Beruflich Dokumente
Kultur Dokumente
DeAngelis Registration Altruism and Trust
Hochgeladen von
isojukouOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
DeAngelis Registration Altruism and Trust
Hochgeladen von
isojukouCopyright:
Verfügbare Formate
Commentaire
Clinical trial registration: a statement from the
International Committee of Medical Journal Editors
Catherine De Angelis, Jeffrey M. Drazen, Frank A. Frizelle, Charlotte Haug, John Hoey,
Richard Horton, Sheldon Kotzin, Christine Laine, Ana Marusic, A. John P.M. Overbeke,
Torben V. Schroeder, Hal C. Sox, Martin B. Van Der Weyden
A
ltruism and trust lie at the heart of research on hu- taneously in all member journals, the International Com-
man subjects. Altruistic individuals volunteer for mittee of Medical Journal Editors (ICMJE) proposes com-
research because they trust that their participation prehensive trials registration as a solution to the problem of
will contribute to improved health for others and that re- selective awareness and announces that all 11 ICMJE
searchers will minimize risks to participants. In return for member journals will adopt a trials-registration policy to
the altruism and trust that make clinical research possible, promote this goal.
the research enterprise has an obligation to conduct re- The ICMJE member journals will require, as a condi-
search ethically and to report it honestly. Honest report- tion of consideration for publication, registration in a pub-
ing begins with revealing the existence of all clinical stud- lic trials registry. Trials must register at or before the onset
ies, even those that reflect unfavourably on a research of patient enrollment. This policy applies to any clinical
sponsor’s product. trial starting enrollment after July 1, 2005. For trials that
Unfortunately, selective reporting of trials does occur, began enrollment before this date, the ICMJE member
and it distorts the body of evidence available for clinical de- journals will require registration by Sept. 13, 2005, before
cision-making. Researchers (and journal editors) are gener- considering the trial for publication. We speak only for
ally most enthusiastic about the publication of trials that ourselves, but we encourage editors of other biomedical
show either a large effect of a new treatment (positive tri- journals to adopt similar policies. For this purpose, the
als) or equivalence of 2 approaches to treatment (non- ICMJE defines a clinical trial as any research project that
inferiority trials). Researchers (and journals) typically are prospectively assigns human subjects to intervention or
less excited about trials that show that a new treatment is comparison groups to study the cause-and-effect relation-
inferior to standard treatment (negative trials) and even less ship between a medical intervention and a health outcome.
interested in trials that are neither clearly positive nor Studies designed for other purposes, such as to study phar-
clearly negative, since inconclusive trials will not in them- macokinetics or major toxicity (e.g., phase I trials), would
selves change practice. Irrespective of their scientific inter- be exempt.
est, trial results that place financial interests at risk are par- The ICMJE does not advocate one particular registry,
ticularly likely to remain unpublished and hidden from but its member journals will require authors to register
public view. The interests of the sponsor or authors not- their trial in a registry that meets several criteria. The reg-
withstanding, anyone should be able to learn of any trial’s istry must be accessible to the public at no charge. It must
existence and its important characteristics. be open to all prospective registrants and managed by a
The case against selective reporting is particularly com- not-for-profit organization. There must be a mechanism
pelling for research that tests interventions that could enter to ensure the validity of the registration data, and the reg-
mainstream clinical practice. Rather than a single trial, it is istry should be electronically searchable. An acceptable
usually a body of evidence, consisting of many studies, that registry must include at minimum the following informa-
changes medical practice. When research sponsors or in- tion: a unique identifying number, a statement of the in-
vestigators conceal the presence of selected trials, these tervention (or interventions) and comparison (or compar-
studies cannot influence the thinking of patients, clinicians, isons) studied, a statement of the study hypothesis,
other researchers, and experts who write practice guidelines definitions of the primary and secondary outcome mea-
or decide on insurance-coverage policy. If all trials are reg- sures, eligibility criteria, key trial dates (registration date,
DOI:10.1503/cmaj.1041281
istered in a public repository at their inception, every trial’s anticipated or actual start date, anticipated or actual date
existence is part of the public record and the many stake- of last follow-up, planned or actual date of closure to data
holders in clinical research can explore the full range of entry, and date trial data considered complete), target
clinical evidence. We are far from this ideal at present, number of subjects, funding source, and contact informa-
since trial registration is largely voluntary, registry data sets tion for the principal investigator. To our knowledge, at
and public access to them vary, and registries contain only a present, only www.clinicaltrials.gov, sponsored by the US
small proportion of trials. In this editorial, published simul- National Library of Medicine, meets these requirements;
606 JAMC • 14 SEPT. 2004; 171 (6)
Commentary
there may be other registries, now or in the future, that This document is not covered by copyright and may be copied or reprinted with-
out permission.
meet all these requirements.
Registration is only part of the means to an end; that All authors are members of the International Committee of Medical Journal Edi-
tors: Catherine De Angelis, Editor-in-Chief, JAMA; Jeffrey M. Drazen, Editor-in-
end is full transparency with respect to performance and Chief, New England Journal of Medicine; Frank A. Frizelle, Editor, The New Zealand
reporting of clinical trials. Research sponsors may argue Medical Journal; Charlotte Haug, Editor-in-Chief, Norwegian Medical Journal; John
Hoey, Editor, CMAJ; Richard Horton, Editor, The Lancet; Sheldon Kotzin, Execu-
that public registration of clinical trials will result in unnec- tive Editor, MEDLINE, National Library of Medicine; Christine Laine, Senior
essary bureaucratic delays and destroy their competitive Deputy Editor, Annals of Internal Medicine; Ana Marusic, Editor, Croatian Medical
Journal; A. John P.M. Overbeke, Executive Editor, Nederlands, Tijdschrift voor Ge-
edge by allowing competitors full access to their research neeskunde (Dutch Journal of Medicine); Torben V. Schroeder, Editor, Journal of the
plans. We argue that enhanced public confidence in the re- Danish Medical Association; Hal C. Sox, Editor, Annals of Internal Medicine; Martin
search enterprise will compensate for the costs of full dis- B. Van Der Weyden, Editor, The Medical Journal of Australia.
closure. Patients who volunteer to participate in clinical tri- Competing interests: None declared.
als deserve to know that their contribution to improving
human health will be available to inform health care deci-
sions. The knowledge made possible by their collective al- Correspondence to: John Hoey, Editor, CMAJ, 1867 Alta
truism must be accessible to everyone. Required trial regis- Vista Dr., Ottawa ON K1G 3Y6; fax 613 565-5471;
tration will advance this goal. john.hoey@cma.ca
CMAJ’s Editorial Fellowship
The CMAJ EDITORIAL FELLOWSHIP, launched in 1998, provides an exciting opportunity for physicians early in
their training to discover the inner workings of a leading medical journal. Applications are invited from recent
medical graduates and residents who are interested in obtaining a rich experience in medical writing, editing and
publishing. The fellow participates in all aspects of journal production, ranging from deciding which manuscripts
to publish and working with authors to soliciting commentaries and review articles. Fellows are also expected to
write extensively and are encouraged to develop theme issues, series or other journal innovations.
The position is full time for one year and “You will be exposed to aspects of medicine that you never had
is based at CMAJ’s offices in Ottawa. occasion to ponder before. And, perhaps, you will better
The salary is based on the equivalent understand why we physicians do what we do.”
residency remuneration in Ontario. — James Maskalyk, fellow 2002
The next round of applications is for
the 2005 fellowship, which begins “The fellowship is an amazing
July 1, 2005. The application deadline opportunity to develop new skills and
is December 15, 2004. learn what it means to be an editor.
For more information, please contact Prepare yourself for an exciting year
Dr. John Hoey, Editor, at as you watch new developments in
john.hoey@cma.ca. modern medicine unfold before your
eyes and help shape how physicians
interpret them.”
— Steve Choi, fellow 2003
CMAJ • SEPT. 14, 2004; 171 (6) 607
Das könnte Ihnen auch gefallen
- Agenda - JC 2008Dokument1 SeiteAgenda - JC 2008isojukouNoch keine Bewertungen
- Statistics Review 7 Correlation and RegressionDokument9 SeitenStatistics Review 7 Correlation and RegressionManish Chandra PrabhakarNoch keine Bewertungen
- ΧρυσοθήρεςDokument1 SeiteΧρυσοθήρεςisojukouNoch keine Bewertungen
- Σύμφωνο των ΝησιώνDokument4 SeitenΣύμφωνο των ΝησιώνisojukouNoch keine Bewertungen
- Statistics Review 6 Non Parametric MethodsDokument5 SeitenStatistics Review 6 Non Parametric MethodsManish Chandra PrabhakarNoch keine Bewertungen
- Statistics Review 11 Assessing RiskDokument5 SeitenStatistics Review 11 Assessing RiskManish Chandra PrabhakarNoch keine Bewertungen
- Wager E - PLoS ONE - JAMA Published Fewer Industry-Funded Studies After Introducing A Requirement For Independent Statistical AnalysisDokument3 SeitenWager E - PLoS ONE - JAMA Published Fewer Industry-Funded Studies After Introducing A Requirement For Independent Statistical AnalysisisojukouNoch keine Bewertungen
- Gotzsche - PLoS Medicine - What Should We Done To Tackle Ghostwriting in The Medical LiteratuteDokument4 SeitenGotzsche - PLoS Medicine - What Should We Done To Tackle Ghostwriting in The Medical LiteratuteisojukouNoch keine Bewertungen
- CONSORT 2010 Statement: Updated Guidelines For Reporting Parallel Group Randomised TrialsDokument8 SeitenCONSORT 2010 Statement: Updated Guidelines For Reporting Parallel Group Randomised TrialsAshwini KalantriNoch keine Bewertungen
- Statistics Non Parametric MethodsDokument4 SeitenStatistics Non Parametric MethodsСварооп СатурнскыNoch keine Bewertungen
- Statistics Review 13 Receiver Operating Characteristic CurvesDokument5 SeitenStatistics Review 13 Receiver Operating Characteristic CurvesManish Chandra PrabhakarNoch keine Bewertungen
- Whitley Statistics Review 3Dokument1 SeiteWhitley Statistics Review 3isojukouNoch keine Bewertungen
- Statistics Review 12 Survival AnalysisDokument6 SeitenStatistics Review 12 Survival AnalysisManish Chandra PrabhakarNoch keine Bewertungen
- Statistics Review 9 One-Way Analysis of VarianceDokument7 SeitenStatistics Review 9 One-Way Analysis of VarianceManish Chandra PrabhakarNoch keine Bewertungen
- 6th Central Pay Commission Salary CalculatorDokument15 Seiten6th Central Pay Commission Salary Calculatorrakhonde100% (436)
- Statistics Review 5 Comparison of MeansDokument5 SeitenStatistics Review 5 Comparison of MeansManish Chandra PrabhakarNoch keine Bewertungen
- Twenty Statistical Errors Even YOU Can Find in Biomedical Research Articles - Tom Lang CMJ 2004Dokument10 SeitenTwenty Statistical Errors Even YOU Can Find in Biomedical Research Articles - Tom Lang CMJ 2004isojukouNoch keine Bewertungen
- Whitley Statistics Review 2Dokument1 SeiteWhitley Statistics Review 2isojukouNoch keine Bewertungen
- CONSORT ExplanationDokument32 SeitenCONSORT Explanationapi-3699557Noch keine Bewertungen
- GPP2Dokument8 SeitenGPP2isojukouNoch keine Bewertungen
- Statistics Review 4 Sample Size CalculationsDokument7 SeitenStatistics Review 4 Sample Size CalculationsManish Chandra PrabhakarNoch keine Bewertungen
- Whitley Statistics Review 1Dokument6 SeitenWhitley Statistics Review 1isojukouNoch keine Bewertungen
- Gpp2 Chris Graf Ismpp Eu May2010Dokument32 SeitenGpp2 Chris Graf Ismpp Eu May2010isojukouNoch keine Bewertungen
- Αθαναζία Μπενέκος Medical WriterDokument14 SeitenΑθαναζία Μπενέκος Medical WriterisojukouNoch keine Bewertungen
- Peer Review Process: Athanasia Benekou Medical WriterDokument21 SeitenPeer Review Process: Athanasia Benekou Medical WriterisojukouNoch keine Bewertungen
- Αζαλαζία Μπελέθνπ Medical WriterDokument11 SeitenΑζαλαζία Μπελέθνπ Medical WriterisojukouNoch keine Bewertungen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- 03 - Gherkin An OverviewDokument19 Seiten03 - Gherkin An OverviewArunkumar KrishnamoorthyNoch keine Bewertungen
- Troubleshooting Lab 1Dokument1 SeiteTroubleshooting Lab 1Lea SbaizNoch keine Bewertungen
- Area Manager ChecklistDokument7 SeitenArea Manager ChecklistUtkarsh RaiNoch keine Bewertungen
- Collection of Books To Read Preparing For ACM ICPCDokument1 SeiteCollection of Books To Read Preparing For ACM ICPCJia Hong100% (2)
- Fop 2.1Dokument11 SeitenFop 2.1Paramita HalderNoch keine Bewertungen
- 5 Overview PsasDokument19 Seiten5 Overview Psasعلي صالحNoch keine Bewertungen
- The Danieli Danarc Plus M Furnace at Abs Meltshop: Aldo A. Fior Danieli C M - Process Engineer Buttrio, ItalyDokument6 SeitenThe Danieli Danarc Plus M Furnace at Abs Meltshop: Aldo A. Fior Danieli C M - Process Engineer Buttrio, ItalyBrandon CoxNoch keine Bewertungen
- 3D Technical Data Package Configuration Management, Modeling and Drawing ProcedureDokument175 Seiten3D Technical Data Package Configuration Management, Modeling and Drawing Procedurejesse_w_petersNoch keine Bewertungen
- Sae Technical Paper Series 2015-36-0353: Static and Dynamic Analysis of A Chassis of A Prototype CarDokument12 SeitenSae Technical Paper Series 2015-36-0353: Static and Dynamic Analysis of A Chassis of A Prototype CarGanesh KCNoch keine Bewertungen
- Design of A Neural Network Function Block For Insertion Into The Function Block Library of A Programmable Logic ControllerDokument4 SeitenDesign of A Neural Network Function Block For Insertion Into The Function Block Library of A Programmable Logic ControllerArmando Fermin PerezNoch keine Bewertungen
- Machine Guarding PrinciplesDokument5 SeitenMachine Guarding Principlesliveconnectionz282Noch keine Bewertungen
- 08 - Truck Driver's Dhobi SinkDokument3 Seiten08 - Truck Driver's Dhobi SinkfebousNoch keine Bewertungen
- QO™ Load Centers - QO124M200PDokument4 SeitenQO™ Load Centers - QO124M200PIsraelNoch keine Bewertungen
- Philips 170v7fbDokument95 SeitenPhilips 170v7fbaposticaaNoch keine Bewertungen
- Usg Sheetrock® Brand Acoustical SealantDokument3 SeitenUsg Sheetrock® Brand Acoustical SealantHoracio PadillaNoch keine Bewertungen
- This Study Resource Was: Practice Questions and Answers Inventory Management: EOQ ModelDokument7 SeitenThis Study Resource Was: Practice Questions and Answers Inventory Management: EOQ Modelwasif ahmedNoch keine Bewertungen
- Paper Velocity String SPE-30197-PADokument4 SeitenPaper Velocity String SPE-30197-PAPablo RaffinNoch keine Bewertungen
- Suggested For You: 15188 5 Years Ago 20:50Dokument1 SeiteSuggested For You: 15188 5 Years Ago 20:50DeevenNoch keine Bewertungen
- Air Blue E-TicketDokument1 SeiteAir Blue E-TicketMuneeb Ahmed100% (3)
- Languages and CommunicationDokument17 SeitenLanguages and CommunicationDERICK REBAYNoch keine Bewertungen
- Hwids - 2012 05 22 - 19 04 00Dokument9 SeitenHwids - 2012 05 22 - 19 04 00RONAL DAMIANO PAREJANoch keine Bewertungen
- Duplichecker Plagiarism Report 3Dokument3 SeitenDuplichecker Plagiarism Report 3Mushfiqur RahmanNoch keine Bewertungen
- ION-CEDI-BR Ion PureDokument8 SeitenION-CEDI-BR Ion PureAndri YantoNoch keine Bewertungen
- IEC 60793-1-30-2001 Fibre Proof TestDokument12 SeitenIEC 60793-1-30-2001 Fibre Proof TestAlfian Firdaus DarmawanNoch keine Bewertungen
- The Electric Field Due To A Continuous Charge Distribution (Worked Examples)Dokument13 SeitenThe Electric Field Due To A Continuous Charge Distribution (Worked Examples)Elias BojagoNoch keine Bewertungen
- List of British StandardsDokument6 SeitenList of British StandardsPankajNoch keine Bewertungen
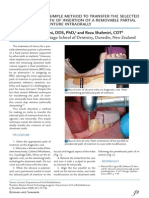
- A Simple Method To Transfer The SelectedDokument2 SeitenA Simple Method To Transfer The SelectedrekabiNoch keine Bewertungen
- Vehicle and Driver Vibration - PPTDokument16 SeitenVehicle and Driver Vibration - PPTAnirban MitraNoch keine Bewertungen
- HydrodynamicsDokument122 SeitenHydrodynamicsIustin Cristian100% (2)