Beruflich Dokumente
Kultur Dokumente
5refrigerant 134a Enters An Adiabatic Compressor As A Saturated Vapor at 120 KPa at A Rate of 0
Hochgeladen von
BryanChavezOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
5refrigerant 134a Enters An Adiabatic Compressor As A Saturated Vapor at 120 KPa at A Rate of 0
Hochgeladen von
BryanChavezCopyright:
Verfügbare Formate
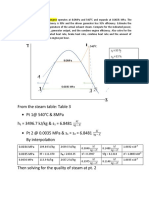
5 Refrigerant 134a enters an adiabatic compressor as a saturated vapor at 120 kPa
at a rate of 0.3 m3/min and exits at 1 MPa pressure. If the isentropic efficiency of
the compressor is 80%, determine (a) the temperature of the refrigerant at the
exit of the compressor and (b) the power input in kW. Also, show the process on
a T-s diagram with respect to the saturation lines.
We assume a steady process with negligible changes in kinetic and potential energies.
We are given that the compressor is adiabatic and we see that it has one inlet and one
outlet. Thus the first law for this compressor reduces to W m
(hin hout ) . We use the
property tables for refrigerant 134a. On a per unit mass basis we can compute the
ideal work, ws = hin – hout,s and the actual work wa = hin – hout.
At the inlet state, a saturated vapor at 120 kPa, we find the following properties: hin =
hg(120 kPa) = 236.97 kJ/kg; sin = sg(120 kPa) = 0.94779 kJ/kg∙K; vin = vg(120 kPa) =
0.16212 m3/kg. We can use the inlet volume flow rate and the inlet specific volume to
compute the mass flow rate.
0.3 m 3 min
V min 60 s 0.03084 kg
m in
vin 0.16212 m 3 s
kg
At the ideal outlet state of Pout = 1 MPa and sout,s = sin = 0.94779 kJ/kg∙K, we find the
ideal outlet state to be between 40oC and 50oC. We would get the ideal outlet enthalpy
by interpolation.
282.74 kJ 271.71 kJ
271.71 kJ kg kg 0.94779 kJ 0.9179 kJ 281.25 kJ
hout
kg 0.9525 kJ 0.9179 kJ
kg K kg K kg
kg K kg K
We can now compute the ideal work per unit mass.
236.97 kJ 281.25 kJ 44.27 kJ
ws hin hout,s
kg kg kg
The negative work confirms out understanding that this is a work input device so that
we have to divide the ideal work by the isentropic efficiency to find the actual work.
Multiplying the actual work by the mass flow rate gives the power.
0.03084 kg 44.27 kJ kW s
m w s kg 1 kJ
W m w s
W 1.707 kW
s 80%
Applying the first law to the actual process gives hout,a as follows.
hout,a hin w hin
W 236.97 kJ
1.707 kW 292.31 kJ
m kg 0.03084 kg kW s kg
s 1 kJ
At the outlet pressure of 1 MPa, the value of hout = 292.31 is between temperatures of
50oC and 60oC. Interpolation gives the corresponding outlet temperature as follows:
60o C 50o C 292.31 kJ 282.74 kJ
Tout 50o C Tout = 59.0oC .
293.38 kJ 282.74 kJ kg kg
kg kg
Das könnte Ihnen auch gefallen
- Book Index The Art of Heavy TransportDokument6 SeitenBook Index The Art of Heavy TransportHermon Pakpahan50% (2)
- Rachel Joyce - A Snow Garden and Other Stories PDFDokument118 SeitenRachel Joyce - A Snow Garden and Other Stories PDFИгорь ЯковлевNoch keine Bewertungen
- From The Steam Table: Table 3 PT 1@ 540 C & 8mpa H 3496.7 KJ/KG & S 6.8481 PT 2 at 0.0035 Mpa & S S 6.8481 by InterpolationDokument5 SeitenFrom The Steam Table: Table 3 PT 1@ 540 C & 8mpa H 3496.7 KJ/KG & S 6.8481 PT 2 at 0.0035 Mpa & S S 6.8481 by InterpolationMark AgusNoch keine Bewertungen
- Chapter 9 Examples&SolutionDokument42 SeitenChapter 9 Examples&SolutionSami ullahNoch keine Bewertungen
- Worded Problems in Thermodynamics 1 2Dokument12 SeitenWorded Problems in Thermodynamics 1 2Jonel Santos60% (10)
- 15me03 Thermodynamics Problems June2017Dokument19 Seiten15me03 Thermodynamics Problems June2017Praveen Vijay100% (1)
- Asteroids Prospective EnergyDokument710 SeitenAsteroids Prospective EnergySlavica Otovic100% (1)
- DJI F450 Construction Guide WebDokument21 SeitenDJI F450 Construction Guide WebPutu IndrayanaNoch keine Bewertungen
- Dharmakirti39s Commentary On ChakrasamvaraDokument15 SeitenDharmakirti39s Commentary On ChakrasamvaraThiago AlbuquerqueNoch keine Bewertungen
- Case AnalysisDokument2 SeitenCase AnalysisJessa San PedroNoch keine Bewertungen
- Unit Three Homework Solutions, September 16, 2010: Mechanical Engineering 370 ThermodynamicsDokument8 SeitenUnit Three Homework Solutions, September 16, 2010: Mechanical Engineering 370 ThermodynamicsPerantara RieyAnaNoch keine Bewertungen
- Unit Three Homework Solutions, September 16, 2010: KG M KG M KG M XV V X VDokument6 SeitenUnit Three Homework Solutions, September 16, 2010: KG M KG M KG M XV V X VApple StarkNoch keine Bewertungen
- Rankine Cycle Diagram: K KG KJ K KG KJ Kpa S Kpa S S XDokument16 SeitenRankine Cycle Diagram: K KG KJ K KG KJ Kpa S Kpa S S Xanon_166336005Noch keine Bewertungen
- Persamaan Energi Pada Beberapa Volume AturDokument35 SeitenPersamaan Energi Pada Beberapa Volume AturHONOR clothNoch keine Bewertungen
- Refrigeration Systems Quiz 1Dokument4 SeitenRefrigeration Systems Quiz 1MartiNoch keine Bewertungen
- 2300 HW 13 SolDokument4 Seiten2300 HW 13 SolFrederick DugayNoch keine Bewertungen
- Unit Eight Homework Solutions, November 2, 2010: M KG M KG M KG M C V C V C V V XDokument7 SeitenUnit Eight Homework Solutions, November 2, 2010: M KG M KG M KG M C V C V C V V XShainee Delle PalmeraNoch keine Bewertungen
- HW 13Dokument5 SeitenHW 13Maria Mikaela PelagioNoch keine Bewertungen
- XSteam V2aDokument9 SeitenXSteam V2aPRABU PERUMALNoch keine Bewertungen
- Test 1 Sol PDFDokument5 SeitenTest 1 Sol PDFabhiNoch keine Bewertungen
- 2nd Law Analysis For A Control VolumeDokument13 Seiten2nd Law Analysis For A Control VolumeSergey ShkapovNoch keine Bewertungen
- InglesDokument6 SeitenInglesdyonathanNoch keine Bewertungen
- Week 6 AssessmentDokument10 SeitenWeek 6 AssessmentVenrick CamposNoch keine Bewertungen
- Second LawDokument12 SeitenSecond Lawcobalt boronNoch keine Bewertungen
- Chemical and Mechanical Engineering 2300 / Thermodynamics I: Problem 1Dokument6 SeitenChemical and Mechanical Engineering 2300 / Thermodynamics I: Problem 1Takishima N VovoNoch keine Bewertungen
- Tutorial 1: Basic Concept of ThermodynamicsDokument4 SeitenTutorial 1: Basic Concept of ThermodynamicsKaka ZettyNoch keine Bewertungen
- 12th PhysucsvipDokument3 Seiten12th Physucsvipphysics a2Noch keine Bewertungen
- Entropy ProbsDokument5 SeitenEntropy ProbsFAzle RAbbyNoch keine Bewertungen
- ENGG1050 Lecture Problem Outline SolutionsDokument15 SeitenENGG1050 Lecture Problem Outline SolutionsclearcastingNoch keine Bewertungen
- Steam Turbinedocx PDF FreeDokument13 SeitenSteam Turbinedocx PDF Freeben richNoch keine Bewertungen
- Solutions ProblemSet8 Sem22007Dokument7 SeitenSolutions ProblemSet8 Sem22007clearcastingNoch keine Bewertungen
- Review On Thermodynamics Plate No. 2: Instruction: Choose The Best AnswerDokument10 SeitenReview On Thermodynamics Plate No. 2: Instruction: Choose The Best AnswerMarcial Jr. Militante100% (1)
- Chapter 1 - ExerciseDokument15 SeitenChapter 1 - Exerciseحسن كميت hassankomeit lNoch keine Bewertungen
- Assignment No. 1 in PpeDokument3 SeitenAssignment No. 1 in PpeJenny Mae PomedaNoch keine Bewertungen
- PPEDokument1 SeitePPEElla AlumbroNoch keine Bewertungen
- Chapter17 PDFDokument42 SeitenChapter17 PDFJason B PamatmatNoch keine Bewertungen
- Powerplant and Industrial Plant Engineering Trial 1: 1 PointsDokument8 SeitenPowerplant and Industrial Plant Engineering Trial 1: 1 PointsJerdNoch keine Bewertungen
- Assumptions 1 Steady Operating Conditions Exist. 2 The Mixing Chamber Is Well-Insulated So That Heat LossDokument1 SeiteAssumptions 1 Steady Operating Conditions Exist. 2 The Mixing Chamber Is Well-Insulated So That Heat LossHafiz Mahar28Noch keine Bewertungen
- Quantity of HeatDokument40 SeitenQuantity of HeatKiel JohnNoch keine Bewertungen
- Solved Board Problems - Gas Turbine Power PlantDokument8 SeitenSolved Board Problems - Gas Turbine Power PlantFAMY Vazzim Soriano100% (1)
- Chapter 7 Continued Entropy: A Measure of Disorder Study Guide in PowerpointDokument53 SeitenChapter 7 Continued Entropy: A Measure of Disorder Study Guide in Powerpointbrayan CortezNoch keine Bewertungen
- Unit Four Homework Solutions, September 23. 2010: Mechanical Engineering 370 ThermodynamicsDokument3 SeitenUnit Four Homework Solutions, September 23. 2010: Mechanical Engineering 370 ThermodynamicsRengganis Putri ParmudyaNoch keine Bewertungen
- Pipe Solved ProbsetDokument115 SeitenPipe Solved ProbsetRemae Garci100% (1)
- Turbines and Pumps Notes in ThermodynamicsDokument5 SeitenTurbines and Pumps Notes in ThermodynamicsJuan HeroNoch keine Bewertungen
- Turbines and Pumps Notes in ThermodynamicsDokument5 SeitenTurbines and Pumps Notes in ThermodynamicsJuan HeroNoch keine Bewertungen
- CH 18Dokument22 SeitenCH 18nallilathaNoch keine Bewertungen
- Thermodynamics Questions and AnswersDokument5 SeitenThermodynamics Questions and AnswersMD SHOEBUDDIN0% (1)
- Tarea 5 TermodinamicaDokument4 SeitenTarea 5 TermodinamicaMario GonzalezNoch keine Bewertungen
- PDF Worded Problems in Thermodynamics 1 2docx DDDokument6 SeitenPDF Worded Problems in Thermodynamics 1 2docx DDErning TutpickNoch keine Bewertungen
- Exercise Sheet 2 SolutionsDokument4 SeitenExercise Sheet 2 SolutionsGonçalo GuerreiroNoch keine Bewertungen
- Problem Set No 1pdfpdf PDF FreeDokument87 SeitenProblem Set No 1pdfpdf PDF FreeMaria Perez100% (1)
- ETD - Question BankDokument6 SeitenETD - Question BankGopinath VNoch keine Bewertungen
- Orca Share Media1519993790396 PDFDokument10 SeitenOrca Share Media1519993790396 PDFSecret SecretNoch keine Bewertungen
- Solutions ProblemSet9 Sem22007Dokument3 SeitenSolutions ProblemSet9 Sem22007clearcastingNoch keine Bewertungen
- Thermo EXAMPLE 7.1-CHAPTER 7 PDFDokument11 SeitenThermo EXAMPLE 7.1-CHAPTER 7 PDFFattihiEkhmalNoch keine Bewertungen
- What Is Kpag?: Table of ContentsDokument2 SeitenWhat Is Kpag?: Table of Contentsangelito bernalNoch keine Bewertungen
- Che198 Thermodynamics DrillsDokument8 SeitenChe198 Thermodynamics DrillsTrebob GardayaNoch keine Bewertungen
- Tutorial - 6 - EntropyDokument7 SeitenTutorial - 6 - EntropyanotherdeobiNoch keine Bewertungen
- Gaspowercycle 150627103029 Lva1 App6892Dokument28 SeitenGaspowercycle 150627103029 Lva1 App6892XADA YADANoch keine Bewertungen
- 8.5 Cents: 0.60 Btu/Lbor, 0.48 Btu/LborDokument5 Seiten8.5 Cents: 0.60 Btu/Lbor, 0.48 Btu/LborKATHLEEN DEL PILARNoch keine Bewertungen
- Question 1. Liquid Water at 200 Kpa and 15: S S M S M T Q DT DsDokument6 SeitenQuestion 1. Liquid Water at 200 Kpa and 15: S S M S M T Q DT Dsfivos_rgNoch keine Bewertungen
- Questions and Answers To Problems Number 11 & 29Dokument1 SeiteQuestions and Answers To Problems Number 11 & 29owl knightNoch keine Bewertungen
- QB Unit 1Dokument6 SeitenQB Unit 1Gaurav GadhesariaNoch keine Bewertungen
- 2W04 Assignment 5 - 2023 Solutions PDFDokument4 Seiten2W04 Assignment 5 - 2023 Solutions PDFas asNoch keine Bewertungen
- Thermodynam TasksDokument1 SeiteThermodynam TasksEhtıram SeyıdovNoch keine Bewertungen
- CarrosDokument1 SeiteCarrosBryanChavezNoch keine Bewertungen
- LluviaDokument1 SeiteLluviaBryanChavezNoch keine Bewertungen
- CovidDokument1 SeiteCovidBryanChavezNoch keine Bewertungen
- GuardarrayasDokument1 SeiteGuardarrayasBryanChavezNoch keine Bewertungen
- Do Not Write On This QuizDokument2 SeitenDo Not Write On This QuizBryanChavezNoch keine Bewertungen
- Freud MascaraDokument1 SeiteFreud MascaraBryanChavezNoch keine Bewertungen
- Bryan Chavez: Part A. Respond To The Statements Two Ways. Check The Examples Above If You Need HelpDokument2 SeitenBryan Chavez: Part A. Respond To The Statements Two Ways. Check The Examples Above If You Need HelpBryanChavezNoch keine Bewertungen
- 4 Level B Units 13-14Dokument1 Seite4 Level B Units 13-14BryanChavezNoch keine Bewertungen
- 4 Level B Units 13-14 Answer SheetDokument1 Seite4 Level B Units 13-14 Answer SheetBryanChavez0% (1)
- Passive Ex SimpleDokument2 SeitenPassive Ex Simplekevin43% (7)
- 4 Level B Units 13-14 AnsweDokument1 Seite4 Level B Units 13-14 AnsweBryanChavezNoch keine Bewertungen
- 4 Level B Units 11-12 Answer Key-1-1Dokument1 Seite4 Level B Units 11-12 Answer Key-1-1BryanChavezNoch keine Bewertungen
- Units 1-2 For Exam Simple PastDokument8 SeitenUnits 1-2 For Exam Simple PastBryanChavezNoch keine Bewertungen
- LinksDokument14 SeitenLinksBryanChavezNoch keine Bewertungen
- 4 LEVEL SB UNITS 15 - 16Dokument2 Seiten4 LEVEL SB UNITS 15 - 16BryanChavezNoch keine Bewertungen
- Do Not Write On This QuizDokument2 SeitenDo Not Write On This QuizBryanChavezNoch keine Bewertungen
- 4 LEVEL SB UNITS 15 - 16Dokument2 Seiten4 LEVEL SB UNITS 15 - 16BryanChavezNoch keine Bewertungen
- Do Not Write On This QuizDokument2 SeitenDo Not Write On This QuizBryanChavezNoch keine Bewertungen
- 4 LEVEL SB UNITS 15-16 ANSWER KEY QUIZDokument1 Seite4 LEVEL SB UNITS 15-16 ANSWER KEY QUIZBryanChavezNoch keine Bewertungen
- 4 LEVEL SB UNITS 15 - 16Dokument2 Seiten4 LEVEL SB UNITS 15 - 16BryanChavezNoch keine Bewertungen
- Homework Name:: As As, Too, EnoughDokument4 SeitenHomework Name:: As As, Too, EnoughBryanChavezNoch keine Bewertungen
- 4 LEVEL SB UNITS 15 - 16Dokument2 Seiten4 LEVEL SB UNITS 15 - 16BryanChavezNoch keine Bewertungen
- Holidays in QuitoDokument1 SeiteHolidays in QuitoBryanChavezNoch keine Bewertungen
- Do Not Write On This QuizDokument2 SeitenDo Not Write On This QuizBryanChavezNoch keine Bewertungen
- Para Lec CombinedDokument83 SeitenPara Lec CombinedClent Earl Jason O. BascoNoch keine Bewertungen
- EXAMPLE 8.6 Veneer Grades and RepairsDokument2 SeitenEXAMPLE 8.6 Veneer Grades and RepairsnickNoch keine Bewertungen
- Theoretical CyclesDokument49 SeitenTheoretical CyclesMariaEzzaSyUyNoch keine Bewertungen
- Multi Organ Dysfunction SyndromeDokument40 SeitenMulti Organ Dysfunction SyndromeDr. Jayesh PatidarNoch keine Bewertungen
- Frye LGD As A Function of The Default Rate 091013 PDFDokument13 SeitenFrye LGD As A Function of The Default Rate 091013 PDFSushant SinghNoch keine Bewertungen
- IBM BladeCenter S RedBookDokument36 SeitenIBM BladeCenter S RedBookGuillermo García GándaraNoch keine Bewertungen
- Precision CatalogDokument256 SeitenPrecision CatalogImad AghilaNoch keine Bewertungen
- Conquest CXAX Air-to-Water Heat PumpDokument6 SeitenConquest CXAX Air-to-Water Heat PumpAlexandre LopesNoch keine Bewertungen
- ContempoDokument4 SeitenContempoPrincess Jonette YumulNoch keine Bewertungen
- Cold Regions Science and TechnologyDokument8 SeitenCold Regions Science and TechnologyAbraham SilesNoch keine Bewertungen
- Exam 3 DynamicsDokument7 SeitenExam 3 DynamicsJulioNoch keine Bewertungen
- Test7 PointersDokument16 SeitenTest7 PointersPratibha DwivediNoch keine Bewertungen
- Hashimoto's Thyroiditis: Veena RedkarDokument10 SeitenHashimoto's Thyroiditis: Veena RedkarSan RedkarNoch keine Bewertungen
- Technical Data Sheet: LPI HVSC PlusDokument2 SeitenTechnical Data Sheet: LPI HVSC PlusNguyễn TấnNoch keine Bewertungen
- Vanish Magic Magazine VANISH MAGIC MAGAZINE 58 May 2019Dokument118 SeitenVanish Magic Magazine VANISH MAGIC MAGAZINE 58 May 2019mick byrnes100% (1)
- Optical Scattering of Gold NanosphereDokument24 SeitenOptical Scattering of Gold NanosphereParas KumarNoch keine Bewertungen
- Adriano Costa Sampaio: Electrical EngineerDokument3 SeitenAdriano Costa Sampaio: Electrical EngineeradrianorexNoch keine Bewertungen
- Đề 17Dokument11 SeitenĐề 17Nguyen CuongNoch keine Bewertungen
- Volvo Penta GensetDokument4 SeitenVolvo Penta GensetafandybaharuddinNoch keine Bewertungen
- Ict 2120 Animation NC Ii Week 11 20 by Francis Isaac 1Dokument14 SeitenIct 2120 Animation NC Ii Week 11 20 by Francis Isaac 1Chiropractic Marketing NowNoch keine Bewertungen
- Preview: Proquest Dissertations and Theses 2002 Proquest Dissertations & Theses Full TextDokument24 SeitenPreview: Proquest Dissertations and Theses 2002 Proquest Dissertations & Theses Full TextFelipe AguilarNoch keine Bewertungen
- Integration ConceptDokument34 SeitenIntegration ConceptJANELLA ALVAREZNoch keine Bewertungen
- 15 Benefits of CyclingDokument8 Seiten15 Benefits of CyclingJoycs PintoNoch keine Bewertungen
- FebvreDokument449 SeitenFebvreIan Pereira AlvesNoch keine Bewertungen