Beruflich Dokumente
Kultur Dokumente
Quiz 11 - Chemical Reactions - Version 2
Hochgeladen von
api-4365974570 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
24 Ansichten3 SeitenOriginaltitel
quiz 11 - chemical reactions - version 2
Copyright
© © All Rights Reserved
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
24 Ansichten3 SeitenQuiz 11 - Chemical Reactions - Version 2
Hochgeladen von
api-436597457Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 3
Name: _______________________________Date: _______________Class: ________________
Total / 22
* QUIZ 11 – Chemical Reactions – Version 2 *
Self-Assess (0 marks)
Before writing your quiz, circle the statements in the boxes that best reflect your situation.
I can identify the type of chemical reaction.
I’m not sure… I think I can do that? I can definitely do that
I can balance a chemical reaction.
I’m not sure… I think I can do that? I can definitely do that
I can predict the products of a reaction.
I’m not sure… I think I can do that? I can definitely do that
I can predict the state of a compound in a reaction.
I’m not sure… I think I can do that? I can definitely do that
Matching (4 marks)
Identify the type of chemical reaction for the following by writing the letter for the
corresponding type of reaction in the blank provided. Letters may be used more than once or
not at all.
F = Formation DR = Double Replacement
D = Decomposition C = Combustion
SR = Single Replacement O = Other
________ H3PO4 (aq) + 3 KOH (aq) à K3PO4 (aq) + 3 H2O (l)
________ 2 HgO (s) à 2 Hg (l) + O2 (g)
________ C2H5OH (l) + 3 O2 (g) à 2 CO2 (g) + 3 H2O (g)
________ 2 FeCl3 (aq) + 3 BaBr2 (aq) à 2 FeBr3 (aq) + 3 BaCl2 (aq)
Fill in the Blanks (4 marks)
Balance the following chemical reactions by putting the correct numbers in the blanks provided.
The number “1” doesn’t need to be included.
_____ CaC2 (s) + _____ AsBr3 (aq) à _____ C (s) + _____ As (s) + _____ CaBr2 (aq)
_____ NaOH (aq) + _____ H2SO4 (aq) à _____ Na2SO4 (aq) + _____ H2O (l)
_____C3H8 (g) + _____O2 (g) à _____CO2 (g) + _____H2O (g)
_____ Pb (s) + _____ O2 (g) à _____ PbO (s)
Short Answer 1 (9 marks)
For each of the following reactions:
- Predict the products by writing them after the arrow in the chemical reaction.
- Indicate the state for the products.
- Balance the reaction.
_____ (NH4)3PO4 (aq) + _____ Ba(OH)2 (aq) à
_____ C2H6 (g) + _____ O2 (g) à
_____ Al (s) + _____ O2 (g) à
Short Answer 2 (3 marks)
For the following statement:
- Write out the chemical equation.
- Indicate the states for all chemicals.
- Balance the chemical reaction.
When heated, potassium chlorate produces potassium chloride and oxygen gas.
Short Answer 3 (2 marks)
In class, we talked about five things that indicate a chemical reaction has occurred. List two of
these below.
1–
2-
Das könnte Ihnen auch gefallen
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Acids and Bases LabDokument3 SeitenAcids and Bases Labapi-436597457Noch keine Bewertungen
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Drama PLP PlanningDokument2 SeitenDrama PLP Planningapi-436597457Noch keine Bewertungen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5795)
- Resume - Deanna FoxDokument1 SeiteResume - Deanna Foxapi-436597457Noch keine Bewertungen
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Circuits Virtual LabDokument6 SeitenCircuits Virtual Labapi-436597457Noch keine Bewertungen
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Classroom Management PlanDokument9 SeitenClassroom Management Planapi-436597457Noch keine Bewertungen
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- PD Development CertificateDokument1 SeitePD Development Certificateapi-436597457Noch keine Bewertungen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Psii PD CertificateDokument1 SeitePsii PD Certificateapi-436597457Noch keine Bewertungen
- MULTIPLE CHOICE. Choose The One Alternative That Best Completes The Statement or Answers The QuestionDokument6 SeitenMULTIPLE CHOICE. Choose The One Alternative That Best Completes The Statement or Answers The QuestionBoshra BoshraNoch keine Bewertungen
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- Lesson Plan Tower of LondonDokument5 SeitenLesson Plan Tower of Londonmacrinabratu4458Noch keine Bewertungen
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Electrical Information: Service Training MechanikDokument22 SeitenElectrical Information: Service Training Mechanikfroilan ochoaNoch keine Bewertungen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Level - 1: Expansion of DeterminantsDokument13 SeitenLevel - 1: Expansion of DeterminantsAtomitronNoch keine Bewertungen
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- The Utopia of The Zero-OptionDokument25 SeitenThe Utopia of The Zero-Optiontamarapro50% (2)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- How To Write A ThesisDokument14 SeitenHow To Write A ThesisPiyushNoch keine Bewertungen
- Simonkucher Case Interview Prep 2015Dokument23 SeitenSimonkucher Case Interview Prep 2015Jorge Torrente100% (1)
- GBS210099-Nguyen Manh Quan - 1004B - As01Dokument33 SeitenGBS210099-Nguyen Manh Quan - 1004B - As01quannmgbs210099Noch keine Bewertungen
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- Kalki ProjectDokument3 SeitenKalki ProjectMandar SohoniNoch keine Bewertungen
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
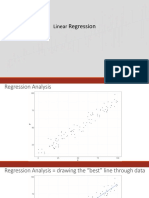
- Slides - Simple Linear RegressionDokument35 SeitenSlides - Simple Linear RegressionJarir AhmedNoch keine Bewertungen
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- DocumentDokument4 SeitenDocumentJuliana ZamorasNoch keine Bewertungen
- Republic Act No. 1125Dokument8 SeitenRepublic Act No. 1125Jazlynn WongNoch keine Bewertungen
- Figures of Speech StylisticsDokument11 SeitenFigures of Speech StylisticsCarmie Lactaotao DasallaNoch keine Bewertungen
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- Project RealDokument4 SeitenProject RealKenneth Jay BagandoNoch keine Bewertungen
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1091)
- TransModeler BrochureDokument12 SeitenTransModeler BrochureedgarabrahamNoch keine Bewertungen
- Reported Speech Rd1Dokument3 SeitenReported Speech Rd1Jose ChavezNoch keine Bewertungen
- ShakespeareDokument12 SeitenShakespeareapi-510189551Noch keine Bewertungen
- Thompson VarelaDokument18 SeitenThompson VarelaGiannis NinosNoch keine Bewertungen
- LAAG4 Elementary Row Operations-3Dokument14 SeitenLAAG4 Elementary Row Operations-3Kamran AliNoch keine Bewertungen
- N Advocates Act 1961 Ankita218074 Nujsedu 20221008 230429 1 107Dokument107 SeitenN Advocates Act 1961 Ankita218074 Nujsedu 20221008 230429 1 107ANKITA BISWASNoch keine Bewertungen
- Credit Transactions Case Digestpdf PDFDokument241 SeitenCredit Transactions Case Digestpdf PDFLexa L. DotyalNoch keine Bewertungen
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- SG Insert SpecificationDokument16 SeitenSG Insert SpecificationRaamkriss Raja100% (2)
- CHAPTER 6 - Adjusting EntriesDokument25 SeitenCHAPTER 6 - Adjusting EntriesMuhammad AdibNoch keine Bewertungen
- Myriam Met. CBLDokument25 SeitenMyriam Met. CBLCamila EscobarNoch keine Bewertungen
- Concept Paper For Business ResearchDokument4 SeitenConcept Paper For Business ResearchRobertchristian RagaNoch keine Bewertungen
- Subiecte Engleza August 2018 - V1Dokument6 SeitenSubiecte Engleza August 2018 - V1DenisNoch keine Bewertungen
- Current Technique in The Audiologic Evaluation of Infants: Todd B. Sauter, M.A., CCC-ADokument35 SeitenCurrent Technique in The Audiologic Evaluation of Infants: Todd B. Sauter, M.A., CCC-AGoesti YudistiraNoch keine Bewertungen
- Sanjay Seth - Once Was Blind But Now Can See Modernity and The Social SciencesDokument16 SeitenSanjay Seth - Once Was Blind But Now Can See Modernity and The Social SciencesQuelen GuedesNoch keine Bewertungen
- 9300 Servo Inverter TRDokument10 Seiten9300 Servo Inverter TRIhsan CanakogluNoch keine Bewertungen
- Chairperson 2012 Bar Examinations Committee: Bar Exam Question 2012 Martin S. Villarama, JRDokument73 SeitenChairperson 2012 Bar Examinations Committee: Bar Exam Question 2012 Martin S. Villarama, JRsejinma0% (1)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)