Beruflich Dokumente
Kultur Dokumente
Mole
Hochgeladen von
Rosita CayananOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Mole
Hochgeladen von
Rosita CayananCopyright:
Verfügbare Formate
Republic of the Philippines Document Code: _____________
Department of Education
Region III Revision: ____________________
SCHOOLS DIVISION OF PAMPANGA
High School Blvd, Brgy. Lourdes, City of San Fernando Effectivity Date: ______________
LESSON PLAN EXEMPLAR IN CONSUMER POTRERO NATIONAL HIGH SCHOOL
CHEMISTRY Bulaon Resettlement, City of San Fernando Pampanga
(045)300-2264 potreronationalhighschool@gmail.com
( 9-STE )
Topic/Title MOLE
Time Allotment 60 MINUTES
Learning Competency/Objective
At the end of the lesson, the learners should be able to:
a. Explain the Mole Concept
b. Apply mole concept in completing the table with the needed information
Elicit
Through discussion explore with the students their understanding of
What a mole is
What does Avogadro’s number represent
What is an atom
And what is a molecule
Engage Materials
Pictures/images
Show to class pictures showing some counting units such as dozen, pair
and ream.
o Shoes, earrings, gloves are sold by pairs
o Eggs and doughnuts by the dozens
Ask students about the counting unit to measure the number of
submicroscopic particles.
Explore Mateirals
Introduce to the class what is mole concept and Avogadro’s number. Periodic table of elements
Discussion.
Calculator
Cite examples on identifying particles (may be atom, ions or molecules).
Individually, let the students answer the activity 1. Please refer to the Activity sheet
attached sheet.
Explain
1. Selected students will present their output and will explain on how they
computed for the number of moles. Manila paper/ pen
2. Teacher’s Input
Elaborate
1. Relate the mole to real life situations:
a. Which has greater number of particles, one mole of cotton or one
mole of salt?
b. How many pesos are there in one mole of pesos? Do you think
Manny Pacquiao will have one mole of pesos? Does Bill gates have
one mole of dollars?
2. Trivia’s: How Big is a Mole?
If you received a mole of pennies on the day you were born, and
spent a million dollars a second until you died at 100, you’d still have
over 99.99% of your money in the bank.
If you had a mole of basketballs, you could create a new planet the
size of the Earth!
A mole of cereal boxes stacked end to end would reach from the Sun
to Pluto 7.5 million times.
A mole of turkeys could form sixteen earths.
Evaluate
In a ½ sheet of paper, compute for the number of mole of a given element or Periodic table of elements
compound.
Calculator
1. How many moleS of acetylene, C₂H₄, are there in 2.00 g acetylene?
a. 0.71 mole b. 0.071 mole c. 0.71 g/mole
2. How many moles are there in 9.52 g N?
a. 0.06 mole b. 0.68 mole c. 0.08 mole
Extend
Measure a beaker of one mole table sugar and a beaker of one mole Beaker, sugar, salt
table salt
How do the piles compare?
Illustrate the piles to show the relative sizes
Prepared by: Checked by: Note by:
ROSITA C. CAYANAN AILEEN C. MANALO EDNA R. GUTIERREZ
I.S. Principal
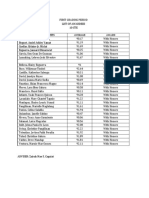
Activity 1
Instruction: Fill out the table with the appropriate value in each cell.
Substance Types of Types of Amount of Molar Moles
Substance Particles substance (g) mass/Atomic
(element / (atom / mass (mole)
compound) molecule) (g/mole)
1. CO2 18 g
2. H 6.16 g
3. Ca 3.5 g
4. H₂O 50 g
5. NaCl 67.5 g
Das könnte Ihnen auch gefallen
- Amazing Kitchen Chemistry Projects: You Can Build YourselfVon EverandAmazing Kitchen Chemistry Projects: You Can Build YourselfBewertung: 4.5 von 5 Sternen4.5/5 (2)
- MolesDokument4 SeitenMolesDaryl CadanillaNoch keine Bewertungen
- 9 MassDokument4 Seiten9 MassxoxkakidoxoxNoch keine Bewertungen
- DLP Q2 Week 6 D4Dokument4 SeitenDLP Q2 Week 6 D4Menchie Yaba50% (2)
- Department of Education Division of Leyte Taberna National High SchoolDokument4 SeitenDepartment of Education Division of Leyte Taberna National High SchoolSheila May Tapales Tabon100% (1)
- Grade 9-2ND QuarterDokument5 SeitenGrade 9-2ND QuarterLorie Ann RatunilNoch keine Bewertungen
- Lesson Plan Science 9 Q3Dokument2 SeitenLesson Plan Science 9 Q3MYLYN PALOMERNoch keine Bewertungen
- 2nd QRTR DLL-MOLE-CONCEPTDokument15 Seiten2nd QRTR DLL-MOLE-CONCEPTChenee Bulawan PontilloNoch keine Bewertungen
- Q2 Week 1 Copy 1Dokument5 SeitenQ2 Week 1 Copy 1Roberto Misola Jr.Noch keine Bewertungen
- DLL Demo g9Dokument4 SeitenDLL Demo g9Marie VicNoch keine Bewertungen
- SCIENCE 9 - Lesson Plan MAGMA (Viscosity)Dokument3 SeitenSCIENCE 9 - Lesson Plan MAGMA (Viscosity)Richelle MasingNoch keine Bewertungen
- 7 E Model Lesson PlanDokument7 Seiten7 E Model Lesson Plangloria tolentinoNoch keine Bewertungen
- SCIENCE 9 (1) - MELC 1 - Reviewed by CommitteeDokument13 SeitenSCIENCE 9 (1) - MELC 1 - Reviewed by CommitteeBayani VicencioNoch keine Bewertungen
- DLL July 15-19, 2019Dokument4 SeitenDLL July 15-19, 2019ROdney BArbaNoch keine Bewertungen
- DLL - SCIENCE - 9 - Q3 - W5 2019finDokument5 SeitenDLL - SCIENCE - 9 - Q3 - W5 2019finMary ApostolNoch keine Bewertungen
- 7e LP On ReadingDokument6 Seiten7e LP On ReadingMERIAM P. PACALSONoch keine Bewertungen
- 2 - Activity Factors Affecting ClimateDokument7 Seiten2 - Activity Factors Affecting ClimateCes Michaela CadividaNoch keine Bewertungen
- Climate Change Lesson PlanDokument3 SeitenClimate Change Lesson PlanApple ArellanoNoch keine Bewertungen
- Electron Configuration Lesson 2Dokument9 SeitenElectron Configuration Lesson 2brian catianNoch keine Bewertungen
- Grade 9 Science Detailed Lesson Plan - Ntot 2018Dokument3 SeitenGrade 9 Science Detailed Lesson Plan - Ntot 2018Jengkie PecanaNoch keine Bewertungen
- Jan 4-6Dokument3 SeitenJan 4-6Vanessa ParayoNoch keine Bewertungen
- Science9 Q2 W7 D1-2Dokument2 SeitenScience9 Q2 W7 D1-2Rovz GC Bin0% (1)
- Percent CompositionstoichiometrypptDokument16 SeitenPercent CompositionstoichiometrypptKazuki Fujiyama100% (1)
- Activity 13 Organic CompoundsDokument1 SeiteActivity 13 Organic CompoundsWENDEL MAYORNoch keine Bewertungen
- Sci 9 DLL Q2 W1Dokument6 SeitenSci 9 DLL Q2 W1Nomar Maigue DarNoch keine Bewertungen
- Weekly Learning Plan 4Dokument8 SeitenWeekly Learning Plan 4Jenny PartozaNoch keine Bewertungen
- dll-10 CODokument3 Seitendll-10 CORochelle UlgadoNoch keine Bewertungen
- DLP Science 9 Q3 Week 3Dokument4 SeitenDLP Science 9 Q3 Week 3Gabriel PachecoNoch keine Bewertungen
- DLL HeatDokument3 SeitenDLL HeatMatet GenerosaNoch keine Bewertungen
- Lawy National High School: Weekly Learning PlanDokument3 SeitenLawy National High School: Weekly Learning PlanMerrie Anne Pascual BagsicNoch keine Bewertungen
- Topic 4 BondingDokument13 SeitenTopic 4 Bondinglobna masadehNoch keine Bewertungen
- DLL-observation - Free Fall MotionDokument5 SeitenDLL-observation - Free Fall Motionleny santosNoch keine Bewertungen
- Iloilo National High School, La Paz, Iloilo City December 12, 2017 Schools Division: Grade Level: Teachers: Learning AreaDokument6 SeitenIloilo National High School, La Paz, Iloilo City December 12, 2017 Schools Division: Grade Level: Teachers: Learning AreaCaryl Ann C. SernadillaNoch keine Bewertungen
- DLL - Science 9 - Q2Dokument37 SeitenDLL - Science 9 - Q2Nazer M. LacaboNoch keine Bewertungen
- Final Revised Las in Science 9 q3w3Dokument4 SeitenFinal Revised Las in Science 9 q3w3Ma.Kristine Ibarreta JazulNoch keine Bewertungen
- Daily Lesson Log Grade 10 2nd Week 2Dokument6 SeitenDaily Lesson Log Grade 10 2nd Week 2Queeny Pantoja-HondradaNoch keine Bewertungen
- Lesson Plan in StoichiometryDokument7 SeitenLesson Plan in StoichiometryLoreen Jane AbogadoNoch keine Bewertungen
- SCIENCE 9 - Q1 - W5 - Mod5 - ADMDokument22 SeitenSCIENCE 9 - Q1 - W5 - Mod5 - ADMdonnaNoch keine Bewertungen
- DLL (Motion in 2 Dimension)Dokument3 SeitenDLL (Motion in 2 Dimension)JeanRachoPaynandosNoch keine Bewertungen
- Factors Affecting Climate: 1.latitude 2.altitude 3.distance From The Sea 4.ocean Currents 5.land TopographyDokument54 SeitenFactors Affecting Climate: 1.latitude 2.altitude 3.distance From The Sea 4.ocean Currents 5.land TopographyMeljean Kalaw CastilloNoch keine Bewertungen
- Weekly-DLL-Science G9 WK 2Dokument6 SeitenWeekly-DLL-Science G9 WK 2Liway Nieles Umaclap CuerdoNoch keine Bewertungen
- DLL Science 9Dokument3 SeitenDLL Science 9Belinda LapsitNoch keine Bewertungen
- Weekly Home Learning Plan For Grade 9-Science Quarter 1, Week 7Dokument1 SeiteWeekly Home Learning Plan For Grade 9-Science Quarter 1, Week 7Enteng ODNoch keine Bewertungen
- Incomplete Dominance and Codominance Lesson Plan PDFDokument3 SeitenIncomplete Dominance and Codominance Lesson Plan PDFRirinNoch keine Bewertungen
- G9 Science Q3 - Week 6 Climate-PhenomenaDokument28 SeitenG9 Science Q3 - Week 6 Climate-PhenomenaJanetMagnayeLapitanNoch keine Bewertungen
- Electron ConfigurationDokument15 SeitenElectron ConfigurationJireh PasiliaoNoch keine Bewertungen
- Matulatula High School: Multiple Choice DIRECTIONS: Choose The Letter of The Correct Answer. EncircleDokument2 SeitenMatulatula High School: Multiple Choice DIRECTIONS: Choose The Letter of The Correct Answer. EncircleANDJELYN M. ABALOSNoch keine Bewertungen
- Volcano 2Dokument3 SeitenVolcano 2christian jade quijanoNoch keine Bewertungen
- DLL Grade 9 Matter 2nd QuarterpdfDokument44 SeitenDLL Grade 9 Matter 2nd QuarterpdfLIWLIWA SUGUITANNoch keine Bewertungen
- 2018-2019 DLL Science 7Dokument3 Seiten2018-2019 DLL Science 7Reihnard RosariioNoch keine Bewertungen
- Lasip National High School: School: Teacher: Year and Section: Subject and Time: Date(s) : I. ObjectiveDokument4 SeitenLasip National High School: School: Teacher: Year and Section: Subject and Time: Date(s) : I. ObjectivePepito Rosario Baniqued, JrNoch keine Bewertungen
- Lesson Plan Demo TeachingDokument3 SeitenLesson Plan Demo TeachingISABEL PARRONoch keine Bewertungen
- DLL Week 2 2nd QuarterDokument4 SeitenDLL Week 2 2nd QuarterWendz ArominNoch keine Bewertungen
- Lesson Plan LightDokument12 SeitenLesson Plan LightRhisia RaborNoch keine Bewertungen
- Grade 9 Mass of An ObjectDokument2 SeitenGrade 9 Mass of An Objectking devesfrutoNoch keine Bewertungen
- Percentage CompositionDokument12 SeitenPercentage CompositionDebbie BacalsoNoch keine Bewertungen
- Daily Lesson PlanDokument8 SeitenDaily Lesson PlanUdani Jaymar100% (1)
- DLL Science 9 SYSTEMSDokument6 SeitenDLL Science 9 SYSTEMSseoraksanNoch keine Bewertungen
- Group 10 Lesson PlanDokument9 SeitenGroup 10 Lesson PlanLerma De DomingoNoch keine Bewertungen
- DLP Q2 Week 6 D3Dokument6 SeitenDLP Q2 Week 6 D3Menchie Yaba100% (1)
- Science 8 Learning Activity Sheet (Week 3)Dokument2 SeitenScience 8 Learning Activity Sheet (Week 3)Rosita CayananNoch keine Bewertungen
- Potrero National High School Semi - Detailed Lesson Plan in Technology and Livelihood Education Grade 8 (Computer System Servicing)Dokument8 SeitenPotrero National High School Semi - Detailed Lesson Plan in Technology and Livelihood Education Grade 8 (Computer System Servicing)Rosita CayananNoch keine Bewertungen
- Science 8 Learning Activity Sheet (Week 4 & 5) : Effect of Temperature To Speed of SoundDokument2 SeitenScience 8 Learning Activity Sheet (Week 4 & 5) : Effect of Temperature To Speed of SoundRosita CayananNoch keine Bewertungen
- Direction: MULTIPLE CHOICE: Write Using Big Letter of The Correct Answer Beside Each NumberDokument3 SeitenDirection: MULTIPLE CHOICE: Write Using Big Letter of The Correct Answer Beside Each NumberRosita CayananNoch keine Bewertungen
- Second Quarter With Honors 8 Ste: Potrero National High SchoolDokument1 SeiteSecond Quarter With Honors 8 Ste: Potrero National High SchoolRosita CayananNoch keine Bewertungen
- Grade 8 STE - MODULE 6 WHLPDokument6 SeitenGrade 8 STE - MODULE 6 WHLPRosita CayananNoch keine Bewertungen
- GRADE-8 Most Essential Learning Competencies Budget of Work School Year 2020-2021Dokument2 SeitenGRADE-8 Most Essential Learning Competencies Budget of Work School Year 2020-2021Rosita CayananNoch keine Bewertungen
- E Class Record 8 Aguinaldo Science R.CAYANANDokument16 SeitenE Class Record 8 Aguinaldo Science R.CAYANANRosita CayananNoch keine Bewertungen
- School Form 2 (SF2) Daily Attendance Report of Learners: 301065 2020 - 2021 Feb-21 Potrero NHS Grade 8 (Year II) 8-STEDokument3 SeitenSchool Form 2 (SF2) Daily Attendance Report of Learners: 301065 2020 - 2021 Feb-21 Potrero NHS Grade 8 (Year II) 8-STERosita CayananNoch keine Bewertungen
- Science 8 Learning Activity Sheet (: Week 1)Dokument5 SeitenScience 8 Learning Activity Sheet (: Week 1)Rosita CayananNoch keine Bewertungen
- Unpacking of MELCS GRADE 8..R. CAYANANDokument1 SeiteUnpacking of MELCS GRADE 8..R. CAYANANRosita CayananNoch keine Bewertungen
- ChcklistDokument2 SeitenChcklistRosita CayananNoch keine Bewertungen
- SF 9 - JHS (Learner's Progress Report Card B) TCDokument4 SeitenSF 9 - JHS (Learner's Progress Report Card B) TCRosita CayananNoch keine Bewertungen
- Science 8 - Q2 - Week 2 - Melc 1-3Dokument31 SeitenScience 8 - Q2 - Week 2 - Melc 1-3Rosita Cayanan50% (2)
- Earthquakes Presentation Teachers Notes PDFDokument6 SeitenEarthquakes Presentation Teachers Notes PDFRosita CayananNoch keine Bewertungen
- Cards Out First Quarter: Schools Division Office of Pampanga Potrero National High School (8-Del Pilar)Dokument2 SeitenCards Out First Quarter: Schools Division Office of Pampanga Potrero National High School (8-Del Pilar)Rosita CayananNoch keine Bewertungen
- Learning Activity in Consumer ChemistryDokument1 SeiteLearning Activity in Consumer ChemistryRosita CayananNoch keine Bewertungen
- Conchem Q2 WK2Dokument14 SeitenConchem Q2 WK2Rosita CayananNoch keine Bewertungen
- Card Template FINALDokument1 SeiteCard Template FINALRosita CayananNoch keine Bewertungen
- 10 STE List of Awardees 1st QuarterDokument1 Seite10 STE List of Awardees 1st QuarterRosita CayananNoch keine Bewertungen
- Learn@Home 112720 PDFDokument1 SeiteLearn@Home 112720 PDFRosita CayananNoch keine Bewertungen
- Science 8: Learning Activity Sheet Module 1 Week 1 Force and MotionDokument2 SeitenScience 8: Learning Activity Sheet Module 1 Week 1 Force and MotionRosita CayananNoch keine Bewertungen
- School Form 1 (SF 1) School RegisterDokument3 SeitenSchool Form 1 (SF 1) School RegisterRosita CayananNoch keine Bewertungen
- Department of Education: Republic of The PhilippinesDokument2 SeitenDepartment of Education: Republic of The PhilippinesRosita CayananNoch keine Bewertungen
- First Quarter Summary Grades of 8 Ste.r.cayananDokument5 SeitenFirst Quarter Summary Grades of 8 Ste.r.cayananRosita CayananNoch keine Bewertungen
- Certificate of Participation: Rosita C.CayananDokument1 SeiteCertificate of Participation: Rosita C.CayananRosita CayananNoch keine Bewertungen
- Melcs in Consumer Chemistry Second QuarterDokument3 SeitenMelcs in Consumer Chemistry Second QuarterRosita Cayanan100% (4)
- E Class Record 9 STE Consumer Chem R. CAYANANDokument12 SeitenE Class Record 9 STE Consumer Chem R. CAYANANRosita CayananNoch keine Bewertungen
- Cards Out First Quarter: Schools Division Office of Pampanga Potrero National High School (8-STE)Dokument2 SeitenCards Out First Quarter: Schools Division Office of Pampanga Potrero National High School (8-STE)Rosita CayananNoch keine Bewertungen
- WebinarDokument3 SeitenWebinarRosita CayananNoch keine Bewertungen
- JEE Main 2018 1Dokument393 SeitenJEE Main 2018 1avinashno1Noch keine Bewertungen
- Maths Cs Form 5Dokument6 SeitenMaths Cs Form 5juriah binti ibrahimNoch keine Bewertungen
- AskelandPhuleNotes CH12PrintableDokument79 SeitenAskelandPhuleNotes CH12PrintablebountymaniNoch keine Bewertungen
- (Doi 10.1016/b978!1!4832-2832-7.50007-1), - ICUMSA Methods of Sugar Analysis - Determination of Sucrose - (By Polarimetry)Dokument6 Seiten(Doi 10.1016/b978!1!4832-2832-7.50007-1), - ICUMSA Methods of Sugar Analysis - Determination of Sucrose - (By Polarimetry)marifa16Noch keine Bewertungen
- Energy TransportDokument16 SeitenEnergy TransportSarun NeamnomNoch keine Bewertungen
- Lecture 6 - Laplace TransformDokument7 SeitenLecture 6 - Laplace TransformSujeet SharmaNoch keine Bewertungen
- Activities 6Dokument4 SeitenActivities 6Zenith DebbarmaNoch keine Bewertungen
- Granulometry of ClinkerDokument18 SeitenGranulometry of ClinkerNael100% (12)
- Modern Physics (Exam:6)Dokument7 SeitenModern Physics (Exam:6)Arnab BhowmikNoch keine Bewertungen
- Boyle's Law: Chavez, Gabreille R. 1. The Combined Gas LawDokument2 SeitenBoyle's Law: Chavez, Gabreille R. 1. The Combined Gas LawGabreille Rullamas ChavezNoch keine Bewertungen
- 3 - H. M. Hsiao & I. M. Daniel - Effect of Fiber Waviness On Stiffness and Strength Reduction of Uniderectional Composites Under Compressive LoadingDokument13 Seiten3 - H. M. Hsiao & I. M. Daniel - Effect of Fiber Waviness On Stiffness and Strength Reduction of Uniderectional Composites Under Compressive LoadingImad Al-din KhattabNoch keine Bewertungen
- Derivation of Gravitational Potential Energy Using Calculus2Dokument7 SeitenDerivation of Gravitational Potential Energy Using Calculus2W-d DomNoch keine Bewertungen
- Handbook of Multiphase Flow Science and Technology (Guan Heng Yeoh (Eds.) )Dokument444 SeitenHandbook of Multiphase Flow Science and Technology (Guan Heng Yeoh (Eds.) )thulyyNoch keine Bewertungen
- !!trends in Pharmaceutical Analysis and Quality Contro - 2022 - TrAC Trends in AnaDokument14 Seiten!!trends in Pharmaceutical Analysis and Quality Contro - 2022 - TrAC Trends in AnaMostafa AfifyNoch keine Bewertungen
- XYZ Dairy EnggDokument7 SeitenXYZ Dairy EnggBhuwesh PantNoch keine Bewertungen
- Tanker Analiz RaporuDokument23 SeitenTanker Analiz Raporuakın ersözNoch keine Bewertungen
- 10 - Grain Size MeasurementDokument27 Seiten10 - Grain Size MeasurementRohib RohibNoch keine Bewertungen
- Ncert Solutions Class 11 Physics Chapter 2 Units and Measurement - 0Dokument29 SeitenNcert Solutions Class 11 Physics Chapter 2 Units and Measurement - 0Aniruddha MishraNoch keine Bewertungen
- 5054 PHYSICS: MARK SCHEME For The May/June 2011 Question Paper For The Guidance of TeachersDokument5 Seiten5054 PHYSICS: MARK SCHEME For The May/June 2011 Question Paper For The Guidance of Teacherskaran79Noch keine Bewertungen
- Structure of An Atom and Its Sub-Atomic Particles: Prepared by Frenchel Aira B. BeloDokument9 SeitenStructure of An Atom and Its Sub-Atomic Particles: Prepared by Frenchel Aira B. BeloScared CreatorNoch keine Bewertungen
- GenPhysics 2 Quarter 3 Module 2023 2024Dokument56 SeitenGenPhysics 2 Quarter 3 Module 2023 2024Cassandra Ayesha CastilloNoch keine Bewertungen
- Hadron - WikipediaDokument5 SeitenHadron - WikipediaSmart ArenaNoch keine Bewertungen
- 1 - Electrostatics - Theory - PDF Module-4Dokument28 Seiten1 - Electrostatics - Theory - PDF Module-4Raju SinghNoch keine Bewertungen
- Static Analysis of Circular Cylindrical Shell Under Hydrostatic and Ring ForcesDokument10 SeitenStatic Analysis of Circular Cylindrical Shell Under Hydrostatic and Ring ForcestevredeNoch keine Bewertungen
- Analysis Tools For The Design of Aluminium Extrusion Dies PDFDokument164 SeitenAnalysis Tools For The Design of Aluminium Extrusion Dies PDFsakthivel100% (1)
- Electric Circuit Analysis PDFDokument53 SeitenElectric Circuit Analysis PDFmasimeriseNoch keine Bewertungen
- BK Pandey 01Dokument70 SeitenBK Pandey 01Anurag SrivastavaNoch keine Bewertungen
- Time-Dependent Perturbation Theory and Time-Dependent PhenomenaDokument14 SeitenTime-Dependent Perturbation Theory and Time-Dependent PhenomenaPhysicist91Noch keine Bewertungen
- Chain FlexibilityDokument38 SeitenChain Flexibilityvgokuul86% (7)
- Bushong: Radiologic Science For Technologists, 10th Edition: Laboratory ExperimentsDokument3 SeitenBushong: Radiologic Science For Technologists, 10th Edition: Laboratory Experimentsmoussa medjahedNoch keine Bewertungen
- Knocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldVon EverandKnocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldBewertung: 3.5 von 5 Sternen3.5/5 (64)
- Summary and Interpretation of Reality TransurfingVon EverandSummary and Interpretation of Reality TransurfingBewertung: 5 von 5 Sternen5/5 (5)
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseVon EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseBewertung: 3.5 von 5 Sternen3.5/5 (69)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincVon EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincBewertung: 3.5 von 5 Sternen3.5/5 (137)
- A Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceVon EverandA Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceBewertung: 4 von 5 Sternen4/5 (51)
- A Brief History of Time: From the Big Bang to Black HolesVon EverandA Brief History of Time: From the Big Bang to Black HolesBewertung: 4 von 5 Sternen4/5 (2193)
- Quantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessVon EverandQuantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessBewertung: 4 von 5 Sternen4/5 (6)
- Machinery of the Mind (An Interview)Von EverandMachinery of the Mind (An Interview)Bewertung: 4.5 von 5 Sternen4.5/5 (28)
- Midnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterVon EverandMidnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterBewertung: 4.5 von 5 Sternen4.5/5 (410)
- Lost in Math: How Beauty Leads Physics AstrayVon EverandLost in Math: How Beauty Leads Physics AstrayBewertung: 4.5 von 5 Sternen4.5/5 (125)
- The End of Everything: (Astrophysically Speaking)Von EverandThe End of Everything: (Astrophysically Speaking)Bewertung: 4.5 von 5 Sternen4.5/5 (157)
- The Power of Eight: Harnessing the Miraculous Energies of a Small Group to Heal Others, Your Life, and the WorldVon EverandThe Power of Eight: Harnessing the Miraculous Energies of a Small Group to Heal Others, Your Life, and the WorldBewertung: 4.5 von 5 Sternen4.5/5 (54)
- Infinite Powers: How Calculus Reveals the Secrets of the UniverseVon EverandInfinite Powers: How Calculus Reveals the Secrets of the UniverseBewertung: 4.5 von 5 Sternen4.5/5 (126)
- Bedeviled: A Shadow History of Demons in ScienceVon EverandBedeviled: A Shadow History of Demons in ScienceBewertung: 5 von 5 Sternen5/5 (5)
- Hyperspace: A Scientific Odyssey Through Parallel Universes, Time Warps, and the 10th DimensionVon EverandHyperspace: A Scientific Odyssey Through Parallel Universes, Time Warps, and the 10th DimensionBewertung: 4.5 von 5 Sternen4.5/5 (3)
- The Holographic Universe: The Revolutionary Theory of RealityVon EverandThe Holographic Universe: The Revolutionary Theory of RealityBewertung: 4.5 von 5 Sternen4.5/5 (78)
- The Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeVon EverandThe Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeNoch keine Bewertungen
- Mastering Logical Fallacies: The Definitive Guide to Flawless Rhetoric and Bulletproof LogicVon EverandMastering Logical Fallacies: The Definitive Guide to Flawless Rhetoric and Bulletproof LogicBewertung: 4 von 5 Sternen4/5 (91)
- The Beginning of Infinity: Explanations That Transform the WorldVon EverandThe Beginning of Infinity: Explanations That Transform the WorldBewertung: 5 von 5 Sternen5/5 (60)
- AP Physics 1 Premium, 2024: 4 Practice Tests + Comprehensive Review + Online PracticeVon EverandAP Physics 1 Premium, 2024: 4 Practice Tests + Comprehensive Review + Online PracticeNoch keine Bewertungen
- Packing for Mars: The Curious Science of Life in the VoidVon EverandPacking for Mars: The Curious Science of Life in the VoidBewertung: 4 von 5 Sternen4/5 (1396)
- Let There Be Light: Physics, Philosophy & the Dimensional Structure of ConsciousnessVon EverandLet There Be Light: Physics, Philosophy & the Dimensional Structure of ConsciousnessBewertung: 4.5 von 5 Sternen4.5/5 (57)
- Quantum Physics: What Everyone Needs to KnowVon EverandQuantum Physics: What Everyone Needs to KnowBewertung: 4.5 von 5 Sternen4.5/5 (49)
- Vibration and Frequency: How to Get What You Want in LifeVon EverandVibration and Frequency: How to Get What You Want in LifeBewertung: 4.5 von 5 Sternen4.5/5 (13)