SeitenDevlabdrawings - Exercise 4Art Julius D. HallazgoNoch keine Bewertungen
- Exercise 6. Chick Development: Egg To 28 Hours of IncubationDokument8 SeitenExercise 6. Chick Development: Egg To 28 Hours of IncubationArt Julius D. HallazgoNoch keine Bewertungen
- Art Hallazgo Developmental Biology YA: Exercise 7: Chick Development: 33-60 Hours EmbryosDokument7 SeitenArt Hallazgo Developmental Biology YA: Exercise 7: Chick Development: 33-60 Hours EmbryosArt Julius D. HallazgoNoch keine Bewertungen
- Art Hallazgo Gram Staining Exercise: Usp SharingDokument1 SeiteArt Hallazgo Gram Staining Exercise: Usp SharingArt Julius D. HallazgoNoch keine Bewertungen
- Identification of Unknown Plant SpecimensDokument29 SeitenIdentification of Unknown Plant SpecimensArt Julius D. HallazgoNoch keine Bewertungen
- Sea Otters (Enhydra Lutris) : A Keystone Species: Submitted byDokument33 SeitenSea Otters (Enhydra Lutris) : A Keystone Species: Submitted byArt Julius D. HallazgoNoch keine Bewertungen
- The Difference Between Homeostasis and MetabolismDokument1 SeiteThe Difference Between Homeostasis and MetabolismArt Julius D. HallazgoNoch keine Bewertungen
- nCoV Orientation As of 2 Feb 2020Dokument23 SeitennCoV Orientation As of 2 Feb 2020Art Julius D. HallazgoNoch keine Bewertungen
- Metabolism: Primary Metabolites Are Directly Involved in Their Growth Development and Reproduction Secondary MetabolitesDokument22 SeitenMetabolism: Primary Metabolites Are Directly Involved in Their Growth Development and Reproduction Secondary MetabolitesArt Julius D. HallazgoNoch keine Bewertungen
- When Performing ChromatographyDokument1 SeiteWhen Performing ChromatographyArt Julius D. HallazgoNoch keine Bewertungen
- Genlab Exp1Dokument3 SeitenGenlab Exp1Art Julius D. HallazgoNoch keine Bewertungen
- Lipids LabdiscussionDokument16 SeitenLipids LabdiscussionArt Julius D. HallazgoNoch keine Bewertungen
- Rna TypesDokument2 SeitenRna TypesArt Julius D. HallazgoNoch keine Bewertungen
- Chem Lab - 1 AnswersDokument2 SeitenChem Lab - 1 AnswersArt Julius D. HallazgoNoch keine Bewertungen
- LipidsDokument71 SeitenLipidsArt Julius D. HallazgoNoch keine Bewertungen
- Exp 2 - Post Lab BiochemDokument5 SeitenExp 2 - Post Lab BiochemArt Julius D. HallazgoNoch keine Bewertungen
- Kenshuquiz AkutalvarezquinonezDokument6 SeitenKenshuquiz AkutalvarezquinonezArt Julius D. HallazgoNoch keine Bewertungen
- MethodsDokument4 SeitenMethodsGianne AbcedeNoch keine Bewertungen
- Eng 5Dokument1 SeiteEng 5Art Julius D. HallazgoNoch keine Bewertungen
- Deficiency Deficiency Deficiency Deficiency Deficiency Deficiency Deficiency Deficiency Deficiency Deficiency Deficiency Deficiency DeficiencyDokument2 SeitenDeficiency Deficiency Deficiency Deficiency Deficiency Deficiency Deficiency Deficiency Deficiency Deficiency Deficiency Deficiency DeficiencyArt Julius D. HallazgoNoch keine Bewertungen
- Inter 2 Physics Success SeriesDokument12 SeitenInter 2 Physics Success SeriesZain AliNoch keine Bewertungen
- Numericals On CapacitorsDokument19 SeitenNumericals On CapacitorsYatn BangadNoch keine Bewertungen
- Lab 1 PH Conductivity TurbidityDokument4 SeitenLab 1 PH Conductivity TurbiditySumit Priyam67% (3)
- CFDeffectoffluidviscosityDokument7 SeitenCFDeffectoffluidviscosityHarsh TekriwalNoch keine Bewertungen
- ESAS Objectives 1Dokument15 SeitenESAS Objectives 1Jayven VillamaterNoch keine Bewertungen
- Be Prepared With Lutz: Safety Is Our ConcernDokument16 SeitenBe Prepared With Lutz: Safety Is Our ConcernJhonatan QuispeNoch keine Bewertungen
- Grout Pressures Around A Tunnel LiningDokument6 SeitenGrout Pressures Around A Tunnel LiningkapolaNoch keine Bewertungen
- Lab Report 10Dokument4 SeitenLab Report 10Rafay MalikNoch keine Bewertungen
- Panasonic TX-40ESR500 LA75Dokument83 SeitenPanasonic TX-40ESR500 LA75André PaivaNoch keine Bewertungen
- Unit 10 Properties and Applications of Engineering MaterialsDokument12 SeitenUnit 10 Properties and Applications of Engineering MaterialsRavishanker Baliga0% (1)
- PHY 310 Modern Physics Course OutlineDokument6 SeitenPHY 310 Modern Physics Course OutlineNur HamizahNoch keine Bewertungen
- Chemguard 3% x 6% AR-AFFF for hydrocarbon and polar solvent fuelsDokument2 SeitenChemguard 3% x 6% AR-AFFF for hydrocarbon and polar solvent fuelsTri Cahyono YuniantoNoch keine Bewertungen
- Geog 213Dokument365 SeitenGeog 213Fatima TwumasiNoch keine Bewertungen
- CH101 Tutorial Sheet 2Dokument2 SeitenCH101 Tutorial Sheet 2Amelia IloNoch keine Bewertungen
- B Bogdanov 2Dokument6 SeitenB Bogdanov 2tonmoyahmed06Noch keine Bewertungen
- Combustion Engine Vs Gas Turbine - Part Load Efficiency and FlexibilityDokument4 SeitenCombustion Engine Vs Gas Turbine - Part Load Efficiency and Flexibilityseif elsaieNoch keine Bewertungen
- Industrial and Genset Cooling System Issue 11Dokument49 SeitenIndustrial and Genset Cooling System Issue 11khoirulfeb.rizmarosNoch keine Bewertungen
- Hempel Topaz SG Enamel 524ME MsdsDokument10 SeitenHempel Topaz SG Enamel 524ME MsdsM.FAIZAN ARSHADNoch keine Bewertungen
- Kolthoff 1929Dokument5 SeitenKolthoff 1929ipark2025Noch keine Bewertungen
- MME 295 Lec 5Dokument20 SeitenMME 295 Lec 5Fahim Faisal RaunaqNoch keine Bewertungen
- Ether (Theory) Module-4Dokument7 SeitenEther (Theory) Module-4Raju SinghNoch keine Bewertungen
- Alexa Riley - Transpiration LabDokument7 SeitenAlexa Riley - Transpiration Labapi-553676905Noch keine Bewertungen
- Design and Evaluation of Floating Drug Delivery Based On Matrix Tablet of Acyclovir PDFDokument9 SeitenDesign and Evaluation of Floating Drug Delivery Based On Matrix Tablet of Acyclovir PDFJemmy Anton Prasetia IgnNoch keine Bewertungen
- Star and GalaxiesDokument32 SeitenStar and GalaxiesMazura AhmadNoch keine Bewertungen
- June 2015 QP - M1 EdexcelDokument15 SeitenJune 2015 QP - M1 EdexcelRishita SinghNoch keine Bewertungen