Beruflich Dokumente
Kultur Dokumente
Jurnal Template
Hochgeladen von
Qurrotul A'yun0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
29 Ansichten6 SeitenOriginaltitel
Jurnal Template.doc
Copyright
© © All Rights Reserved
Verfügbare Formate
DOC, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
© All Rights Reserved
Verfügbare Formate
Als DOC, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
29 Ansichten6 SeitenJurnal Template
Hochgeladen von
Qurrotul A'yunCopyright:
© All Rights Reserved
Verfügbare Formate
Als DOC, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 6
Synthesis and Characterization of Nano TiO2 with
Sol-Gel Method as Self-Cleaning Agent on Acrylic
Paint
*Note: Sub-titles are not captured in Xplore and should not be used
XXX-X-XXXX-XXXX-X/XX/$XX.00 ©20XX IEEE
line 1: 1st Given Name Surname
line 1: 2nd Given Name Surname line 1: 3rd Given Name Surname
line 2: dept. name of organization
line 2: dept. name of organization line 2: dept. name of organization
(of Affiliation)
(of Affiliation) (of Affiliation)
line 3: name of organization
line 3: name of organization line 3: name of organization
(of Affiliation)
(of Affiliation) (of Affiliation)
line 4: City, Country
line 4: City, Country line 4: City, Country
line 5: email address
line 5: email address line 5: email address
line 1: 4th Given Name Surname
line 1: 5th Given Name Surname line 1: 6th Given Name Surname
line 2: dept. name of organization
line 2: dept. name of organization line 2: dept. name of organization
(of Affiliation)
(of Affiliation) (of Affiliation)
line 3: name of organization
line 3: name of organization line 3: name of organization
(of Affiliation)
(of Affiliation) (of Affiliation)
line 4: City, Country
line 4: City, Country line 4: City, Country
line 5: email address
line 5: email address line 5: email address
Abstract— Metal oxide TiO 2 has photocatalytic properties suspension (sol) forming a continuous liquid phase (gel). The
that make TiO2 amphilic, that is, hydrophobic in the dark advantages of the sol-gel method for the synthesis of metal
(without UV light) and hydrophilic in the light (there is UV oxide nanoparticles include forming a transparent oxide layer
light). The photocatalytic properties of TiO 2 can be utilized to that adheres perfectly to the fabric, resulting in uniform
develop self-cleaning materials in paints. Self-cleaning material particle size distribution and a large surface area [3].
is a material capable of cleaning itself both by avoiding it and
by degrading the dirt that clings to it with the help of UV rays. Based on this information, a self-cleaning material from
The TiO2 nano particle synthesis process uses the sol-gel TiO2 surface paint will be made in this study. Mixing paint
method with the precursor tetrabuthyl titanate (TBT). The with TiO2 will be carried out with several different volume
purpose of this research is to synthesize TiO 2 nano particles as compositions, namely the mass (gr) ratio of TiO 2 / paint
self-cleaning material in paint with the most appropriate mixture of 0.5: 9.5; 1: 9; 1.5: 8.5; and 2:8. In this research
composition. The results obtained from the synthesis of nano the characterization of TiO2 nanoparticles will be tested using
TiO2 particles were tested by FTIR and appeared at a peak of X-Ray Diffraction (XRD) and Fourier Transfrom Infra Red
467.50 cm-1 indicating the presence of TiO 2 groups. In addition, (FTIR) instruments. The self-cleaning test will be measured
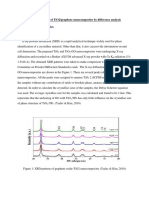
characterization using XRD was also produced which by the strength of the hydrophobic activity obtained by
produced the anatase crystalline phase of TiO 2 with a crystal measuring the angle of contact of water with the paint / TiO 2
size of 24.32 nm. Surface morphology was analyzed by
coated glass surface.
Scanning Electron Microscopy (SEM). The ability of self-
cleaning properties in paint was measured through
hydrophobic properties through contact angle measurements II. METHODS
and the best contact angle values obtained on TiO 2 / paint (2: 8)
were 102.2o. A. Tool
The tools used in this study are glass, dropper pipette,
Keywords nano TiO2, sol-gel, self-cleaning, hidrofobic
beaker, measuring cup, hot plate stirrer, magnetic stirrer,
spatula, watch glass, mortal and pestle, analytical balance.
I. INTRODUCTION Furnace, oven (Binder, Germani), scanning electron
Currently the development of nanoparticle technology microscopy (SEM-EDX, Philips XL30; Germany), Fourier
has been widely used in various purposes, one of which is as Transform Infrared Spectrophotometer (Shimadzu 8021),
a developer of self-cleaning properties. Self-cleaning universal pH indicator, X-Ray Diffraction (Shimadzu XRD
properties are properties where a material is able to clean 6000).
itself both by avoiding it and by degrading the dirt that
adheres to itself. One of these self-cleaning properties can be B. Material
applied to wall paints. Wall paint used by the community is The materials used in this research are TBT (Tetrabuthyl
often difficult to clean from dirt or ink so self-cleaning Titanate) (Sigma-Aldrich), white acrylic paint, ethanol pro-
properties need to be developed in the paint to simplify the analyst, glacial acetic acid and aquades.
maintenance process.
Development of the properties of paint is needed to III. PROCEDURE
overcome the above problems. The paint is expected to
degrade the dirt or stains on the wall itself. This property is A. Synthesis of TiO2 nanosols
called "self-cleaning". Self-cleaning properties on the surface The manufacture of nano TiO2 particles was carried out
of the fabric work with the photocatalytic principle that by the sol-gel method using TBT as precursors, aquades, p.a
works when the surface gets ultraviolet (UV) radiation. The ethanol and glacial acetic acid. Nano sol was obtained by
photocatalysis process makes TiO2 hydrophilic, which is mixing Tetrabuthyl Titanate (TBT) and ethanol p.a and then
very like water when exposed to UV rays, the impact of sterilizing it for 30 minutes at room temperature and a clear
nano-sized TiO2 surface remains transparent and not foggy yellow solution was obtained. The solution was then added
when exposed to water vapor, while in the dark (no UV rays) to a mixture of glacial acetic acid, distilled water and ethanol
TiO2 is hydrophobic or does not like water. In a world of p.a dropwise (± 1 drop / second) with strong stirring. The
light and dark cycles, TiO2 behaves amphilic, hydrophobic mixture is then stirred for 1 hour and a clear yellow light sol
when dark and hydrophilic when bright [1]. is produced. The solution is then left in ambient conditions
The mechanism of the amphilic properties of TiO2 occurs for 24 hours. After being simulated for 24 hours, the sol-gel
due to the presence of UV light. The presence of UV light on Tis dried at 110ºC to convert the sol-gel phase to powder and
TiO2 results in the transition of electrons (e-) from the is ground for 2 hours at 500ºC. The powder formed was
valence band to the conduction band, thus producing a characterized by FTIR and XRD [4].
vacancy (h+) on the valence band and electrons (e-) on the
conduction band. Electrons reduce Ti (IV) to Ti (III), while a B. Chemical Characterization of TiO2 nanosols with FTIR
vacuum (h+) oxidizes O2- to O2 resulting in an oxygen The dried TiO2 powder was taken several mg to be mixed
vacuum on the surface. The oxygen vacuum is filled with with a few mg of dry KBr to be made pellet then analyzed
water molecules that bind with Ti (III) which then using FTIR.
decomposes into hydroxo on the surface. Furthermore, the
hydroxso undergoes condensation by releasing water to form C. Characterization of TiO2 nanosols by X-ray diffraction
a hydrophobic Ti-O-Ti bond [2]. (XRD)
The development of TiO2 nano particle synthesis method The dried TiO2 powder is taken several mg for testing
is needed to control the crystalline phase, size, and and analyzed using X-ray diffraction (XRD) at an angle of 2θ
morphology of TiO2 nanocrystals. Synthesis of TiO2 10º-100º.
nanoparticles can be done by the sol-gel method. The sol-gel
method is a method of synthesis of nanoparticles where in
the process a phase change occurs from a colloidal
D. Mixing TiO2 with Acrylic Paint and Coating on Glass OH buckling vibrations 1638,21
Media from Ti-OH
TiO2 powder that has been planted is then mixed with Stretching OH vibrations 3418,39
acrylic paint with a composition (gram) TiO 2 / Cat 0.5: 9.5; from Ti-OH and ethanol
1: 9; 1.5: 8.5; and 2: 8. Each mixing result is added with 2 C. Characterization of TiO2 nanosols by X-ray diffraction
mL of distilled water. The paint is then stirred with a spatula (XRD)
until evenly distributed and coated on glass that has been
washed with distilled water and ethanol. Coating of paint / The results of the peak angle XRD test that appeared
TiO2 on the glass is done by the smear method. Ti-O were matched with JCPDS and the anatase phase was
obtained. This was evidenced by the presence of typical
E. Self-Cleaning Test with Contact Angle measurement anatase peaks seen at an angle of 2θ = 25.0707º with an
Paint with the addition of TiO2 coated glass is then intensity of 100% and at several other peaks corresponding
measured by contact angle by means of a test of water drops to JCPDS anatase with number 21- 1272. To calculate the
on the glass surface. The drip results are then photographed size of the TiO2 FHHM crystals in Figure 2 are included in
using a Canon Ixus 230HS camera. Images from this shoot the following Scherrer equation :
are then analyzed using the AutoCAD application to measure
the angle of contact between water and glass surface.
IV. RESULTS AND DISCUSSION
D = crystal size
A. Synthesis of TiO2 nanosols λ = wavelength of X-ray beam
In the TiO2 nanosol synthesis process hydrolysis and β = FWHM (full width half maximum) /
condensation reactions occur. In the hydrolysis stage, the intensity in radians
reaction takes place quickly where there is a change of θ = large angle from peak with high intensity.
alkoxide groups with nucleophilic hydroxyl groups. At the Obtained size of the synthesized TiO2 crystal is 24.32 nm.
condensation stage it will produce the formation and growth
of TiO2.xH2O [5].
B. Characterization of TiO2 nanosols with FTIR
Chemical characteristics of TiO2 nanosols from the
analysis of the chemical functional groups of TiO2 nanosols
using FTIR spectrophotometer are shown in Figure 1.
Figure 2. Synthesis of XRD TiO2 data (XRD
Philips X-pert Powder Diffractometer)
D. Self-Cleaning Test Results with Contact Angle
measurements
The ability of self-cleaning paint with the addition of
Figure 1. FTIR spectra of TiO2 synthesized by TiO2 TiO2 can be measured by how high the hydrophobic value of
the paint produced when coating a media. Paint / TiO 2
Based on the results obtained from the FTIR test in coated glass media are measured at the contact angle and the
Figure 1 shows the peaks that appear on several waves. The data produced in the Table 2 are:
FTIR spectrum on TiO2 shows the peak at wave number Table 2. Data of contact angle test result
3418.39 cm-1 which is the absorption of the stretching -OH Sample Drop Test Results Contact Angle
vibration, while the absorption of 1638.21 cm-1 which is the
Cat tanpa 62,6º
absorption of the bend -OH vibration. Based on the research
that has been carried out the bend -OH vibration of the Ti- TiO2
OH bond at the peak of 1650 cm -1 resulted from H2O
molecular adsorbent that is not completely released after
synthesis by the sol-gel method, whereas, at the peak of 3250 Cat + TiO2 67,6º
cm-1 is stretching vibration absorption -OH group of Ti-OH. 0,25 gr
The next spectrum appears at the peak of 467.50 cm -1
indicating the presence of Ti-O bonds.
Cat + TiO2 61,0º
Table 1. Functional group data on TiO2 nanosols 0,50 gr
Functional group Wave Number
Vibratory Ti-O from TiO2 467,50
Cat + TiO2 86,1º in the contact angle values when the paint is tested for
1,00 gr hydrophobic properties between one surface and another.
SEM-EDX analysis of paint with the addition of TiO2.
Cat + TiO2 72,3º
1,50 gr
Cat + TiO2 102,2º
2,00 gr
Figure 4. Analysis of EDX paint with the addition of
TiO2
The results of the test drops on paint without TiO 2 Figure 4 shows that TiO2 is found in paint. In paint with
showed that the glass has a small contact angle because the addition of TiO2 shows that there is TiO 2 in the paint.
when the water drops the paint is not round and the angle is This shows that the process of adding TiO 2 to the paint was
62.6 °. The 62.6 ° angle shows that this glass is not successful because of the TiO 2 that was embedded in the
hydrophobic because there are still many interactions of paint even though the results were less than maximal at only
water with the glass surface. The condition for an object to 3.4%.
be called hydrophobic is if it forms an angle of ˃90 °, CONCLUSIONS AND SUGGESTIONS
whereas for an object having a contact angle <90 ° then the
object is hydrophilic [6] Conclusion
The results of the test drops on the glass coated with Based on the results of research and discussion it can be
paint/TiO2 with several variations of the mixture showed concluded that the XRD analysis of TiO2 synthesis results
that the glass coated with paint/TiO2 with several variations has a crystal size of 24.32 nm. FTIR test shows the existence
of the mixture produced contact angles with a greater value of Ti-O group at wave number 467.5. The hydrophobic
compared to the contact angle values produced by the paint nature test shows that the more TiO2 is added to the paint, the
alone although the results were not significant. Paint / TiO 2 better the hydrophobic properties.
coated glass (9.25: 0.25), (9.5: 0.5), (9: 1), (8.5: 1.5), and (8: Suggestion
2), produce a value The contact angles were 67.6°, 61.0°,
86.1°, 72.2° and 102.2° respectively. The contact angle It is recommended to vary the mixing of paint with more
value in the paint / TiO2 composition has exceeded 90° so TiO2 so that the best results can be known.
that the resulting paint is already hydrophobic.
The hydrophobic nature of the paint increases with REFERENCES
increasing mass of TiO2 added. This happens because the
more TiO2 is added to the paint, the more nano TiO 2 coats [1] Mukti, K. H., Hastiawan, I., Rakhmawati, D., & Noviyanti, A. R.
the surface so that nano-sized TiO2 will hold water from 2013. Preparasi Fotokatalis Barium Bismut Titanat
dripping so it won't wet the surface. This is based on the Terprotonasi(Hbbt) Untuk Fotodegradasi Metilen Biru. Prosiding
Seminar Nasional Sains dan Teknologi Nuklir PTNBR BATAN
lotus effect on taro leaves. Taro leaves have a nano-sized Bandung.
layer, this layer makes taro leaves and lotus leaves not wet. [2] Sutrisno, Hari. 2009. Tinjauan Mikrostruktur Kereaktifan Anatas Dan
The same principle is applied to surfaces coated with TiO 2 Rutil Sebagai Material Superfotohidrofil Permukaan. Prosiding
with nano size [7]. Seminar Nasional Penelitian, Pendidikan dan Penerapan MIPA,
Fakultas MIPA, Universitas Negeri Yogyakarta.
E. Morphological analysis of paint using Scanning [3] Maharani, D. K., Kartini, I., Aprilita, N. H. 2010. Nanosilica-Chitosan
Electron Microscopy (SEM) Composite Coating on Cotton Fabrics. AIP Conference Proceedings
1284, 87.
Analysis using SEM was conducted to determine the [4] Tian, J., Chen, L., Yin, Y., Wang, X., Dai, J., Zhu, Z., Liu, X., & Wu,
surface morphology that formed on the surface of the paint. P. 2009. Photocatalyst of TiO2/ZnO Nano Composite Film:
Preparation, Characterization, and Photodegradation Activity Of
Methyl Orange. Surface and Coating Technology, Vol. 204 No. 1,
205-214..
[5] Haryati, T. dan Mulyono T. 2013. Sintesis dan karakterisasi Core-
Shell ZnO/TiO2 sebagai Material Fotoanoda Dye Sensitized Solar
Cell (DSSC).
[6] Pravita S, Anggi, dan Dahlan, Dahyunir. 2013 . Sintesis Lapisan TiO2
Menggunakan Prekursor TiCl4 untuk Aplikasi Kaca Self Cleaning
dan Anti Fogging . Jurnal Fisika Unand Vol. 2, No. 2, ISSN 2302-
Figure 3. Paint surface morphology with the addition of 8491.
TiO2 at 10,000 times magnification [7] Putri, T.A., Ratnawulan., & Ramli. 2018. Sintesis Lapisan
Hydrophobic Nanokomposit Mangan Oksida/Polystyrene (MnO2/PS)
The surface of the paint with the addition of TiO 2 is still untuk Aplikasi Self-Cleaning. Pillar of Physic. Vol. 11., No. 2. Hal: 1-
a lump which indicates that the mixing of the paint and TiO 2 8.
is still not evenly distributed perfectly, causing a difference
Das könnte Ihnen auch gefallen
- JMO Solutions 2008Dokument4 SeitenJMO Solutions 2008ichkhuyNoch keine Bewertungen
- Bryophytes MorphologyDokument9 SeitenBryophytes Morphologyrachna singh0% (1)
- 19 Free Amigurumi Crochet Patterns: MaterialsDokument4 Seiten19 Free Amigurumi Crochet Patterns: MaterialsLucica Diaconu100% (1)
- Synthesis and Characterization of Bismuth Oxide Doped Titanium Dioxide and Its Antibacterial ActivityDokument16 SeitenSynthesis and Characterization of Bismuth Oxide Doped Titanium Dioxide and Its Antibacterial ActivityShinta Novita Sari100% (1)
- TotSK 3.0Dokument22 SeitenTotSK 3.0PedroNoch keine Bewertungen
- Soal Pat Bahasa Inggris Kelas 5Dokument5 SeitenSoal Pat Bahasa Inggris Kelas 5Tini Bastuti Joyolaksono100% (1)
- Industrial Chemistry of Oxides for Emerging ApplicationsVon EverandIndustrial Chemistry of Oxides for Emerging ApplicationsNoch keine Bewertungen
- Self-Cleaning Finish On Cotton Textile Using Sol-Gel Derived Tio2 Nano FinishDokument5 SeitenSelf-Cleaning Finish On Cotton Textile Using Sol-Gel Derived Tio2 Nano FinishS BiswasNoch keine Bewertungen
- Self-Cleaning Finish On Cotton Textile Using Sol-Gel Derived Tio2 Nano FinishDokument5 SeitenSelf-Cleaning Finish On Cotton Textile Using Sol-Gel Derived Tio2 Nano FinishS BiswasNoch keine Bewertungen
- Calcination Temperature Effect On Titanium Oxide (TiO2)Dokument27 SeitenCalcination Temperature Effect On Titanium Oxide (TiO2)SaiFunNoch keine Bewertungen
- 607 SanjeevDokument13 Seiten607 SanjeevRITWIsNoch keine Bewertungen
- Assignment: Name of The StudentsDokument15 SeitenAssignment: Name of The StudentsRishabh MishraNoch keine Bewertungen
- Surface & Coatings TechnologyDokument6 SeitenSurface & Coatings TechnologySegundo Jonathan Rojas FloresNoch keine Bewertungen
- Photocatalytic Degradation of Eriochrome Black-T by The PDFDokument30 SeitenPhotocatalytic Degradation of Eriochrome Black-T by The PDFPedro Reynaldo MartinezNoch keine Bewertungen
- 1Dokument5 Seiten1Fadime Gedik AteşNoch keine Bewertungen
- Ngon CS 2023 0033Dokument6 SeitenNgon CS 2023 0033Phúc Nguyễn BáNoch keine Bewertungen
- Characterization and Synthesis of Nanosized Tio Particles: M.U. Shahab, T.A.Tabish, B. Zaman, Zahra Tariq, M. KamranDokument4 SeitenCharacterization and Synthesis of Nanosized Tio Particles: M.U. Shahab, T.A.Tabish, B. Zaman, Zahra Tariq, M. Kamranrajbharaths1094Noch keine Bewertungen
- Titanium Oxide Nano Colloids For DSSC and Other Applications v1 G24 PowerDokument5 SeitenTitanium Oxide Nano Colloids For DSSC and Other Applications v1 G24 Powersugar marmelateNoch keine Bewertungen
- TiO2 CleaningDokument7 SeitenTiO2 CleaningRAHUL GNoch keine Bewertungen
- Nanocomposites Coating of Cotton Fabrics With Antibacterial and Self-Cleaning PropertiesDokument17 SeitenNanocomposites Coating of Cotton Fabrics With Antibacterial and Self-Cleaning PropertiesZabed HossainNoch keine Bewertungen
- Study of Nano Sio2/Tio2 Superhydrophobic Self-Cleaning Surface Produced by Sol-GelDokument5 SeitenStudy of Nano Sio2/Tio2 Superhydrophobic Self-Cleaning Surface Produced by Sol-GelIyed HaouariNoch keine Bewertungen
- NANV03I03P0169Dokument6 SeitenNANV03I03P0169nano_journalNoch keine Bewertungen
- GO-TiO2 2016Dokument5 SeitenGO-TiO2 2016Rafif QuthronadaNoch keine Bewertungen
- Seminar Report On CDI ProcessDokument9 SeitenSeminar Report On CDI ProcessAkash kushwahaNoch keine Bewertungen
- Use of Optical Density and TiO2 Light Scattering To Identify Optimization Potential in Architectural CoatingsDokument9 SeitenUse of Optical Density and TiO2 Light Scattering To Identify Optimization Potential in Architectural CoatingsMajd M. KhalilNoch keine Bewertungen
- Novel Sol-Gel Method of Synthesis of Pure and Aluminum Doped Tio NanoparticlesDokument4 SeitenNovel Sol-Gel Method of Synthesis of Pure and Aluminum Doped Tio NanoparticlesFahim ShahriarNoch keine Bewertungen
- Photocatalytic Degradation of Methyl Orange Using Tio2/Sno2 Binary Nano CompositeDokument7 SeitenPhotocatalytic Degradation of Methyl Orange Using Tio2/Sno2 Binary Nano CompositeasdaNoch keine Bewertungen
- 2012 HindawiDokument8 Seiten2012 HindawiA. M. SHAREQUENoch keine Bewertungen
- Photo-Catalysis in Self Cleaning: Ankip Kumar, Department of Physics (2013PH10834)Dokument4 SeitenPhoto-Catalysis in Self Cleaning: Ankip Kumar, Department of Physics (2013PH10834)Ankip KumarNoch keine Bewertungen
- Nanotechnology and Energy Storage Lab Manual - 2Dokument30 SeitenNanotechnology and Energy Storage Lab Manual - 2Sunskrati PandeyNoch keine Bewertungen
- Photocatalytic Degradation of Methylene Blue Using TiO2 Impregnated DiatomiteDokument8 SeitenPhotocatalytic Degradation of Methylene Blue Using TiO2 Impregnated DiatomitechemengNoch keine Bewertungen
- Photocatalytic Activity of Nano-Tio On Glass in Building EnvelopeDokument7 SeitenPhotocatalytic Activity of Nano-Tio On Glass in Building EnvelopeNABIL HUSSAINNoch keine Bewertungen
- Superlattices and Microstructures: P. Klankaw, C. Chawengkijwanich, N. Grisdanurak, Siriluk ChiarakornDokument10 SeitenSuperlattices and Microstructures: P. Klankaw, C. Chawengkijwanich, N. Grisdanurak, Siriluk ChiarakornIsna NurhidayatiNoch keine Bewertungen
- Materials Research Bulletin: Yang You, Long Wan, Shiying Zhang, Difa XuDokument5 SeitenMaterials Research Bulletin: Yang You, Long Wan, Shiying Zhang, Difa XuChemist.AlchemistNoch keine Bewertungen
- Nano-TiO2 Production Using A Spinning Disc Reactor PDFDokument6 SeitenNano-TiO2 Production Using A Spinning Disc Reactor PDFAyush53Noch keine Bewertungen
- Textile Research Journal 2012 Zhang 747 54Dokument9 SeitenTextile Research Journal 2012 Zhang 747 54Rushil KhuranaNoch keine Bewertungen
- Formation and Characterization of Nano Sized Tio Powder by Sol-Gel MethodDokument9 SeitenFormation and Characterization of Nano Sized Tio Powder by Sol-Gel MethoddmaheswariNoch keine Bewertungen
- Icst 1013Dokument4 SeitenIcst 1013International Jpurnal Of Technical Research And ApplicationsNoch keine Bewertungen
- Hydrothermal Solvothermal Synthesis andDokument69 SeitenHydrothermal Solvothermal Synthesis andmhshimul13Noch keine Bewertungen
- TiO2 Micro Wave Sol-Gel Method PDFDokument5 SeitenTiO2 Micro Wave Sol-Gel Method PDFBommineediLakshmanKumarNoch keine Bewertungen
- Dye Sensitized Solar Cell Lesson Plan: PurposeDokument8 SeitenDye Sensitized Solar Cell Lesson Plan: PurposeDeva RajNoch keine Bewertungen
- Effect of Nano Tio Pretreatment On Functional Properties of Cotton FabricDokument6 SeitenEffect of Nano Tio Pretreatment On Functional Properties of Cotton FabricIJERDNoch keine Bewertungen
- Influenceof Dye Adsorbtion Time On Tio Dye-Sensitized Solar Cells With Krokot Extract (Portulaca Oleracea L.) As A Natural SensitizerDokument8 SeitenInfluenceof Dye Adsorbtion Time On Tio Dye-Sensitized Solar Cells With Krokot Extract (Portulaca Oleracea L.) As A Natural SensitizerdidikkrisNoch keine Bewertungen
- IIT Delhi 2013ME20795Dokument8 SeitenIIT Delhi 2013ME20795RAhulSidhNoch keine Bewertungen
- Scholars Research Library: ISSN: 2278 - 0041Dokument6 SeitenScholars Research Library: ISSN: 2278 - 0041SaiNoch keine Bewertungen
- 2022 Aug 29 MSAT-11-e-proceedings - Rev0-85-88Dokument4 Seiten2022 Aug 29 MSAT-11-e-proceedings - Rev0-85-88Pheeraphong BunroekNoch keine Bewertungen
- Titanium Dioxide Tio2Dokument6 SeitenTitanium Dioxide Tio2api-316630268Noch keine Bewertungen
- Eibc 2335Dokument39 SeitenEibc 2335jhenyNoch keine Bewertungen
- Titanium Oxide Dispersed On Natural Zeolite (Tio /zeolite) and Its Application For Congo Red PhotodegradationDokument5 SeitenTitanium Oxide Dispersed On Natural Zeolite (Tio /zeolite) and Its Application For Congo Red PhotodegradationAddien WiditaNoch keine Bewertungen
- Extraction of Betalain Dye From Beetroot and Preparation of Organic DSSCDokument4 SeitenExtraction of Betalain Dye From Beetroot and Preparation of Organic DSSCrobel kassawNoch keine Bewertungen
- Asish Anatase Tio2Dokument11 SeitenAsish Anatase Tio2Jhasaketan NayakNoch keine Bewertungen
- Advance Material Draft Version1.0Dokument16 SeitenAdvance Material Draft Version1.0Yz LowNoch keine Bewertungen
- 2018 - Prakash Et AlDokument5 Seiten2018 - Prakash Et AlDương Minh MẫnNoch keine Bewertungen
- PartIIISessionAV4 PDFDokument388 SeitenPartIIISessionAV4 PDFdangthNoch keine Bewertungen
- Anatase and Rutile TiO2 Composites Influence of The Mixing Ratio On The Photocatalytic DegradationDokument15 SeitenAnatase and Rutile TiO2 Composites Influence of The Mixing Ratio On The Photocatalytic DegradationNếck XùNoch keine Bewertungen
- Fabrication of Dye-Sensitized Solar Cells by Spray Pyrolysis Deposition (SPD) TechniqueDokument6 SeitenFabrication of Dye-Sensitized Solar Cells by Spray Pyrolysis Deposition (SPD) TechniqueanueshaNoch keine Bewertungen
- Jurnal Fisika FluxDokument8 SeitenJurnal Fisika FluxDEWI PRATIWINoch keine Bewertungen
- Shivam SynthesisDokument15 SeitenShivam SynthesisSachin GoyalNoch keine Bewertungen
- Xin 2011Dokument5 SeitenXin 2011Claudio Gallardo-ArayaNoch keine Bewertungen
- 1 s2.0 S223878541830303X Main PDFDokument6 Seiten1 s2.0 S223878541830303X Main PDFDevy Lestary IINoch keine Bewertungen
- Preparation of Sub-Micron Size Anatase Tio Particles For Use As Light-Scattering Centers in Dye-Sensitized Solar CellDokument5 SeitenPreparation of Sub-Micron Size Anatase Tio Particles For Use As Light-Scattering Centers in Dye-Sensitized Solar CellvirparaNoch keine Bewertungen
- 1 s2.0 S2214785319334170 MainDokument6 Seiten1 s2.0 S2214785319334170 Mainedcr23Noch keine Bewertungen
- Molecules: Ffect of The Titanium Isopropoxide:AcetylacetoneDokument14 SeitenMolecules: Ffect of The Titanium Isopropoxide:AcetylacetoneSasitharan MNoch keine Bewertungen
- Dual Morphology Titanium Dioxide For Dye Sensitized Solar CellsDokument40 SeitenDual Morphology Titanium Dioxide For Dye Sensitized Solar CellsksmithulpranavNoch keine Bewertungen
- Design of Visible Light Active Photocatalyst For Environmental and Energy ApplicationsDokument73 SeitenDesign of Visible Light Active Photocatalyst For Environmental and Energy ApplicationsSnehal AbhyankarNoch keine Bewertungen
- Materi 3Dokument1 SeiteMateri 3Qurrotul A'yunNoch keine Bewertungen
- 1jadwal Piket Ramadhan 5 Hari TerakhirDokument2 Seiten1jadwal Piket Ramadhan 5 Hari TerakhirQurrotul A'yunNoch keine Bewertungen
- Measurement Conditions:: Teknik Material Dan Metalurgi N 1 10/19/2019Dokument4 SeitenMeasurement Conditions:: Teknik Material Dan Metalurgi N 1 10/19/2019Qurrotul A'yunNoch keine Bewertungen
- Measurement Conditions:: Teknik Material Dan Metalurgi N 1 10/19/2019Dokument3 SeitenMeasurement Conditions:: Teknik Material Dan Metalurgi N 1 10/19/2019Qurrotul A'yunNoch keine Bewertungen
- Universitas Negeri Surabaya: Kementerian Riset, Teknologi, Dan Pendidikan TinggiDokument1 SeiteUniversitas Negeri Surabaya: Kementerian Riset, Teknologi, Dan Pendidikan TinggiQurrotul A'yunNoch keine Bewertungen
- Unit Test 7 (PDF)Dokument1 SeiteUnit Test 7 (PDF)emirelliucNoch keine Bewertungen
- BÀI TẬP TA 9 THEO CHUYÊN ĐỀ NGỮ PHÁPDokument213 SeitenBÀI TẬP TA 9 THEO CHUYÊN ĐỀ NGỮ PHÁPhoangmaiNoch keine Bewertungen
- 0.6m (2ft) Low Profile Antennas Microwave Antenna SpecificationsDokument15 Seiten0.6m (2ft) Low Profile Antennas Microwave Antenna SpecificationsDarwin Lopez AcevedoNoch keine Bewertungen
- Site AnalysisDokument20 SeitenSite AnalysisCarlo RosaioNoch keine Bewertungen
- China Care Foundation - Fall 2010 NewsletterDokument8 SeitenChina Care Foundation - Fall 2010 NewsletterChinaCareNoch keine Bewertungen
- Aluminum Alloy 6351 T6 Sheet SuppliersDokument10 SeitenAluminum Alloy 6351 T6 Sheet Supplierssanghvi overseas incNoch keine Bewertungen
- Nav Bharat Nirman: Indispensable Ideas For Green, Clean and Healthy IndiaDokument4 SeitenNav Bharat Nirman: Indispensable Ideas For Green, Clean and Healthy IndiaRishabh KatiyarNoch keine Bewertungen
- 8 Field Quality PlanDokument18 Seiten8 Field Quality PlanRamaKrishna ANoch keine Bewertungen
- Unit-I: Digital Image Fundamentals & Image TransformsDokument70 SeitenUnit-I: Digital Image Fundamentals & Image TransformsNuzhath FathimaNoch keine Bewertungen
- Full Download Test Bank For Environmental Economics and Management Theory Policy and Applications 6th Edition Callan PDF Full ChapterDokument27 SeitenFull Download Test Bank For Environmental Economics and Management Theory Policy and Applications 6th Edition Callan PDF Full Chapterscissionrideau941m100% (20)
- ASTM A581 A581M-95bDokument3 SeitenASTM A581 A581M-95bFeteneNoch keine Bewertungen
- Lexus JTJBT20X740057503 AllSystemDTC 20230702045631Dokument2 SeitenLexus JTJBT20X740057503 AllSystemDTC 20230702045631Venerable DezzyNoch keine Bewertungen
- Alzheimer's Disease Inhalational Alzheimer's Disease An UnrecognizedDokument10 SeitenAlzheimer's Disease Inhalational Alzheimer's Disease An UnrecognizednikoknezNoch keine Bewertungen
- Contactor - SchniderDokument28 SeitenContactor - SchniderPramod DixitNoch keine Bewertungen
- It Park Design Submission PDFDokument20 SeitenIt Park Design Submission PDFSAKET TYAGI100% (1)
- SMA - Core 1 - IEC62109-2 - 0 Test ReportDokument6 SeitenSMA - Core 1 - IEC62109-2 - 0 Test ReportFurqan HamidNoch keine Bewertungen
- Igorot Village: Get To Know..Dokument11 SeitenIgorot Village: Get To Know..Elain RagosNoch keine Bewertungen
- AN-PFC-TDA 4863-3 Calculation-Tool For PFC-Preconverter Using TDA 4863Dokument9 SeitenAN-PFC-TDA 4863-3 Calculation-Tool For PFC-Preconverter Using TDA 4863NaciConSolNoch keine Bewertungen
- Flooding Deagon Flood Flag MapDokument1 SeiteFlooding Deagon Flood Flag MapNgaire TaylorNoch keine Bewertungen
- 0707-Passive VoiceDokument6 Seiten0707-Passive VoiceKhôi TrầnNoch keine Bewertungen
- Propagare - Threshold Degradation - AbateriDokument72 SeitenPropagare - Threshold Degradation - AbateriAndrada AdaNoch keine Bewertungen
- Phase-Locked Loop Independent Second-Order Generalized Integrator For Single-Phase Grid SynchronizationDokument9 SeitenPhase-Locked Loop Independent Second-Order Generalized Integrator For Single-Phase Grid SynchronizationGracella AudreyNoch keine Bewertungen
- Nestle Internship ResumeDokument2 SeitenNestle Internship ResumeHasnain AshrafNoch keine Bewertungen
- TSBDokument3 SeitenTSBnoe dela vegaNoch keine Bewertungen
- Beauty Therapy Thesis SampleDokument8 SeitenBeauty Therapy Thesis Samplerachelvalenzuelaglendale100% (2)