Beruflich Dokumente
Kultur Dokumente
MSS 1718MockPaper1A
Hochgeladen von
Kelvin ChowCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
MSS 1718MockPaper1A
Hochgeladen von
Kelvin ChowCopyright:
Verfügbare Formate
Marymount Secondary School
HKDSE Mock Examination 2017 – 2018
Chemistry
Paper 1
Secondary 6 Date : 25 January 2018
Time Allowed : 2 hours 30 minutes
Total Marks : 120
GENERAL INSTRUCTIONS:
1. There are TWO sections, A and B, in this Paper. You are advised to finish Section A in about
45 minutes.
2. Section A consists of multiple-choice questions in this question paper, while Section B
contains conventional questions printed separately in Question-Answer Book B.
3. Answers to Section A should be marked on the Multiple-choice Answer Sheet while answers
to Section B should be written in the spaces provided in Question-Answer Book B. The
Answer Sheet for Section A and the Question-Answer Book for Section B will be
collected separately at the end of the examination.
4. A Periodic Table is printed on the last page of Question-Answer Book B. Atomic numbers
and relative atomic masses of elements can be obtained from the Periodic Table.
INSTRUCTIONS FOR SECTION A (MULTIPLE-CHOICE QUESTIONS):
1. Read carefully the instructions on the Answer Sheet. No extra time will be given to candidates
for filling in the name, class and class number after the ‘Time is up’ announcement.
2. When told to open this book, you should check that all the questions are there. Look for the
words ‘END OF SECTION A’ after the last question.
3. All questions carry equal marks.
4. ANSWER ALL QUESTIONS. You are advised to use an HB pencil to mark all the answers
on the Answer Sheet, so that wrong marks can be completely erased with a clean rubber.
You must mark the answers clearly; otherwise you will lose marks if the answers cannot be
captured.
5. You should mark only ONE answer for each question. If you mark more than one answer,
you will receive NO MAKRS for that question.
6. No marks will be deducted for wrong answers.
S6/Chemistry/Mock Examination (Paper 1A) Page 1
This section consists of two parts. There are 24 questions in PART I and 12 questions in PART II.
Choose the best answer for each question.
Candidates may refer to the Periodic Table printed on the last page of Question-Answer Book B.
PART I
1. Which of the following pairs of molecules have the same number of lone pairs of electrons ?
A. Nitrogen, water
B. Oxygen, fluorine
C. Chlorine, hydrogen chloride
D. Hydrogen, carbon dioxide
2. Consider the following experiment :
After the experiment, the residue in the combustion tube is dissolved in water. An alkaline
solution is obtained. X could be
A. carbon.
B. gold.
C. magnesium.
D. silver.
3. In cinnabar, mercury exists mainly as a
A. carbonate.
B. chloride.
C. sulphate.
D. sulphide.
4. W, X, Y and Z are metals. X displaces Y from YCl(aq). Z displaces X from X(NO3)2(aq). W has no
reactions with XSO4(aq), YCl(aq) and ZNO3(aq). Which of the following is the ascending order of
the oxidizing power of the cations ?
A. Z+ < X2+ < Y+ < W2+
B. W2+ < Y+ < X2+ < Z+
C. Y+ < X2+ < Z+ < W2+
D. Y+ < W2+ < X2+ < Z+
5. When 50.0 cm3 of 1.0 M KOH(aq) is mixed with 25.0 cm3 of 1.0 M H2SO4(aq), the temperature of
the mixture rises by 11.3C. Which of the following pairs of reactants, when mixed under the
same conditions, would give a similar temperature rise ?
A. 25.0 cm3 of 0.5 M KOH(aq) and 12.5 cm3 of 0.5 M H2SO4(aq)
B. 40.0 cm3 of 0.8 M KOH(aq) and 20.0 cm3 of 0.8 M H2SO4(aq)
C. 70.0 cm3 of 1.0 M KOH(aq) and 35.0 cm3 of 1.0 M H2SO4(aq)
D. 100.0 cm3 of 2.0 M KOH(aq) and 50.0 cm3 of 2.0 M H2SO4(aq)
6. M is a metal that forms a phosphate with the formula Ma(PO4)b. The formula of the oxide of M
is
A. MbOa.
B. M2bOa.
C. M3bO2a.
D. M2aO3b.
S6/Chemistry/Mock Examination (Paper 1A) Page 2
7. Consider the following reaction:
aA + bB cC
Given that the formula masses of A and B are 20 and 80 respectively. What is the mass of
A that required to produce 100 g of C ?
100 a
A. g
a 4b
200 a
B. g
a 2b
200a
C. g
c
200b
D. g
ac
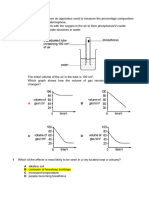
8. Consider the following set-ups.
(1) (2)
beaker beaker
oil distilled water
iron nail iron nail
(3)
zinc container
distilled water
iron nail
In which of the above set ups would the iron nail NOT rust ?
A. (1) only
B. (2) only
C. (1) and (3) only
D. (2) and (3) only
9. Which of the following reagents can decolorize bromine water ?
(1) But-1-ene
(2) Sodium sulphite solution
(3) Sodium chloride solution
A. (1) and (2) only
B. (1) and (3) only
C. (2) and (3) only
D. (1), (2) and (3)
10. 10 cm3 of ethanol (density = 0.789 g cm-3) is added to 100 cm3 of water. If 25 cm3 of the
solution is withdrawn from the solution, what is the molarity of this portion of solution ?
A. 1.56 M
B. 1.72 M
C. 6.88 M
D. 17.2 M
S6/Chemistry/Mock Examination (Paper 1A) Page 3
11. In an experiment, 2.0 M sodium hydroxide solution was added to 20.0 cm3 of 1.0 M sulphuric
acid until the acid was completely neutralized. What is the concentration of sodium sulphate in
the resultant solution ?
A. 0.25 M
B. 0.33 M
C. 0.50 M
D. 1.00 M
12. 1.5 M sulphuric acid was added to 30.0 cm3 of 2.0 M potassium hydroxide solution until the
alkali was completely neutralized. What is the concentration of potassium sulphate in the
resultant solution ?
A. 0.20 M
B. 0.40 M
C. 0.60 M
D. 0.80 M
13. 80.0 cm3 of NaOH(aq) is mixed with 120.0 cm3 of HCl(aq) of the same concentration in a beaker.
The pH of the resultant solution is 2. What is the initial concentration of HCl(aq) ?
A. 0.01 M
B. 0.05 M
C. 0.50 M
D. 0.80 M
14. When 1.0 mol of methane is allowed to react with chlorine in the presence of sunlight, a mixture
of products is obtained and is shown below :
Product Amount (mol)
CH2Cl2 0.1
CHCl3 0.3
CCl4 0.5
Which of the following combinations is correct ?
Methane left (mol) Chlorine consumed (mol)
A. 0 0.9
B. 0 3.1
C. 0.1 0.9
D. 0.1 3.1
15. A bottle of pure ethanol (C2H5OH) undergoes complete combustion to give a certain mass of
carbon dioxide and 61.2 g of water. What is the volume of the bottle of ethanol ?
(Density of ethanol = 0.789 g cm-3)
A. 52.0 cm3
B. 65.9 cm3
C. 156 cm3
D. 198 cm3
S6/Chemistry/Mock Examination (Paper 1A) Page 4
16. The set-up below shows a simple chemical cell :
salt bridge
iron electrode X iron electrode Y
0.50 M FeSO4(aq) 1.0 M FeSO4(aq)
After a while, the concentrations of iron(II) ions in the two half cells become equal. Which of
the following statements about this set-up is correct ?
A. The salt bridge allows electrons to flow between the two half cells.
B. Electrons flow from electrode X to electrode Y in the external circuit.
C. The mass of X increases while that of Y decreases.
D. The voltage is constant throughout the experiment.
17. Consider the following chemical cell:
salt bridge
graphite electrode A graphite electrode B
K2S2O8(aq) KI(aq)
Which of the following statements about the chemical cell are correct ?
(1) K2S2O8(aq) is a stronger oxidizing agent than I2(aq).
(2) The solution in the right half cell changes from colourless to brown.
(3) Electrons flow from electrode B to electrode A through the external circuit.
A. (1) and (2) only
B. (1) and (3) only
C. (2) and (3) only
D. (1), (2) and (3)
18. A reaction between magnesium and dilute hydrochloric acid is carried out in a stoppered flask.
The heat change is found to be 433.8 kJ. If the same reaction is carried out in a flask without a
stopper, what would be the heat change ?
A. Lower than 433.8 kJ, as there is energy lost to the surroundings.
B. 433.8 kJ, as the difference in work done between two cases is negligible.
C. Higher than 433.8 kJ, as the latter case does not have work done against the wall of flask.
D. 433.8 kJ, as the heat change is independent of the pressure in the flask.
S6/Chemistry/Mock Examination (Paper 1A) Page 5
19. Given that:
ΔHʅf [Sb2O3(s)] = p kJ mol1
ΔHʅf [CO(g)] = q kJ mol1
ΔHʅf [Sb(l)] = r kJ mol1
ΔHʅf [CO2(g)] = s kJ mol1
(Given: p, q, r and s are non-zero values.)
Which of the following statements about the following reaction are correct ?
Sb2O3(s) + 3CO(g) 2Sb(l) + 3CO2(g)
(1) The standard enthalpy change of the above reaction is 2r + 3s – p – 3q.
(2) ΔHʅf [Sb(l)] is not zero as Sb(l) is not the standard state of Sb.
(3) The above reaction is a redox reaction.
A. (1) and (2) only
B. (1) and (3) only
C. (2) and (3) only
D. (1), (2) and (3)
20. A solution with a chemical formula, K4[Fe(CN)6](aq), can be obtained by electrolysis of saturated
potassium hexacyanoferrate(III) solution (K3[Fe(CN)6](aq)). Which of the following
combinations is correct ?
Oxidation number of Fe in Electrode at which K4[Fe(CN)6]
K4[Fe(CN)6] is produced
A. +2 Anode
B. +2 Cathode
C. +3 Anode
D. +3 Cathode
21. Consider the following reactions:
13
CH3CH2CH2CH3(g) + O2(g) 4CO2(g) + 5H2O(l) ΔH1 = 2878 kJ mol1
2
13
(CH3)2CHCH3(g) + O2(g) 4CO2(g) + 5H2O(l) ΔH2 = 2869 kJ mol1
2
Which of the following statements are correct ?
(1) The energy stored in butane is less than that in 2-methylpropane.
(2) Both reactions involve breaking CH bonds.
(3) Both reactions are exothermic.
A. (1) and (2) only
B. (1) and (3) only
C. (2) and (3) only
D. (1), (2) and (3)
S6/Chemistry/Mock Examination (Paper 1A) Page 6
22. 1.0 g of each of the following substances was burned. The following table shows the amount of
energy released during the combustion of the substances.
Substance Energy released (kJ g1)
C(graphite) 32.8
H2(g) 143.0
C4H10(g) 49.6
What is the standard enthalpy change of formation of C4H10(g) ?
A. +2197 kJ mol1
B. 127.4 kJ mol1
C. 2197 kJ mol1
D. 5881 kJ mol1
Directions :
Each question below (Question Nos. 23 to 24) consists of two separate statements. Decide
whether each of the two statements is true or false; if both are true, then decide whether or not
the second statement is a correct explanation of the first statement. Then select one option from
A to D according to the following table:
A. Both statements are true and the 2nd statement is a correct explanation of the 1st statement.
B. Both statements are true but the 2nd statement is NOT a correct explanation of the 1st statement.
C. The 1st statement is false while the 2nd statement is true.
D. Both statements are false.
1st statement 2nd statement
23. When excess tetrachloromethane is added In general, hydrogen bonds are stronger than
to a beaker of ethanol, the temperature of van der Waals’ forces.
the beaker does not change.
24. Adding copper(II) sulphate solution to Copper(II) hydroxide is a blue insoluble
sodium hydroxide solution will cause a solid.
decrease in pH.
S6/Chemistry/Mock Examination (Paper 1A) Page 7
PART II
For Questions 25 and 26, refer to the following information :
An experiment was performed to study the rate of reaction between hydrochloric acid and
calcium carbonate by measuring the change in mass of the reaction mixture. 2.0 g of calcium
carbonate granules were added to four conical flasks, P, Q, R and S, each containing
hydrochloric acid which was prepared as follows :
Conical flask Hydrochloric acid Volume of water (cm3)
Concentration (M) Volume (cm3)
P 1.0 10 90
Q 1.5 60 30
R 2.0 50 40
S 0.5 70 20
25. In which of the conical flasks does the reaction proceed at the highest rate ?
A. P
B. Q
C. R
D. S
26. Besides using the conical flask, which of the following apparatus would be used when carrying
out the experiment ?
(1) Electronic balance
(2) Cotton wool
(3) Stop watch
A. (1) and (2) only
B. (1) and (3) only
C. (2) and (3) only
D. (1), (2) and (3)
27. Cortisone is one of the main hormones in our body. It has the following structure :
Which of the following statements about cortisone are correct ?
(1) It has a hydroxyl group for tertiary alcohol.
(2) It can change acidified potassium dichromate solution from orange to green.
(3) It has three ketone groups.
A. (1) and (2) only
B. (1) and (3) only
C. (2) and (3) only
D. (1), (2) and (3)
S6/Chemistry/Mock Examination (Paper 1A) Page 8
28. The reaction between C2O42- ions and MnO4- ions proceeds in two stages that can be represented
by the following equations :
Stage 1: 4Mn2+(aq) + MnO4-(aq) + 8H+(aq) 5Mn3+(aq) + 4H2O(l)
Stage 2: 2Mn3+(aq) + C2O42-(aq) 2Mn2+(aq) + 2CO2(g)
The reaction can also take place in a reverse order. Which of the following statements are
correct ?
(1) Either Mn2+ ions or Mn3+ ions act as a catalyst in the reaction.
(2) If the reaction takes place in an order as shown above, the overall equation for the
reaction is MnO4-(aq) + 8H+(aq) + 2C2O42-(aq) Mn3+(aq) + 4H2O(l) + 4CO2(g).
(3) C2O42- ions are oxidized in the reaction.
A. (1) and (2) only
B. (1) and (3) only
C. (2) and (3) only
D. (1), (2) and (3)
29. If excess acidified potassium dichromate solution is added to 0.3 mol of each of the following
compounds, which of them can reduce the greatest number of moles of acidified potassium
dichromate solution ?
A. CH3CH2CHO
B. CH3CH2OH
C. C6H5COOH
D. CH3CH=CHCH2CH=CHCH2CH=CH2
30. Neotame is an artificial sweetener. It has the following structure :
Which of the following functional groups is/are present in neotame ?
(1) Ester
(2) Ketone
(3) Amide
A. (1) only
B. (2) only
C. (1) and (3) only
D. (2) and (3) only
S6/Chemistry/Mock Examination (Paper 1A) Page 9
31. E, F and G are oxides of Period 3 elements. Three experiments are conducted on each oxide.
The results of the experiments are shown in the table below :
Solubility Adding the oxide to Adding the oxide to
Oxide
in water dilute HCl(aq) dilute NaOH(aq)
E Soluble pH increases No change in the pH
F Insoluble pH increases pH decreases
G Soluble No change in the pH pH decreases
Which of the following statements are correct ?
(1) E and F are metal oxides while G is a non-metal oxide.
(2) F is an amphoteric oxide.
(3) Among the three oxides, G has the lowest melting point.
A. (1) and (2) only
B. (1) and (3) only
C. (2) and (3) only
D. (1), (2) and (3)
32. Ferrate(VI) ions (FeO42-) can be prepared by heating iron(III) hydroxide with sodium
hypochlorite in alkaline solution. The equation for the reaction involved is shown below:
xFe(OH)3(s) + 3OCl-(aq) + 4OH-(aq) ∏ xFeO42-(aq) + 5H2O(l) + 3Cl-(aq)
Which of the following statements is/are correct ?
(1) Iron is oxidized.
(2) The value of x is 3.
(3) The yield of FeO42- ions decreases when an acid is added.
A. (1) only
B. (2) only
C. (1) and (3) only
D. (2) and (3) only
33. The complete combustion of an alkane produces 9 dm3 of carbon dioxide and 10 dm3 of water
vapour. Which of the following is probably the molecular formula of this alkane ?
A. C7H16
B. C8H18
C. C9H20
D. C10H22
34. Two pieces of calcium of the same size react with dilute hydrochloric acid and dilute sulphuric
acid respectively.
Reaction 1 2.0 g of Ca + 100 cm3 of 1 M HCl
Reaction 2 2.0 g of Ca + 100 cm3 of 1 M H2SO4
Which of the following statements about the two reactions are INCORRECT ?
(1) Both reactions give a clear solution at the end of the reaction.
(2) Both reactions have the same initial rate.
(3) Both reactions generate the same volume of hydrogen.
A. (1) and (2) only
B. (1) and (3) only
C. (2) and (3) only
D. (1), (2) and (3)
S6/Chemistry/Mock Examination (Paper 1A) Page 10
35. Methanoic acid ionizes slightly in water to give methanoate ions and hydrogen ions :
HCOOH(aq) ∏ HCOO-(aq) + H+(aq)
At 25C, the equilibrium constant (Kc) of the above reaction is 1.78 × 10-4 mol dm-3. What is
the pH of 1.0 M methanoic acid at 25C ?
A. 1.00
B. 1.88
C. 2.00
D. 3.75
36 Methanoic acid ionizes slightly in water to give methanoate ions and hydrogen ions :
HCOOH(aq) ∏ HCOO-(aq) + H+(aq)
Which of the following statements is/are correct ?
(1) An increase in pressure does not affect the pH of the solution.
(2) After adding a few drops of sodium methanoate solution to methanoic acid, the pH of the
resultant solution decreases.
(3) After adding a few drops of aqueous ammonia to methanoic acid, the pH of the resultant
solution increases.
A. (1) only
B. (2) only
C. (1) and (3) only
D. (2) and (3) only
END OF SECTION A
S6/Chemistry/Mock Examination (Paper 1A) Page 11
Das könnte Ihnen auch gefallen
- F3 Maths 1314 1stexam Paper 1Dokument3 SeitenF3 Maths 1314 1stexam Paper 1伊貝P-0% (1)
- Introduction To Water Chemistry in FreshwasterDokument4 SeitenIntroduction To Water Chemistry in FreshwasterpomodoroNoch keine Bewertungen
- NSS Chemistry Part 2 Microscopic World I - LQDokument22 SeitenNSS Chemistry Part 2 Microscopic World I - LQFelix YueNoch keine Bewertungen
- NSS Chemistry Part 7 Redox Reactions Chemical Cells and Electrolysis - LQDokument38 SeitenNSS Chemistry Part 7 Redox Reactions Chemical Cells and Electrolysis - LQミーチェルNoch keine Bewertungen
- HKDSE Chemistry MC Chapter 13Dokument8 SeitenHKDSE Chemistry MC Chapter 13ScribdNoch keine Bewertungen
- Suggested Answers To In-Text Activities and Unit-End Exercises Topic 3 Unit 12Dokument21 SeitenSuggested Answers To In-Text Activities and Unit-End Exercises Topic 3 Unit 12ミーチェルNoch keine Bewertungen
- Principles of Crop Growth Simulation ModellingDokument57 SeitenPrinciples of Crop Growth Simulation ModellingManuel P. Marcaida IIINoch keine Bewertungen
- Waste-To-Energy Plant Process Safety ChallengesDokument5 SeitenWaste-To-Energy Plant Process Safety Challengessomesh sharmaNoch keine Bewertungen
- HKDSE Chem FX ExamS5 2011 Set1 EngDokument27 SeitenHKDSE Chem FX ExamS5 2011 Set1 Eng12376590Noch keine Bewertungen
- MSS 1718MockPaper2Dokument8 SeitenMSS 1718MockPaper2Kelvin ChowNoch keine Bewertungen
- NSS Chemistry Part 4 Acids and Bases - LQDokument40 SeitenNSS Chemistry Part 4 Acids and Bases - LQFelix YueNoch keine Bewertungen
- Exam Paper - M1 (By Topic)Dokument19 SeitenExam Paper - M1 (By Topic)Henry Leung100% (1)
- NSS Chemistry Part 4 Acids and Bases - MCDokument35 SeitenNSS Chemistry Part 4 Acids and Bases - MCFelix YueNoch keine Bewertungen
- NSS Chemistry Part 11 Chemistry of Carbon CompoundsDokument47 SeitenNSS Chemistry Part 11 Chemistry of Carbon CompoundsFelix YueNoch keine Bewertungen
- NSS Chemistry Part 7 Redox Reactions Chemical Cells and Electrolysis - MCDokument37 SeitenNSS Chemistry Part 7 Redox Reactions Chemical Cells and Electrolysis - MCFelix YueNoch keine Bewertungen
- NSS Chemistry Part 9 Rate of ReactionsDokument26 SeitenNSS Chemistry Part 9 Rate of ReactionsFelix YueNoch keine Bewertungen
- NSS Chemistry Part 12 Patterns in Chemical WorldDokument7 SeitenNSS Chemistry Part 12 Patterns in Chemical WorldFelix YueNoch keine Bewertungen
- NSS Chemistry Part 3 Metals - MCDokument20 SeitenNSS Chemistry Part 3 Metals - MCFelix YueNoch keine Bewertungen
- SSGS 17-18 F.6 Final Exam 1 and 2 CHEMDokument37 SeitenSSGS 17-18 F.6 Final Exam 1 and 2 CHEMKelvin ChowNoch keine Bewertungen
- (Answer Key) Calculation Exercise - 元素の貓 - 免費dse化學練習Dokument6 Seiten(Answer Key) Calculation Exercise - 元素の貓 - 免費dse化學練習Belladonna Lee100% (1)
- NSS Chemistry Part 13 Industrial Chemistry - IIDokument21 SeitenNSS Chemistry Part 13 Industrial Chemistry - IIFelix YueNoch keine Bewertungen
- 2015 F6 Mock P2 PDFDokument7 Seiten2015 F6 Mock P2 PDFKaylie WongNoch keine Bewertungen
- QB 1B Ch07e FDokument40 SeitenQB 1B Ch07e FRyan KwokNoch keine Bewertungen
- Strength of Acids and AlkalisDokument16 SeitenStrength of Acids and AlkalisRyanNoch keine Bewertungen
- Reacting MassesDokument2 SeitenReacting MassesTsz Wai WONGNoch keine Bewertungen
- Movement of Substances Across Cell Membrane: Multiple-Choice QuestionsDokument51 SeitenMovement of Substances Across Cell Membrane: Multiple-Choice QuestionsRyanNoch keine Bewertungen
- HKDSE Biology S5 Exam (2020)Dokument28 SeitenHKDSE Biology S5 Exam (2020)FuchingAuntie100% (1)
- Corrosion of Metals and Their Protection: Learning GoalDokument36 SeitenCorrosion of Metals and Their Protection: Learning GoalRyanNoch keine Bewertungen
- Atomicstructurequestions PDFDokument42 SeitenAtomicstructurequestions PDFNfor KlinsmanNoch keine Bewertungen
- Mole Concept ExerciseDokument2 SeitenMole Concept Exercisechong56100% (1)
- Past Paper - Microscopic WorldDokument7 SeitenPast Paper - Microscopic Worldapi-3739994100% (1)
- New Senior Secondary MASTERING BIOLOGY OXFORD Mock - Bio - Set6 - e - AnsDokument7 SeitenNew Senior Secondary MASTERING BIOLOGY OXFORD Mock - Bio - Set6 - e - AnsChun Kit LauNoch keine Bewertungen
- Chapter 3 Change of State: Multiple-Choice QuestionsDokument63 SeitenChapter 3 Change of State: Multiple-Choice Questionssuperpooh-1Noch keine Bewertungen
- HKDSE Chem FX Mock Exam Paper 1 2012 Set 1 Eng AnsDokument10 SeitenHKDSE Chem FX Mock Exam Paper 1 2012 Set 1 Eng Ansleung_ting_2Noch keine Bewertungen
- NSS Chemistry Part 2 Microscopic World I - MCDokument32 SeitenNSS Chemistry Part 2 Microscopic World I - MCFelix YueNoch keine Bewertungen
- Keep It Simple Science 3 - MetalsDokument13 SeitenKeep It Simple Science 3 - Metalsricerocketz1231231Noch keine Bewertungen
- NSS Chemistry Part 15 Analytical Chemistry - LQDokument42 SeitenNSS Chemistry Part 15 Analytical Chemistry - LQFelix Yue100% (1)
- Baptist Lui Ming Choi Secondary School First Term Examination (2012-2013) Form 3 ChemistryDokument12 SeitenBaptist Lui Ming Choi Secondary School First Term Examination (2012-2013) Form 3 ChemistryyuNoch keine Bewertungen
- The Ultimate Question Bank: Dse Chem MasteryDokument48 SeitenThe Ultimate Question Bank: Dse Chem MasteryYip AvaNoch keine Bewertungen
- Acid and AlkaliDokument9 SeitenAcid and Alkali云吸仓鼠吉尼斯保持者Noch keine Bewertungen
- F.3 Heat NoteDokument12 SeitenF.3 Heat Noteskywalker_handsomeNoch keine Bewertungen
- Afterschool Mole Calculation Exercise Ans.Dokument32 SeitenAfterschool Mole Calculation Exercise Ans.J TNoch keine Bewertungen
- Structured Questions: HKDSE Chemistry A Modern View Part VIII Chemical Reactions and EnergyDokument21 SeitenStructured Questions: HKDSE Chemistry A Modern View Part VIII Chemical Reactions and EnergyNg Swee Loong StevenNoch keine Bewertungen
- Hkdse Chemistry - A Modern View (Chemistry) : Coursebook 3 Suggested AnswersDokument71 SeitenHkdse Chemistry - A Modern View (Chemistry) : Coursebook 3 Suggested AnswersDennis Tik Hei FungNoch keine Bewertungen
- Chapter 04 QuestionDokument31 SeitenChapter 04 Questionapi-19650882Noch keine Bewertungen
- Po Leung Kuk No.1 W.H.Cheung College Yearly Examination (2021-2022) FORM 6 CHEMISTRY PAPER 2 Suggested AnswersDokument4 SeitenPo Leung Kuk No.1 W.H.Cheung College Yearly Examination (2021-2022) FORM 6 CHEMISTRY PAPER 2 Suggested AnswersChun Kit LauNoch keine Bewertungen
- Temperature and Thermometers: Multiple Choice QuestionsDokument27 SeitenTemperature and Thermometers: Multiple Choice QuestionsWaSx3lyNoch keine Bewertungen
- Cs Chem Hkdse Mock Section B EeDokument12 SeitenCs Chem Hkdse Mock Section B Eeleung_ting_2Noch keine Bewertungen
- Plkno1whcc Chemistry 2122 P1aDokument8 SeitenPlkno1whcc Chemistry 2122 P1aChun Kit LauNoch keine Bewertungen
- Chapter 1 Temperature and ThermometersDokument4 SeitenChapter 1 Temperature and ThermometersZhu JiankunNoch keine Bewertungen
- Gas Exchange in Humans: Multiple-Choice QuestionsDokument56 SeitenGas Exchange in Humans: Multiple-Choice QuestionsZ s2021 4C Ma Ka Ki Margaret 4C16Noch keine Bewertungen
- Assignment AC U2 Final eDokument28 SeitenAssignment AC U2 Final eYuenHei KwokNoch keine Bewertungen
- Hkdse Chemistry - A Modern View: (Second Edition) (Reprinted With Minor Amendments 2019)Dokument25 SeitenHkdse Chemistry - A Modern View: (Second Edition) (Reprinted With Minor Amendments 2019)Tat LNoch keine Bewertungen
- Chemsheets GCSE 1282 Revision 18 ANSDokument2 SeitenChemsheets GCSE 1282 Revision 18 ANSchinkey lolNoch keine Bewertungen
- Munsang College 2013-2014 Second Term Examination F. 5 Mathematics Extended Part - Module 1 (Calculus and Statistics)Dokument28 SeitenMunsang College 2013-2014 Second Term Examination F. 5 Mathematics Extended Part - Module 1 (Calculus and Statistics)Anson Ka Kin ChanNoch keine Bewertungen
- Air and AtmosphereDokument12 SeitenAir and Atmospherebob leowNoch keine Bewertungen
- HKDSE Chemistry 2014 Paper1ADokument12 SeitenHKDSE Chemistry 2014 Paper1ABILLYNG33050% (2)
- Reactivity of Metals: Learning GoalDokument36 SeitenReactivity of Metals: Learning GoalRyanNoch keine Bewertungen
- Concentration of SolutionsDokument20 SeitenConcentration of SolutionsRyanNoch keine Bewertungen
- HKDSE Chemistry MC Chapter 10Dokument7 SeitenHKDSE Chemistry MC Chapter 10ScribdNoch keine Bewertungen
- Evolution II: From Short-Necked To Long-NeckedDokument32 SeitenEvolution II: From Short-Necked To Long-NeckedBernardNoch keine Bewertungen
- QC - 2019-20 - Mock - S6 - Chem 1ADokument12 SeitenQC - 2019-20 - Mock - S6 - Chem 1AOof GucciNoch keine Bewertungen
- SSGS 17-18 F.6 Final Exam 1 and 2 CHEMDokument37 SeitenSSGS 17-18 F.6 Final Exam 1 and 2 CHEMKelvin ChowNoch keine Bewertungen
- Ecosoc UsaDokument1 SeiteEcosoc UsaKelvin ChowNoch keine Bewertungen
- Ecosoc UsaDokument1 SeiteEcosoc UsaKelvin ChowNoch keine Bewertungen
- Ecosoc UsaDokument1 SeiteEcosoc UsaKelvin ChowNoch keine Bewertungen
- HKDSE Biology SBA (Antimicrobial Test)Dokument8 SeitenHKDSE Biology SBA (Antimicrobial Test)Kelvin ChowNoch keine Bewertungen
- Ecosoc UsaDokument1 SeiteEcosoc UsaKelvin ChowNoch keine Bewertungen
- NTSE Stage 1 State Level Model Paper 10Dokument30 SeitenNTSE Stage 1 State Level Model Paper 10Om Prakash100% (1)
- Lecture 3 GlycosidesDokument18 SeitenLecture 3 Glycosidessami ullahNoch keine Bewertungen
- Chromatography PharmacyDokument41 SeitenChromatography PharmacyfarisaNoch keine Bewertungen
- Construction Safety ProgramDokument108 SeitenConstruction Safety Programalvin100% (3)
- 10 Scientist Contributed in ChemistryDokument4 Seiten10 Scientist Contributed in ChemistryJefferd PaetNoch keine Bewertungen
- Spesifikasi Material Finishing Unit ApartemenDokument34 SeitenSpesifikasi Material Finishing Unit ApartemenArif GumelarNoch keine Bewertungen
- Carbon Dioxide Capture by Amines Increasing The Efficiency by Amine Structure Modification PDFDokument2 SeitenCarbon Dioxide Capture by Amines Increasing The Efficiency by Amine Structure Modification PDFJorgeSantosAquinoNoch keine Bewertungen
- Sales Contract Bangladesh Complete and Signed by Jolly 22 July 2021Dokument18 SeitenSales Contract Bangladesh Complete and Signed by Jolly 22 July 2021Fantania BerryNoch keine Bewertungen
- Embedded Enzymatic Biomaterial Degradation: 6836 Macromolecules 2009, 42, 6836-6839Dokument4 SeitenEmbedded Enzymatic Biomaterial Degradation: 6836 Macromolecules 2009, 42, 6836-6839hhkkllNoch keine Bewertungen
- 3 PRE BOARD GENERAL EDUCATION Some College StudentsDokument14 Seiten3 PRE BOARD GENERAL EDUCATION Some College StudentsMary-Rose CasuyonNoch keine Bewertungen
- 51314-3985-Methanol-Induced Internal Stress CorrosDokument18 Seiten51314-3985-Methanol-Induced Internal Stress CorrosMahmoud GamalNoch keine Bewertungen
- Nafees Nastaleeq v1.02Dokument2 SeitenNafees Nastaleeq v1.02latifshaikh20Noch keine Bewertungen
- 6.0 Biology Lab ManualDokument49 Seiten6.0 Biology Lab ManualJacob SmithNoch keine Bewertungen
- Waste Management AustriaDokument34 SeitenWaste Management AustriaregiapursofNoch keine Bewertungen
- Magneto Hydro Dynamic GeneratorDokument19 SeitenMagneto Hydro Dynamic GeneratorKarthik ViratNoch keine Bewertungen
- Principles of Topical Therapy: Presented By: DR .Anjali Singh Junior Resident Department of DermatologyDokument25 SeitenPrinciples of Topical Therapy: Presented By: DR .Anjali Singh Junior Resident Department of DermatologyRiyaSinghNoch keine Bewertungen
- Chemical Changes LabDokument5 SeitenChemical Changes LabGildardo SalazarNoch keine Bewertungen
- J.ultsonch.2014.08.022 InglesDokument48 SeitenJ.ultsonch.2014.08.022 InglesBrayan LuisNoch keine Bewertungen
- Series LF210-5 Specification SheetDokument2 SeitenSeries LF210-5 Specification SheetWattsNoch keine Bewertungen
- Advantages of Green BiotechnologyDokument9 SeitenAdvantages of Green BiotechnologyDanica JuanNoch keine Bewertungen
- Gravimetric Analysis Laboratory ReportDokument9 SeitenGravimetric Analysis Laboratory ReportShawn RizalNoch keine Bewertungen
- Effect of Corrosion in StructuresDokument32 SeitenEffect of Corrosion in StructuresasvihariNoch keine Bewertungen
- Adsc of Amorphous Sugar - Mettler ToledoDokument3 SeitenAdsc of Amorphous Sugar - Mettler ToledoMarthaLuceroPerezNoch keine Bewertungen
- Inherited Overflow Metabolic Overflow RenalDokument11 SeitenInherited Overflow Metabolic Overflow RenalChrissa Mae Tumaliuan CatindoyNoch keine Bewertungen
- Masel Catalog - WiresDokument30 SeitenMasel Catalog - WiresOrtho OrganizersNoch keine Bewertungen
- Biological ManagementDokument27 SeitenBiological ManagementpatrickkayeNoch keine Bewertungen