Beruflich Dokumente
Kultur Dokumente
Answer 78936 PDF
Hochgeladen von
Malik Mohammad AsifOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Answer 78936 PDF
Hochgeladen von
Malik Mohammad AsifCopyright:
Verfügbare Formate
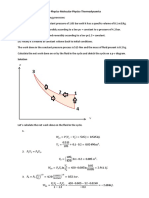
Answer on Question #78936, Physics / Molecular Physics | Thermodynamics
Question. 1 𝑘𝑔 of a fluid expands reversibly according to a linear law from 4.2 𝑏𝑎𝑟 to 1.4 𝑏𝑎𝑟; the initial and
final volumes are 0.004 𝑚3 and 0.02 𝑚3. The fluid is then cooled reversibly at constant pressure, and finally
compressed reversibly according to a law 𝑝𝑉 = 𝑐𝑜𝑛𝑠𝑡𝑎𝑛𝑡 back to the initial conditions of 4.2 𝑏𝑎𝑟 and
0.004 𝑚3 . Calculate the work done in each process and the net work of the cycle. Sketch the cycle on a 𝑝 − 𝑉
diagram.
Given. 𝑚 = 1 𝑘𝑔; 𝑝1 = 4.2 𝑏𝑎𝑟 = 4.2 ∙ 105 𝑃𝑎; 𝑝2 = 1.4 𝑏𝑎𝑟 = 1.4 ∙ 105 𝑃𝑎; 𝑉1 = 0.004 𝑚3 ; 𝑉2 = 0.02 𝑚3.
Find. 𝑊12 , 𝑊23 , 𝑊31 , 𝑊−?
Solution
1 1
𝑊12 (𝑎𝑟𝑒𝑎 𝑜𝑓 𝑡ℎ𝑒 𝑡𝑟𝑎𝑝𝑒𝑧𝑜𝑖𝑑) = (𝑝1 + 𝑝2 )(𝑉2 − 𝑉1 ) = (4.2 ∙ 105 + 1.4 ∙ 105 )(0.02 − 0.004) = 4480 𝐽
2 2
The work done by the fluid.
𝑊23 (𝑎𝑟𝑒𝑎 𝑜𝑓 𝑡ℎ𝑒 𝑟𝑒𝑐𝑡𝑎𝑛𝑔𝑙𝑒) = 𝑝2 (𝑉3 − 𝑉2 )
𝑝3 𝑉3 = 𝑝1 𝑉1 𝑎𝑛𝑑 𝑝3 = 𝑝2
𝑝1 4.2 ∙ 105
𝑉3 = 𝑉1 = 5
0.004 = 0.012 𝑚3
𝑝2 1.4 ∙ 10
𝑊23 = 1.4 ∙ 105 (0.012 − 0.02) = −1120 𝐽
The work done on the fluid.
𝑉1 𝑉1
𝑚 𝑅𝑇 𝑚 𝑉1 𝑉1 𝑉1 0.004
𝑊31 = ∫ 𝑝𝑑𝑉 = ∫ 𝑑𝑉 = 𝑅𝑇 ln = 𝑝3 𝑉3 ln = 𝑝1 𝑉1 ln = 4.2 ∙ 105 ∙ 0.004 ∙ ln =
𝑀 𝑉 𝑀 𝑉3 𝑉3 𝑉3 0.012
𝑉3 𝑉3
= −1845 𝐽
The work done on the fluid.
𝑊 = 𝑊12 + 𝑊23 + 𝑊31 = 4480 − 1120 − 1845 = 1515 𝐽
The work done by the fluid.
Answer. 𝑊12 = 4480 𝐽; 𝑊23 = −1120 𝐽; 𝑊31 = −1845 𝐽; 𝑊 = 1515 𝐽.
Answer provided by https://www.AssignmentExpert.com
Das könnte Ihnen auch gefallen
- Stirred Tank HeaterDokument34 SeitenStirred Tank HeaterIman Haerudin100% (1)
- Residual Properties by Equations of StateDokument2 SeitenResidual Properties by Equations of Statehameed1966Noch keine Bewertungen
- Unitops - ch5 ProblemsDokument6 SeitenUnitops - ch5 ProblemsfsamperizNoch keine Bewertungen
- FLASH ADIABÁTICO - Separation Process Principles - SEADER - 3rdedDokument5 SeitenFLASH ADIABÁTICO - Separation Process Principles - SEADER - 3rdedMaykkkowNoch keine Bewertungen
- Feedback SystemDokument3 SeitenFeedback SystemSeanne Cruz100% (1)
- Henrys Law Solved ProblemsDokument3 SeitenHenrys Law Solved ProblemsayushNoch keine Bewertungen
- H.W II Mass 2Dokument5 SeitenH.W II Mass 2ⵃⴰⵎⵣⴰ ⵖⵉⵢⵜNoch keine Bewertungen
- Chapter 4 - Methods of Analysis of Resistive CircuitsDokument52 SeitenChapter 4 - Methods of Analysis of Resistive CircuitsGeoFurriel100% (2)
- Lab-6-Binomail and Poisson DistributionDokument13 SeitenLab-6-Binomail and Poisson DistributionRakib Khan100% (1)
- HW Set 1Dokument6 SeitenHW Set 1GsusKrystNoch keine Bewertungen
- 344W13FinalExam Solution PDFDokument22 Seiten344W13FinalExam Solution PDFTysir SarhanNoch keine Bewertungen
- PsoDokument17 SeitenPsoKanishka SahniNoch keine Bewertungen
- The Bernoulli EquationDokument4 SeitenThe Bernoulli Equationdether acopiadoNoch keine Bewertungen
- Assignment - 1 - finaFPP L - Fluid - & - Particle - FinalDokument24 SeitenAssignment - 1 - finaFPP L - Fluid - & - Particle - FinalKharkhodaNoch keine Bewertungen
- Internal Forced ConvectionDokument18 SeitenInternal Forced ConvectionMohd Jamal Mohd MoktarNoch keine Bewertungen
- Univerity of West Indies Lab ReportDokument12 SeitenUniverity of West Indies Lab ReportvrajendraupadhyayNoch keine Bewertungen
- PR 1-5Dokument18 SeitenPR 1-5Febryan CaesarNoch keine Bewertungen
- J. Chem. Thermodynamics: J. Soujanya, B. Satyavathi, T.E. Vittal PrasadDokument4 SeitenJ. Chem. Thermodynamics: J. Soujanya, B. Satyavathi, T.E. Vittal PrasadAngie Paola AcostaNoch keine Bewertungen
- Certain Numerical Problems Chemical Engineering MATLABDokument44 SeitenCertain Numerical Problems Chemical Engineering MATLABvadseries0% (1)
- 1 Units and Dimensions 54Dokument24 Seiten1 Units and Dimensions 54SSNoch keine Bewertungen
- External FlowDokument27 SeitenExternal Flowraghu.entrepreneurNoch keine Bewertungen
- RTD Studies in PFRDokument13 SeitenRTD Studies in PFRSiddarthNoch keine Bewertungen
- Test 3 Solution 2010 PDFDokument4 SeitenTest 3 Solution 2010 PDFManishaa Varatha RajuNoch keine Bewertungen
- Hunter NashDokument14 SeitenHunter NashSata AjjamNoch keine Bewertungen
- Heat Transfer Lab ReportDokument6 SeitenHeat Transfer Lab ReportZeenat RanaNoch keine Bewertungen
- Solution Thermodynamics 2020Dokument49 SeitenSolution Thermodynamics 2020Esha ChohanNoch keine Bewertungen
- Chapter 5Dokument36 SeitenChapter 5Lucy BrownNoch keine Bewertungen
- PCHEMDokument11 SeitenPCHEMMika PelagioNoch keine Bewertungen
- Heat Exchanger ReportDokument8 SeitenHeat Exchanger Reportarslan shahidNoch keine Bewertungen
- STA 247 - Answers For Practice Problem Set #1Dokument5 SeitenSTA 247 - Answers For Practice Problem Set #1aakasNoch keine Bewertungen
- 03 Equilibria (I)Dokument11 Seiten03 Equilibria (I)David LevisteNoch keine Bewertungen
- Heat Exchangers: DR Ali JawarnehDokument46 SeitenHeat Exchangers: DR Ali Jawarnehprasanthi100% (1)
- Tutorial Questions 2Dokument21 SeitenTutorial Questions 2Lv Liska71% (7)
- Solutions From OnlineDokument36 SeitenSolutions From OnlineNiniGooseNoch keine Bewertungen
- Try MeDokument9 SeitenTry MeKrizzete HernandezNoch keine Bewertungen
- Applications of Laplace Transform Unit Step Functions and Dirac Delta FunctionsDokument8 SeitenApplications of Laplace Transform Unit Step Functions and Dirac Delta FunctionsJASH MATHEWNoch keine Bewertungen
- Concentric Tube Heat ExchangerDokument10 SeitenConcentric Tube Heat Exchangeramirhazwan93% (14)
- User ManualDokument26 SeitenUser ManualRodney CutinhoNoch keine Bewertungen
- Answer Key-CHE 4353-Exam 3-Fall 14Dokument9 SeitenAnswer Key-CHE 4353-Exam 3-Fall 14Kalmah3636Noch keine Bewertungen
- Tugas Pap Kel3Dokument9 SeitenTugas Pap Kel316-125 Ruth Ria RistaNoch keine Bewertungen
- CHE4162 Particle Technology November 2010 Exam SolutionsDokument14 SeitenCHE4162 Particle Technology November 2010 Exam SolutionsPa1 Kumar MNoch keine Bewertungen
- Shell & Tube Heat Exchanger: - DesigningDokument18 SeitenShell & Tube Heat Exchanger: - DesigningKusmakar100% (1)
- 221-03 ++Dokument7 Seiten221-03 ++dangerous0Noch keine Bewertungen
- Refriger Ac I OnDokument15 SeitenRefriger Ac I OnMiguel Angel MartinezNoch keine Bewertungen
- Part 3Dokument25 SeitenPart 3Zyber ColcolNoch keine Bewertungen
- Homework 1Dokument9 SeitenHomework 1AgithaNoch keine Bewertungen
- ODE Solver in MATLABDokument15 SeitenODE Solver in MATLABKailasham RamalingamNoch keine Bewertungen
- Experiment #2 HinchleyDokument5 SeitenExperiment #2 HinchleyTalha AhmadNoch keine Bewertungen
- Tutorial 1 - SolutionDokument9 SeitenTutorial 1 - SolutionerewrewrNoch keine Bewertungen
- MUCLecture 2022 42228583Dokument2 SeitenMUCLecture 2022 42228583Muhammad Ayan Malik100% (1)
- Camphor BallsDokument8 SeitenCamphor BallsGurunath EpiliNoch keine Bewertungen
- A Step Change of Magnitude 4 Is Introduced Into A System Having The Transfer FunctionDokument8 SeitenA Step Change of Magnitude 4 Is Introduced Into A System Having The Transfer FunctionFarid SarrafNoch keine Bewertungen
- Ionic Equlibrium PDFDokument58 SeitenIonic Equlibrium PDFAniruddha KawadeNoch keine Bewertungen
- Answer On Question #44801-Physics-Molecular Physics-ThermodynamicsDokument2 SeitenAnswer On Question #44801-Physics-Molecular Physics-ThermodynamicsRamish TechNoch keine Bewertungen
- ARABACA - XDD - Module 3 Activity No. 4 - REFSYSDokument4 SeitenARABACA - XDD - Module 3 Activity No. 4 - REFSYSXAVIERDOMINIC ARABACANoch keine Bewertungen
- Lesson 2 Exercises Problem 1Dokument3 SeitenLesson 2 Exercises Problem 1esclitoarhonNoch keine Bewertungen
- Orifice and Jet Trajectory TestDokument8 SeitenOrifice and Jet Trajectory Testali najat0% (2)
- CHE406 - Past Exam QuestionsDokument16 SeitenCHE406 - Past Exam QuestionsCamila Shaine BelmonteNoch keine Bewertungen
- Lab 1Dokument1 SeiteLab 1Malik Mohammad AsifNoch keine Bewertungen
- Bridge Minimum Strands ResultDokument1 SeiteBridge Minimum Strands ResultMalik Mohammad AsifNoch keine Bewertungen
- Matlab Code Bisection MethodDokument2 SeitenMatlab Code Bisection MethodMalik Mohammad AsifNoch keine Bewertungen
- Rigid Jointed Frame AnalysisDokument6 SeitenRigid Jointed Frame AnalysisMalik Mohammad AsifNoch keine Bewertungen
- Lab 1Dokument1 SeiteLab 1Malik Mohammad AsifNoch keine Bewertungen
- Example PDFDokument6 SeitenExample PDFMalik Mohammad AsifNoch keine Bewertungen
- C511 zswf1139Dokument3 SeitenC511 zswf1139Michael PEtrovNoch keine Bewertungen
- D 4643 - 00Dokument5 SeitenD 4643 - 00Camila CastanedaNoch keine Bewertungen
- D2216 (Water Content) PDFDokument5 SeitenD2216 (Water Content) PDFAlif FauzanNoch keine Bewertungen
- Determination of Moisture ContentDokument10 SeitenDetermination of Moisture ContentOmar ViewNoch keine Bewertungen
- D 854 - 14Dokument8 SeitenD 854 - 14Jimmy WalkerNoch keine Bewertungen
- Department of Civil Engineering CE-119 MECHANICS OF SOLIDS - I (Spring 2019) Home Work # 2Dokument2 SeitenDepartment of Civil Engineering CE-119 MECHANICS OF SOLIDS - I (Spring 2019) Home Work # 2Malik Mohammad AsifNoch keine Bewertungen
- D2216 (Water Content) PDFDokument5 SeitenD2216 (Water Content) PDFAlif FauzanNoch keine Bewertungen
- Ce3 PDFDokument2 SeitenCe3 PDFMalik Mohammad AsifNoch keine Bewertungen
- Add Forces Par Law PDFDokument27 SeitenAdd Forces Par Law PDFJam Uly GastyNoch keine Bewertungen
- Astm c40 TestDokument1 SeiteAstm c40 TestMalik Mohammad AsifNoch keine Bewertungen
- C511 zswf1139Dokument3 SeitenC511 zswf1139Michael PEtrovNoch keine Bewertungen
- Answer On Question #11567-Physics-Molecular Physics-ThermodynamicsDokument3 SeitenAnswer On Question #11567-Physics-Molecular Physics-ThermodynamicsMuhammad Haris KhattakNoch keine Bewertungen
- Properties of Nonmetals.: Temperature 10 Substance C W/M C KG/M KJ/KG C M /s Structural and Heat-Resistant MaterialsDokument3 SeitenProperties of Nonmetals.: Temperature 10 Substance C W/M C KG/M KJ/KG C M /s Structural and Heat-Resistant MaterialsMalik Mohammad AsifNoch keine Bewertungen
- YehshsDokument15 SeitenYehshsNadiaNoch keine Bewertungen
- Department of Civil Engineering CE-119 MECHANICS OF SOLIDS - I (Spring 2019) Home Work # 2Dokument2 SeitenDepartment of Civil Engineering CE-119 MECHANICS OF SOLIDS - I (Spring 2019) Home Work # 2Malik Mohammad AsifNoch keine Bewertungen
- Technological Institute of The Philippines College of Engineering and Architecture CE 001 Problem Set # 1Dokument2 SeitenTechnological Institute of The Philippines College of Engineering and Architecture CE 001 Problem Set # 1Malik Mohammad AsifNoch keine Bewertungen
- Urban Dynamics ModelDokument13 SeitenUrban Dynamics ModelMalik Mohammad AsifNoch keine Bewertungen
- Properties of Nonmetals.: Temperature 10 Substance C W/M C KG/M KJ/KG C M /s Structural and Heat-Resistant MaterialsDokument3 SeitenProperties of Nonmetals.: Temperature 10 Substance C W/M C KG/M KJ/KG C M /s Structural and Heat-Resistant MaterialsMalik Mohammad AsifNoch keine Bewertungen
- Properties of Nonmetals.: Temperature 10 Substance C W/M C KG/M KJ/KG C M /s Structural and Heat-Resistant MaterialsDokument3 SeitenProperties of Nonmetals.: Temperature 10 Substance C W/M C KG/M KJ/KG C M /s Structural and Heat-Resistant MaterialsMalik Mohammad AsifNoch keine Bewertungen
- Technological Institute of The Philippines College of Engineering and Architecture CE 001 Problem Set # 1Dokument2 SeitenTechnological Institute of The Philippines College of Engineering and Architecture CE 001 Problem Set # 1Malik Mohammad AsifNoch keine Bewertungen
- 1st Semester PDFDokument5 Seiten1st Semester PDFMalik Mohammad AsifNoch keine Bewertungen
- 1st Semester PDFDokument5 Seiten1st Semester PDFMalik Mohammad AsifNoch keine Bewertungen