Beruflich Dokumente
Kultur Dokumente
CDC: Understanding The Difference Between Masks
Hochgeladen von
Ed PraetorianOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
CDC: Understanding The Difference Between Masks
Hochgeladen von
Ed PraetorianCopyright:
Verfügbare Formate
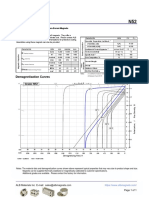
Understanding the Difference
! WARNING
Lorem ipsum dolor sit amet,
consectetur adipiscing elit. Nullam
scelerisque leo et eros convallis
condimentum. Phasellus tincidunt,
volutpat vitae.
Elastomeric Half
Surgical Mask N95 Respirator Facepiece Respirator
Testing and Cleared by the U.S. Food and Evaluated, tested, and Evaluated, tested, and
Approval Drug Administration (FDA) approved by NIOSH as per the approved by NIOSH as per the
requirements in requirements in
42 CFR Part 84* 42 CFR Part 84
Intended Use and Fluid resistant and provides the Reduces wearer’s exposure to Reusable device made of
Purpose wearer protection against large particles including small particle synthetic or rubber material
droplets, splashes, or sprays of aerosols and large droplets (only
bodily or other hazardous fluids. non-oil aerosols)
Protects the patient from the
wearer’s respiratory emissions.
Face Seal Fit Loose-fitting Tight-fitting Tight-fitting
Fit Testing No Yes Yes
Requirement
Designed for No No Yes
Reuse
User Seal Check No Yes. Required each time the Yes. Required each time the
respirator is donned (put on) respirator is donned (put on)
Filtration Does NOT provide the wearer Filters out at least 95% of May be equipped with filters
with a reliable level of protection airborne particles including large that block 95%, 99%, or 100%
from inhaling smaller airborne and small particles of very small particulates. Also
particles and is not considered may be equipped to protect
respiratory protection against vapors/gases.
Leakage Leakage occurs around the edge When properly fitted and When properly fitted and
of the mask when user inhales donned, minimal leakage occurs donned, minimal leakage occurs
around edges of the respirator around edges of the respirator
when user inhales when user inhales
Use Limitations Disposable. Discard after each Ideally should be discarded after Reusable and must be cleaned/
patient encounter. each patient encounter and after disinfected and stored between
aerosol-generating procedures. each patient interaction
It should also be discarded
when it becomes damaged
or deformed; no longer forms
an effective seal to the face;
becomes wet or visibly dirty;
breathing becomes difficult;
or if it becomes contaminated
with blood, respiratory or nasal
secretions, or other bodily fluids.
*
As of July 2, 2018, NIOSH evaluates N95 FFRs intended for use in healthcare for
biocompatibility, flammability, and fluid resistance to ensure conformity to relevant
standards during the approval process. These tasks were previously performed by the FDA.
Resources:
Centers for Disease Control Hospital Respiratory Protection Program Toolkit
and Prevention http://www.cdc.gov/niosh/docs/2015-117/pdfs/2015-117.pdf
National Institute for Occupational Implementing Hospital Respiratory Protection Programs: Strategies from the Field
Safety and Health
https://www.jointcommission.org/assets/1/18/Implementing_Hospital_RPP_2-19-15.pdf
Das könnte Ihnen auch gefallen
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- FINAL REPORT-Durham Racial Equity Task Force 7.22.20Dokument67 SeitenFINAL REPORT-Durham Racial Equity Task Force 7.22.20Ed PraetorianNoch keine Bewertungen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Mental Health Training and Intervention: A Critical Component of Police ReformDokument14 SeitenMental Health Training and Intervention: A Critical Component of Police ReformEd Praetorian100% (2)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Police Mental Health CollaborationsDokument24 SeitenPolice Mental Health CollaborationsEd Praetorian100% (3)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Autism InfographicDokument1 SeiteAutism InfographicEd PraetorianNoch keine Bewertungen
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (894)
- Interagency Legal Advisory On UAS Detection and Mitigation TechnologiesDokument9 SeitenInteragency Legal Advisory On UAS Detection and Mitigation TechnologiesEd PraetorianNoch keine Bewertungen
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- NLEOMF 2020 Mid Year Fatality ReportDokument13 SeitenNLEOMF 2020 Mid Year Fatality ReportEd PraetorianNoch keine Bewertungen
- 10 Essential Actions To Improve School SafetyDokument48 Seiten10 Essential Actions To Improve School SafetyEd Praetorian100% (1)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Report On Police Reform and Racial JusticeDokument40 SeitenReport On Police Reform and Racial Justicejudith retanaNoch keine Bewertungen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Report On Police Reform and Racial JusticeDokument40 SeitenReport On Police Reform and Racial Justicejudith retanaNoch keine Bewertungen
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- Directive 2020 11Dokument13 SeitenDirective 2020 11Ed PraetorianNoch keine Bewertungen
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- 07.20.20 Letter To DOJ and DHSDokument2 Seiten07.20.20 Letter To DOJ and DHSEd PraetorianNoch keine Bewertungen
- 5f07cb77956f0 FileDokument2 Seiten5f07cb77956f0 FileEd PraetorianNoch keine Bewertungen
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- Georgia V Atlanta Mask Mandate LawsuitDokument124 SeitenGeorgia V Atlanta Mask Mandate LawsuitEd PraetorianNoch keine Bewertungen
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Reopen Schools F 2Dokument24 SeitenReopen Schools F 2Ed PraetorianNoch keine Bewertungen
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Pew Research Report On Public Perception of PoliceDokument25 SeitenPew Research Report On Public Perception of PoliceEd PraetorianNoch keine Bewertungen
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- CHS Call To Action Pandemic V Schools Final 7-9-20Dokument49 SeitenCHS Call To Action Pandemic V Schools Final 7-9-20Ed PraetorianNoch keine Bewertungen
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- California DOJ Report On Sacramento PDDokument104 SeitenCalifornia DOJ Report On Sacramento PDEd Praetorian100% (1)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- Open Letter From Mpls Police Leaders To EveryoneDokument1 SeiteOpen Letter From Mpls Police Leaders To EveryoneEd PraetorianNoch keine Bewertungen
- Fact Sheet HR 2 Moving Forward Act FINALDokument5 SeitenFact Sheet HR 2 Moving Forward Act FINALEd PraetorianNoch keine Bewertungen
- Budget Equity ToolDokument22 SeitenBudget Equity ToolEd PraetorianNoch keine Bewertungen
- Hate Crime Statutes June 2018Dokument2 SeitenHate Crime Statutes June 2018Ed PraetorianNoch keine Bewertungen
- FI Certification Standards - June 2020Dokument18 SeitenFI Certification Standards - June 2020Ed Praetorian100% (3)
- June 5 Class DocumentDokument38 SeitenJune 5 Class DocumentEd PraetorianNoch keine Bewertungen
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- On Civil .Dokument50 SeitenOn Civil .Ed Praetorian100% (1)
- Executive Order On Safe Policing For Safe CommunitiesDokument8 SeitenExecutive Order On Safe Policing For Safe CommunitiesEd Praetorian100% (3)
- State V BessDokument15 SeitenState V BessEd PraetorianNoch keine Bewertungen
- NC School Health Reopening GuidanceDokument26 SeitenNC School Health Reopening GuidanceEd PraetorianNoch keine Bewertungen
- 2020 05 27 ELW Facts About Voting by MailDokument29 Seiten2020 05 27 ELW Facts About Voting by MailEd PraetorianNoch keine Bewertungen
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Multi-Family BuildingsDokument152 SeitenMulti-Family BuildingsEERI New York-Northeast Regional ChapterNoch keine Bewertungen
- Ready To Respond Tabletop ExerciseDokument103 SeitenReady To Respond Tabletop ExerciseEd PraetorianNoch keine Bewertungen
- Densification and Microstructure of Si3N4-TiN Ceramic CompositesDokument5 SeitenDensification and Microstructure of Si3N4-TiN Ceramic CompositesThiago Do Santos FerreiraNoch keine Bewertungen
- Chapter 7 PDFDokument36 SeitenChapter 7 PDFRbtl BañosNoch keine Bewertungen
- Molecular Biology IB ReviewerDokument28 SeitenMolecular Biology IB ReviewerCeline Garin ColadaNoch keine Bewertungen
- Mechanical Properties For Stainless Steel FastenersDokument3 SeitenMechanical Properties For Stainless Steel FastenersGonzalo MazaNoch keine Bewertungen
- QS607 220310Dokument3 SeitenQS607 220310Jet ToledoNoch keine Bewertungen
- Hysys 8.8 - ManualDokument606 SeitenHysys 8.8 - ManualCarlos Vaz88% (8)
- Atomic Force Microscope (AFM)Dokument36 SeitenAtomic Force Microscope (AFM)s11925877Noch keine Bewertungen
- Feasibility Study of Cumene ProductionDokument4 SeitenFeasibility Study of Cumene ProductionIntratec SolutionsNoch keine Bewertungen
- N52 Grade Neodymium Magnets DataDokument1 SeiteN52 Grade Neodymium Magnets DataSteve HsuNoch keine Bewertungen
- Cyclones ExerciseDokument4 SeitenCyclones ExerciseValeria cNoch keine Bewertungen
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- MWPA404 Cathodic Protection Guideline Rev 0Dokument44 SeitenMWPA404 Cathodic Protection Guideline Rev 0허윤호Noch keine Bewertungen
- Powder Metallurgy Process and ApplicationsDokument32 SeitenPowder Metallurgy Process and ApplicationsChandan KumarNoch keine Bewertungen
- Manufacturing and Metal Working Process ClassificationDokument5 SeitenManufacturing and Metal Working Process ClassificationPeeka Prabhakara RaoNoch keine Bewertungen
- Thermodynamics For EngineersDokument620 SeitenThermodynamics For Engineersgsuresh40100% (8)
- Prodinfo Antox-75-E Eng FinalDokument2 SeitenProdinfo Antox-75-E Eng FinalKumar RamanNoch keine Bewertungen
- A Simplified Method For The Cultivation of Extreme Anaerobic Archaea Based SULFIDE 2000 !!!!Dokument6 SeitenA Simplified Method For The Cultivation of Extreme Anaerobic Archaea Based SULFIDE 2000 !!!!Vera Brok-VolchanskayaNoch keine Bewertungen
- SuperPur Product InformationDokument1 SeiteSuperPur Product InformationRamNoch keine Bewertungen
- HRSG1 (终版)Dokument120 SeitenHRSG1 (终版)Atif KhanNoch keine Bewertungen
- DP Level Measurement BasicsDokument2 SeitenDP Level Measurement Basicsjsrplc7952Noch keine Bewertungen
- Materials Used in Automotive Manufacture and Material Selection Using Ashby ChartsDokument15 SeitenMaterials Used in Automotive Manufacture and Material Selection Using Ashby ChartsHanumantNoch keine Bewertungen
- Chap 7 PDFDokument45 SeitenChap 7 PDFSuharto Masacal AmpasoNoch keine Bewertungen
- Nuclear Power: Pros, Cons and FutureDokument4 SeitenNuclear Power: Pros, Cons and FutureSamarthNoch keine Bewertungen
- Effectiveness of Liquid Oxygen BleachDokument4 SeitenEffectiveness of Liquid Oxygen BleachSingh GurleenNoch keine Bewertungen
- Msds - Marpozol W-505 (GHS) Eng 130409Dokument5 SeitenMsds - Marpozol W-505 (GHS) Eng 130409Syafarul Mohammad100% (1)
- Comenius - CodDokument13 SeitenComenius - CodsridharancNoch keine Bewertungen
- Mark Scheme (Results) Summer 2015: GCE Chemistry (6CH01/01) The Core Principles of ChemistryDokument21 SeitenMark Scheme (Results) Summer 2015: GCE Chemistry (6CH01/01) The Core Principles of ChemistryAmeenIbrahimNoch keine Bewertungen
- Medical Entrance Exam AnswersDokument24 SeitenMedical Entrance Exam AnswersSanskruti ChavanNoch keine Bewertungen
- 31.PEAK Depressurization RATEDokument1 Seite31.PEAK Depressurization RATEDILIP MATALNoch keine Bewertungen
- Problem #2b: Chromium Crystallizes With A Body-Centered Cubic Unit Cell. The Radius of ADokument8 SeitenProblem #2b: Chromium Crystallizes With A Body-Centered Cubic Unit Cell. The Radius of ARadica AyuNoch keine Bewertungen
- Advion MSDSDokument6 SeitenAdvion MSDSmoespestcontrol_mnNoch keine Bewertungen
- Epic Measures: One Doctor. Seven Billion Patients.Von EverandEpic Measures: One Doctor. Seven Billion Patients.Bewertung: 4 von 5 Sternen4/5 (13)
- Uncontrolled Spread: Why COVID-19 Crushed Us and How We Can Defeat the Next PandemicVon EverandUncontrolled Spread: Why COVID-19 Crushed Us and How We Can Defeat the Next PandemicNoch keine Bewertungen
- Summary: The Myth of Normal: Trauma, Illness, and Healing in a Toxic Culture By Gabor Maté MD & Daniel Maté: Key Takeaways, Summary & AnalysisVon EverandSummary: The Myth of Normal: Trauma, Illness, and Healing in a Toxic Culture By Gabor Maté MD & Daniel Maté: Key Takeaways, Summary & AnalysisBewertung: 4 von 5 Sternen4/5 (9)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeVon EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeBewertung: 5 von 5 Sternen5/5 (1)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeVon EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeBewertung: 4.5 von 5 Sternen4.5/5 (3)
- Do You Believe in Magic?: The Sense and Nonsense of Alternative MedicineVon EverandDo You Believe in Magic?: The Sense and Nonsense of Alternative MedicineNoch keine Bewertungen
- The Wisdom of Plagues: Lessons from 25 Years of Covering PandemicsVon EverandThe Wisdom of Plagues: Lessons from 25 Years of Covering PandemicsBewertung: 4.5 von 5 Sternen4.5/5 (5)
- The HPV Vaccine On Trial: Seeking Justice For A Generation BetrayedVon EverandThe HPV Vaccine On Trial: Seeking Justice For A Generation BetrayedBewertung: 4.5 von 5 Sternen4.5/5 (13)